Back to Journals » OncoTargets and Therapy » Volume 12
Combination treatment with cetuximab in advanced nasopharyngeal carcinoma patients: a meta-analysis
Authors Shen J, Sun C, Zhou M, Zhang Z
Received 1 November 2018
Accepted for publication 1 February 2019
Published 3 April 2019 Volume 2019:12 Pages 2477—2494
DOI https://doi.org/10.2147/OTT.S193039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Jia Shen,1,* Changling Sun,1,* Min Zhou,2 Zhen Zhang1,3
1Department of Otolaryngology Head and Neck Surgery, Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu, People’s Republic of China; 2Department of Traditional Chinese Medicine, Nanjing Medical University Affiliated Cancer Hospital, Nanjing, Jiangsu, People’s Republic of China; 3Department of Integrated Traditional Chinese Medicine & Western Medicine Oncology, Affiliated Hospital of Jiangnan University, Wuxi, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Purpose: Cetuximab, an anti-epidermal growth factor receptor monoclonal antibody, carries the potential for combination treatment against nasopharyngeal carcinoma (NPC). We conducted a meta-analysis to assess the possible benefits and safety between the combination treatment with cetuximab and conventional treatment in NPC patients. Skin toxicity (ST) associated with additional cetuximab was evaluated as well.
Methods: We performed a systematic search (PubMed, Embase, Cochrane library, China National Knowledge Infrastructure, and WanFang Data) for studies comparing combination treatment with cetuximab versus conventional treatment in NPC patients. The selected studies included completely or partly reported clinical outcomes including survivals, complete and partial responses, and adverse reactions (ST). The pooled HR, relative risk (RR), and respective 95% CI were estimated by using fixed effects model or random effects model.
Results: A total of 23 relevant studies with available data were included in the final analysis. According to the pooled data, combination treatment with cetuximab showed improved efficacy on increased objective response rate (studies with cetuximab treatment: RR: 1.39, 95% CI: 1.29–1.50; concurrent chemoradiotherapy with or without cetuximab: RR: 1.39, 95% CI: 1.25–1.54) and prolonged survival (studies with cetuximab treatment: the pooled HR for OS was 0.70, 95% CI: 0.55–0.89; concurrent chemoradiotherapy with or without cetuximab: the pooled HR for OS was 0.64, 95% CI: 0.49–0.84) compared with conventional treatment. Moreover, the improved efficacy was invariably accompanied by an increased occurrence of ST (studies with cetuximab treatment: RR: 2.46, 95% CI: 1.81–3.34; concurrent chemoradiotherapy with or without cetuximab: RR: 1.84, 95% CI: 1.02–3.31). However, the majority of adverse reactions exhibited similar occurrence rates between the different treatments.
Conclusion: Patients with NPC receiving additional cetuximab treatment can benefit more from this systemic comprehensive therapy, while the efficiency of conventional treatment for NPC is limited. ST associated with cetuximab may be used as a potential on-treatment marker to guide treatment with cetuximab against NPC.
Keywords: nasopharyngeal carcinoma, cetuximab, combination treatment, clinical outcomes
Introduction
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck cancers in Southeast Asia and frequently diagnosed in the southern provinces of China. According to the 2017 National Comprehensive Cancer Network guidelines for the treatment of head and neck cancer, radiotherapy consisