Back to Journals » Drug Design, Development and Therapy » Volume 11
Combinatorial and sequential delivery of gemcitabine and oseltamivir phosphate from implantable poly(D,L-lactic-co-glycolic acid) cylinders disables human pancreatic cancer cell survival
Authors Allison Logan S , Brissenden AJ , Szewczuk MR , Neufeld RJ
Received 24 March 2017
Accepted for publication 23 June 2017
Published 31 July 2017 Volume 2017:11 Pages 2239—2250
DOI https://doi.org/10.2147/DDDT.S137934
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Georgios Panos
Stephanie Allison Logan,1 Amanda J Brissenden,1 Myron R Szewczuk,2 Ronald J Neufeld1
1Department of Chemical Engineering, 2Department of Biomedical and Molecular Sciences, Queen’s University, Kingston, ON, Canada
Abstract: Combination therapies against multiple targets are currently being developed to prevent resistance to a single chemotherapeutic agent and to extirpate pre-existing resistance in heterogeneous cancer cells in tumors due to selective pressure from the single agent. Gemcitabine (GEM), a chemotherapeutic agent, is the current standard of care for patients with pancreatic cancer. Patients with pancreatic cancer receiving GEM have a low progression-free survival. Given the poor response rate to GEM, cancer cells are known to develop rapid resistance to this drug. Metronomic chemotherapy using combinatorial and sequential delivery systems are novel developmental approaches to disrupt tumor neovascularization, reduce systemic drug toxicity, and increase the sensitivity of chemotherapeutics in cancer. Here, implantable double-layered poly(D,L-lactic-co-glycolic acid) (PLGA) cylinders were engineered to sequentially release GEM in combination with oseltamivir phosphate (OP) over an extended time. Double-layered PLGA cylindrical implants loaded with these active hydrophilic drugs were fabricated with minimal loss of drugs during the formulation, enabling extensive control of drug loading and establishing uniform drug distribution throughout the polymer matrix. OP is used in the formulation because of its anticancer drug properties targeting mammalian neuraminidase 1 (Neu1) involved in multistage tumorigenesis. OP and GEM encapsulated in inner/outer GEMin/OPout or OPin/GEMout implantable PLGA double-layered cylinders displayed sustained near linear release over 30 days. OP and GEM released from the double-layered cylinders effectively reduced cell viability in pancreatic cancer cell line PANC1 and its GEM-resistant variant for up to 15 days.
Keywords: pancreatic cancer, oseltamivir phosphate, gemcitabine, PLGA, chemoresistance
Introduction
Implantable chemotherapeutic delivery systems are designed to provide sustained release of a drug at the tumor site, providing an optimal dosing for a continuous therapeutic effect while reducing adverse effects associated with systemic chemotherapy.1 Metronomic chemotherapy, which involves lower, more frequent dosing of drug, has resulted in reduced tumor volumes and fewer adverse effects than standard chemotherapy in murine models of pancreatic cancer.2–4 Furthermore, in patients with pancreatic ductal adenocarcinoma (PDAC), there is an elevated frequency of acquired chemoresistance, which has been linked to highly penetrant genetic mutations at various loci, including Kirsten ras (KRAS) oncogene, tumor suppressor p53, cyclin-dependent kinase inhibitor 2A (CDKN2A), and mothers against decapentaplegic homolog-4/deleted in pancreatic cancer-4 (smad4/DPC4).5 Gemcitabine (GEM) hydrochloride (20,20-difluoro-20-deoxycytidine or dFdC) is a hydrophilic chemotherapeutic drug, which is used as the standard of care for patients with unresectable pancreatic cancer. However, cancer resistance to GEM is a major problem during patient treatment.6 Recently, we reported that oseltamivir phosphate (OP) is an effective anticancer agent capable of sensitizing GEM-resistant pancreatic cancer cells to GEM, thereby increasing the efficacy of the chemotherapeutic agent.7,8 In addition, we have reported that poly(D,L-lactic-co-glycolic acid) (PLGA)-loaded OP cylinders surgically implanted at the tumor site in a RAG2xCγ double mutant mouse model of human pancreatic cancer inhibited not only tumor growth but also tumor neovascularization and metastasis to the liver and lungs compared with the untreated cohort over the 30 days release period.9 The xenograft human pancreatic tumors from PLGA-OP-treated cohorts also expressed significantly higher levels of E-cadherin with concomitant reduced N-cadherin and host CD31+ endothelial cells compared to the untreated cohort. We also reported sustained release of OP over 30 days from the implantable PLGA-OP cylinder.9 Despite difficulties with the encapsulation of small hydrophilic drugs, particulate OP encapsulation within polymeric PLGA cylinders using the formulation method described in these studies resulted in full retention of the drug. These results clearly indicated that OP delivered from PLGA cylinders surgically implanted at the tumor site shows promise as an effective treatment therapy for pancreatic cancer. To this end, we defined the optimal combinations and/or sequences of GEM with the novel OP therapy, which may be a more effective treatment regimen than with GEM alone in preventing acquired chemoresistance.
One of the important challenges in drug delivery is the difficulty inherent in the full encapsulation and retention, followed by long-term and targeted delivery of small molecular weight, hydrophilic therapeutics at a tumor site. PLGA is a copolymer composed of lactic and glycolic acid monomers and has been used as a drug delivery vehicle. PLGA is susceptible to hydrolytic degradation of the ester linkage on the polymer backbone, which results in release of the encapsulated drug.10 PLGA has been used to encapsulate a wide range of therapeutics, and several PLGA drug delivery applications, such as Lupron Depot, Risperdal Conta, and Zoladex, have been approved by the US Food and Drug Administration, European Medicine Agency, and Health Canada.11 However, therapeutics demonstrating extended term, sustained release (weeks or longer) from PLGA polymer are hydrophobic in nature with molecular weights of at least 400 g/mol, with most being >1,000 g/mol. Extended term, sustained release of small molecular weight hydrophilic chemotherapeutics from PLGA is thus a challenge to achieve.
In the present study, double-layered PLGA GEM and OP-loaded cylindrical implants were designed and engineered to provide an optimal combinatorial and sequential sustained release of the hydrophilic drugs for 30 days. This report describes the efficacy of this unique delivery system in the treatment of pancreatic cancer cells and its ability to disable the survival mechanism of pancreatic cancer with acquired chemoresistance. Here, OP and GEM encapsulated in distinct inner/outer layers of implantable double-layered PLGA cylinders disabled pancreatic cancer cell survival and increased sensitivity to therapy involving combinatorial and sequential delivery of two small molecular weight hydrophilic chemotherapeutics.
Materials and methods
Reagents
GEM hydrochloride was obtained from Sigma-Aldrich Co. (St Louis, MO, USA). OP with 98% purity was obtained from Hangzhou DayangChem Co., Ltd (Hangzhou, China). PLGA (50/50) was obtained from Purac Biomaterials (Gorinchem, the Netherlands). The molecular weight was determined by gel permeation chromatography (Viscotek GPCmas VE 2001; Malvern Instruments, Malvern, UK) and calibrated with poly(ethylene oxide) standards. PLGA was determined to have a molecular weight (MW) of 16,400 (MW/Mn =1.58).
Cell lines
PANC1 (human epithelioid carcinoma, epithelial-like, ATCC® CRL-1469™) was purchased from the American Type Culture Collection (ATCC) (Manassas, VA, USA). Cells were grown at 37°C with 5% CO2 and cultured with 1× Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS) and 5 μg/mL Plasmocin™. PANC1 cells resistant to GEM (PANC1-GEMR) were developed by culturing PANC1 cells in 1× DMEM containing 10% FBS and 5 μg/mL Plasmocin and gradually increasing the concentrations of GEM up to 0.01 μM. PANC1-GEMR cells are stable and have been cultured in conditioned medium containing 0.01 μM GEM for >3 years.
OP and GEM detection
A 1260 Infinity high performance liquid chromatography (HPLC) and a Poroshell 120 SB-C18 threaded column (Agilent Technologies, Santa Clara, CA, USA) 4.6×50 mm (2.7 μm) were used. OP (Hangzhou Dayang Chem Co., Ltd) and GEM were dissolved in HPLC grade methanol. The mobile phase was 60% HPLC grade methanol and 40% 0.04 M ammonium acetate buffer (pH 5.2) at a flow rate of 1 mL/min. The column temperature was 25°C, the injection volume was 20 μL, and OP and GEM were detected at 230 nm.
Formulation of cylinders containing OP and GEM
PLGA (80 mg), 4–6 mg sorbitan monooleate (Span 80), and the desired drug were mixed together in 400 μL acetone. The solution was ejected dropwise onto a Teflon sheet and then stored at 20°C for 72 h. The polymer film was removed from the Teflon sheet using a razor blade and rolled around a 16 G needle tip. The razor blade and needle tip were lubricated with glycerol to prevent the film from sticking. Once rolled, the hollow polymer cylinder was removed from the needle tip. To form a double cylinder, a second layer was rolled around the first cylinder. OP and GEM double-layered PLGA cylinders were produced and compared with blank control cylinders that did not contain drug.
Scanning electron microscopy (SEM)
Samples were fixed to aluminum inserts with carbon tape and gold sputtered. SEM was performed using a JEOL 840 (Tokyo, Japan) with an accelerating voltage of 10 kV.
Release kinetic experiments
PLGA cylinders were suspended in 5 mL of 0.1 M sodium phosphate buffer (pH 7.4) and stored at 37°C. Supernatants were extracted periodically and replaced with fresh buffer. Supernatants were stored at −20°C pending analysis. OP and GEM released from cylinders were reported as a percent of the cumulative drug released after 30 days.
PLGA cylinders in cell culture
PANC1 and PANC1-GEMR cells were plated in 25 cm2 cell culture flasks at ~112,500 and 45,000 cells per flask, respectively, and incubated overnight. PANC1-GEMR cells were plated at a lower density due to a faster growth rate. Experiments were carried out for 3, 6, 10, or 15 days. Experimental cohorts contained a control (no drug) or drug-loaded double-layered PLGA cylinder and untreated cells not exposed to a cylinder. Drug-loaded cylinders contained 3 mg of GEM and 16 mg of OP. Medium was changed every 3 days. On the final day, cells were lifted using TrypLE Express (Thermo Fisher Scientific, Waltham, MA, USA) and counted twice using a hemocytometer. Trypan blue stain was added to cells prior to counting to ensure that only viable cells were counted. Cell viability was measured as cell count, percentage of the control.
Statistical analysis
Graphing and statistical analysis were carried out using GraphPad Prism 5. Statistical analyses of the data used unpaired t-tests at 95% confidence.
Results and discussion
Fabrication and characterization of double-layered PLGA cylinders loaded with OP and GEM
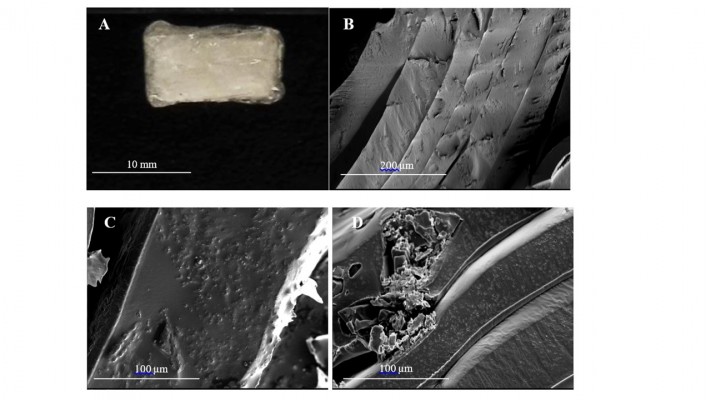
Double-layered PLGA cylinders loaded with OP and GEM at different doses were fabricated using a previous reported method as depicted in Figure 1 as described for single-layered cylinders by Hrynyk et al.9 Implantable double-layered PLGA cylinders were produced first by dissolving PLGA in acetone. OP and GEM are insoluble in acetone and, thus, were dispersed into the PLGA solution as insoluble particulates facilitated by addition of Span 80. The suspension was then ejected dropwise onto a Teflon sheet, forming a thin film enabling evaporation of the acetone. After drying, films were formed into single- or double-layered PLGA cylinders around a 16 G needle. Cylinders were loaded with 16 mg of OP or 3 mg of GEM, the dosage of which is based on prior preclinical in vivo animal studies. Granular OP and GEM were distributed throughout the polymer matrix (Figure 2). The double-layered PLGA cylinders measured ~10×5 mm (Figure 2A). A cross-section of a control cylinder without drug is shown in Figure 2B, which illustrates the distinct polymer layers resulting from the formulation method. The control empty cylinder appears smooth in Figure 2B. SEM micrographs of cross-section, surface, and internal morphologies of PLGA-OP/GEM cylinders show evenly dispersed particles of pure OP of ~1–5 μm visibly embedded in the polymer film in Figure 2C. Larger GEM particles are visible embedded in the polymer film in Figure 2D. The cylinder appears smooth and continuous as seen in Figure 2A.
  | Figure 1 Therapeutic design of OP- and GEM-loaded double-layered PLGA cylindrical implants. |
The degradable double-layered PLGA implants were designed to release OP and GEM for a period extending up to 30 days. Release profiles of OP and GEM from PLGA were determined, and the stability of the respective drugs in terms of the sustained ability to elicit a cancer cell response was also determined. It is noteworthy that both OP and GEM have not been investigated for long-term stability due to their usual route of administration. In particular, OP is administered orally as a tablet while GEM is administered intravenously over a period of ~30 min. To this end, it was necessary to determine the stability of these hydrophilic drugs over an extended release period before encapsulation in double-layered PLGA cylinders. GEM and OP with chemical structures depicted in Figure 3 were assayed for their stability in phosphate buffer (pH 7.4) at 37°C for 30 days using HPLC analysis.
No significant change in the concentration of GEM was detected by HPLC over 30 days. However, a slight 30% linear reduction in the concentration of OP was observed concomitant with the emergence of a new peak at a retention time of 1.3 min with a linear profile (Figure 4). OP stability was also determined in 0.1% trifluoroacetic acid as stabilizer, showing a consistent stability over the same 30-day period. Clinically, OP used as an antiviral drug is administered orally as a prodrug, which is subsequently converted to oseltamivir carboxylate (OC) through ester bond hydrolysis by liver enzymes.12 OC was analyzed using HPLC and found to have a retention time of 1.3 min, matching that of the emerging peak when testing OP stability. The shorter retention time of OC compared to OP was expected, as OC is a more polar molecule. Thus, it is likely that the apparent decrease in OP over time is due to gradual hydrolysis to OC; yet, 70% of the OP remains detectable, even after 30 days at 37°C. While the OC form is effective as an antiviral, the OP form has been shown most effective when directed against tumor tissues.7
It is noteworthy that OP- and GEM-loaded cylinders following incubation in cell culture medium for 15 days showed full stability of retained OP and GEM (Figure 4). Thus, PLGA encapsulation shows a dramatic stabilizing effect on the particulate form of OP for extended term prior to release.
Release of OP and GEM from double-layered PLGA cylinders
Several formulations were developed to optimize OP and GEM release from PLGA cylinders and are summarized in Table 1. The goal in designing the cylinders was to achieve a release profile that paralleled preclinical animal in vivo experiments involving injectable OP in combination with GEM (unpublished data, Szewczuk’s lab, August 2016). A frequent issue was a high release of OP, and to a lesser extent GEM, from the cylinders within the first 3 days. Films were initially held at 4°C overnight prior to rolling, which were found sticky suggesting residual acetone present in the films. To this end, residual acetone may diffuse out of the cylinder in aqueous buffer, leaving pores and channels to allow for the permeation of water, resulting in premature diffusion and release of the drugs from the cylinder. OP may be affected more than GEM because OP particles were found to be smaller in size as seen in Figure 2. To test this hypothesis, cylinders were made from films, which had been kept at 20°C for 72 h. The resulting films were no longer sticky and more rigid than those kept at 4°C for overnight. Two double-layered PLGA cylinders showed sustained release over 30 days and were further studied and selected for in vitro cell testing.
The selected cylinders based on the results from Table 1 were double-layered with one drug contained in the inner layer, and the other drug in the outer layer, made from films kept for 72 h at room temperature prior to rolling. Individual layers were determined to contain either 16 mg of OP or 3 mg of GEM. Cylinders were identified as inner/outer OPin/GEMout or GEMin/OPout. All double-layered PLGA cylinders were fabricated with 80 mg of PLGA and 4–6 mg of Span 80 for each layer. After 30 days in media, PLGA cylinders were found to be completely degraded.
The simultaneous release of OP and GEM from OPin/GEMout or GEMin/OPout cylinders is shown in Figure 5. OPin/GEMout cylinders resulted in a higher initial release of GEM than OP with 39% of GEM and 15.5% of OP released during the initial 24 h. GEM was then released at a constant rate until day 25 at which point 99% GEM was released from the cylinder. In addition, most of the OP was released between days 3 and 16 before beginning to plateau as seen in Figure 5A.
GEMin/OPout cylinders showed a slower release of GEM as shown in Figure 5B. After 72 h, only 2.5% of GEM had been released from the cylinder. GEM release was linear from day 3 until day 25, when it slowed until day 30. OP was released more quickly, with 13, 34, and 53% released from the cylinder after 24, 48, and 72 h, respectively. OP release was then linear until day 20, after which 97% of OP was released with the remaining amount released over the final 10 days.
The release of OP and GEM from double-layered PLGA cylinders was compared with the projected theoretical release of OP and GEM from a cylinder containing 16 mg of OP and 3 mg of GEM, assuming a linear rate of release over 30 days, as shown in Figure 6. Since the projected release was based on injectable drug treatment dosages that resulted in the stagnation or reduction of tumor volume in a heterotropic xenograft model of human pancreatic cancer in mice (unpublished data, Szewczuk’s lab, August 2016), cylinders with the release of OP and GEM that closely matched the projected release were thought most likely to be effective. Indeed, the release profile of GEM from both types of cylinders is closely matched to the projected GEM release. The release of OP from both cylinders is similar to the projected release for the first 16–20 days, before the rate of release decreases and plateaus. Overall, both cylinders are similar to the projected release profile and selected for cell viability assays.
Viability of PANC1 and GEM-resistant PANC1 cells exposed to OP and GEM released from PLGA cylinders
The potential antitumor effects of OP and GEM released from OPin/GEMout or GEMin/OPout cylinders on the viability of human pancreatic PANC1 carcinoma cells and their GEM resistant variants (PANC1-GEMR) were studied by monitoring numbers of viable cells, following exposure. Cell viability assays were initially performed using WST-1 reagent; however, interactions with the acidic polymer degradation products (glycolic acid and lactic acid) led to aberrant results. Instead, culture medium containing empty cylinder, OPin/GEMout cylinder, or GEMin/OPout cylinder was changed every 3 days, and trypan blue was used to count viable stained cells. Viable cells are shown as percent of the control empty PLGA cylinder group.
Images of PANC1 cells over 15 days culture depicted in Figure 7A show cells exposed to drug released from OPin/GEMout or GEMin/OPout cylinders began to display an altered morphology from day 6 onward. Cells became elongated with an increase in spindle-like projections. When exposed to an empty blank cylinder, they appeared to have the same morphological feature as the untreated cells at each indicated time points, suggesting that degradable PLGA polymer alone had no direct effect on PANC1 cell morphology.
Cells treated with GEMin/OPout cylinder showed the lowest viable cell numbers at 3 and 6 days with 15.2 and 3.9%, respectively, compared with untreated cells as seen in Figure 7B. For OPin/GEMout exposed cells, the viable cell numbers on days 3 and 6 were 49.0 and 21.5%, respectively, compared to that of untreated cells and, thus, GEMin/OPout cylinders appeared to be more effective in preventing the cell growth of PANC1 over 3–6 days. However, PANC1 cells exposed to OPin/GEMout cylinders had lower viable cell counts after 10 and 15 days, showing 10.8 and 1.2% of viable cells, respectively. When exposed to OPin/GEMout cylinders, they remained viable compared to the untreated cells, while 11.7 and 12.2% of cells exposed to GEMin/OPout cylinders remained viable.
High cell viability and no visible changes in cell morphology were observed in PANC1 exposed to blank cylinders compared to the untreated cells. PLGA alone does not appear to have an effect on PANC1 cells, and thus, any changes in morphology or cell viability following treatment with cylinders are due to the effect of released OP and GEM.
In the case of GEM-resistant cells (PANC1-GEMR) exposed to OPin/GEMout or GEMin/OPout cylinders, little cell growth appeared to occur after 3 days as seen in Figure 8A. Thus, few cells survived following exposure >3 days, as seen in Figure 8B. PANC1-GEMR cells exposed to blank cylinders showed a similar morphology to untreated cells, indicating again that the PLGA did not affect the viability of the drug-resistant variant cells.
OP and GEM released from OPin/GEMout and GEMin/OPout cylinders greatly reduced the number of PANC1-GEMR viable cells at all time points. For PANC1 cells, viability was lower for cells treated with the GEMin/OPout cylinders compared to the OPin/GEMout cylinders after 3 days of exposure. However, treatment with OPin/GEMout cylinders resulted in equal or lower cell counts compared to GEMin/OPout cylinders at all other time points.
Release of OP and GEM from the OPin/GEMout cylinders appeared to be most effective at reducing cell viability for both PANC1 and PANC1-GEMR cells over the 15 days period. The experiments described earlier were repeated with the OPin/GEMout cylinders on both cell types, showing reproducible similar results (data not shown). The OPin/GEMout cylinders dramatically reduced the number of viable cells for both cell types and at each time point.
In addition to demonstrating reduction in PANC1 and PANC1-GEMR target cell viability during exposure to the combinatorial and sequential release OP and GEM, it is instructive to consider the potential for concomitant migratory or metastatic phenotype. Previously, O’Shea et al8 treated PANC1 and PANC1-GEMR with 600 μg/mL OP for 24 h and observed a significant increase in E-cadherin expression as well as a significant decrease in N-cadherin expression, consistent with epithelial–mesenchymal transition (EMT), indicating a more metastatic phenotype than untreated PANC1 cells.13,14 In the present study, differences in the response to OP and GEM released from the OPin/GEMout cylinders compared to the GEMin/OPout cylinders may be due to the different cylinder release profiles as shown in Figure 5. The GEMin/OPout cylinders have a higher initial release of OP and a lower initial release of GEM than the OPin/GEMout cylinders. Therefore, it is proposed that the higher initial release of OP from GEMin/OPout cylinders could also induce a reversal of EMT.
Chemotherapeutic treatment of pancreatic cancer is often hindered by chemoresistance, which is often linked to EMT in many cancers,13,15 including pancreatic cancer.16–18 EMT is characterized by reduced cell–cell adhesion, reorganization of the cytoskeleton, and loss of cell polarity, resulting in cells that have a more migratory phenotype.19 Cells undergoing EMT have decreased expression of epithelial markers such as E-cadherin, occludin, cytokeratins, and desmoplakin, while showing an increased expression of the mesenchymal markers N-cadherin, vimentin, and fibronectin.13,20–22 Transcription factors, such as Slug, Snail, Twist, and Zeb-1, are repressors of E-cadherin, and their upregulation has also been linked to chemoresistance and EMT.15,17,18,23 We have previously demonstrated that OP can restore chemosensitivity and induce mesenchymal to epithelial transition.8 In the present work, we have observed that the images of the surviving cells following OPin/GEMout exposure from PLGA cylinders showed altered morphologies that could be consistent with sustained chemosensitivity and induced mesenchymal to epithelial transition.
Conclusion
The double-layered PLGA cylinders developed in the present study provide the proof of concept of long-term delivery of OP and GEM from a biodegradable, implantable device for the possible treatment of cancer. Double-layered PLGA cylinders containing OP and GEM showed not only a sustained release of both drugs over 30 days but also stability of the drugs. OP and GEM released from OPin/GEMout and GEMin/OPout cylinders strongly reduced PANC1 and PANC1-GEMR cell viability over a period of 3–15 days, with some evidence supporting previous observations of sustained chemosensitivity and metastatic phenotype of PANC1 cells. The double-layered PLGA cylinders are an effective delivery system for the sustained release of OP and GEM due to a simple fabrication process, the high loading of the small molecular weight hydrophilic drugs, the stability of the drugs while encapsulated, and the potential for implantation and localized drug delivery.
Acknowledgments
This study was supported in part by grants to MRS and RJN from the Natural Sciences and Engineering Research Council of Canada and private sector cancer funding from the Josefowitz Family to MRS. SAL was the recipient of the Queen’s Graduate Award (QGA). Present address of SAL: Polymer Science Group, Department of Chemical and Biomolecular Engineering, University of Melbourne, Parkville, Victoria 3010, Australia.
Author contributions
SAL and AJB fabricated and characterized the double-layered PLGA cylinders. SAL generated and maintained cancer cell line, confirmed the release studies of OP and GEM, and carried out in vitro study with the cells in the presence of PLGA. MRS and RJN supervised the research design and prepared the manuscript with SAL. All authors contributed toward data analysis, drafting, and revising the article and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
Obara K, Ishihara M, Ozeki Y, et al. Controlled release of paclitaxel from photocrosslinked chitosan hydrogels and its subsequent effect on subcutaneous tumor growth in mice. J Control Release. 2005;110(1):79–89. | ||
Yapp DT, Wong MQ, Kyle AH, et al. The differential effects of metronomic gemcitabine and antiangiogenic treatment in patient-derived xenografts of pancreatic cancer: treatment effects on metabolism, vascular function, cell proliferation, and tumor growth. Angiogenesis. 2016;19(2):229–244. | ||
Laquente B, Lacasa C, Ginesta MM, et al. Antiangiogenic effect of gemcitabine following metronomic administration in a pancreas cancer model. Mol Cancer Ther. 2008;7(3):638–647. | ||
Cham KK, Baker JH, Takhar KS, et al. Metronomic gemcitabine suppresses tumour growth, improves perfusion, and reduces hypoxia in human pancreatic ductal adenocarcinoma. Br J Cancer. 2010;103(1):52–60. | ||
Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549–554. | ||
Burris H, Storniolo AM. Assessing clinical benefit in the treatment of pancreas cancer: gemcitabine compared to 5-fluorouracil. Eur J Cancer. 1997;33(suppl 1):S18–S22. | ||
Amith SR, Jayanth P, Franchuk S, et al. Dependence of pathogen molecule-induced toll-like receptor activation and cell function on Neu1 sialidase. Glycoconj J. 2009;26(9):1197–1212. | ||
O’Shea LK, Abdulkhalek S, Allison S, Neufeld RJ, Szewczuk MR. Therapeutic targeting of Neu1 sialidase with oseltamivir phosphate (Tamiflu(R)) disables cancer cell survival in human pancreatic cancer with acquired chemoresistance. Onco Targets Ther. 2014;7:117–134. | ||
Hrynyk M, Ellis JP, Haxho F, et al. Therapeutic designed poly (lactic-co-glycolic acid) cylindrical oseltamivir phosphate-loaded implants impede tumor neovascularization, growth and metastasis in mouse model of human pancreatic carcinoma. Drug Des Devel Ther. 2015;9:4573–4586. | ||
Nair LS, Laurencin CT. Biodegradable polymers as biomaterials. Prog Polym Sci. 2007;32(8–9):762–798. | ||
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Preat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161(2):505–522. | ||
Shi D, Yang J, Yang D, et al. Anti-influenza prodrug oseltamivir is activated by carboxylesterase human carboxylesterase 1, and the activation is inhibited by antiplatelet agent clopidogrel. J Pharmacol Exp Ther. 2006;319(3):1477–1484. | ||
Creighton CJ, Gibbons DL, Kurie JM. The role of epithelial–mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res. 2013;5:187–195. | ||
Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell. 2009;139(5):871–890. | ||
Martin TA, Goyal A, Watkins G, Jiang WG. Expression of the transcription factors snail, slug, and twist and their clinical significance in human breast cancer. Ann Surg Oncol. 2005;12(6):488–496. | ||
Shah AN, Summy JM, Zhang J, Park SI, Parikh NU, Gallick GE. Development and characterization of gemcitabine-resistant pancreatic tumor cells. Ann Surg Oncol. 2007;14(12):3629–3637. | ||
Arumugam T, Ramachandran V, Fournier KF, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69(14):5820–5828. | ||
Hotz B, Arndt M, Dullat S, Bhargava S, Buhr HJ, Hotz HG. Epithelial to mesenchymal transition: expression of the regulators snail, slug, and twist in pancreatic cancer. Clin Cancer Res. 2007;13(16):4769–4776. | ||
Hugo H, Ackland ML, Blick T, et al. Epithelial–mesenchymal and mesenchymal–epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213(2):374–383. | ||
Weber CE, Li NY, Wai PY, Kuo PC. Epithelial–mesenchymal transition, TGF-beta, and osteopontin in wound healing and tissue remodeling after injury. J Burn Care Res. 2012;33(3):311–318. | ||
Bhowmick NA, Ghiassi M, Bakin A, et al. Transforming growth factor-beta1 mediates epithelial to mesenchymal transdifferentiation through a RhoA-dependent mechanism. Mol Biol Cell. 2001;12(1):27–36. | ||
Nieman MT, Prudoff RS, Johnson KR, Wheelock MJ. N-cadherin promotes motility in human breast cancer cells regardless of their E-cadherin expression. J Cell Biol. 1999;147(3):631–644. | ||
Cano A, Perez-Moreno MA, Rodrigo I, et al. The transcription factor snail controls epithelial–mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.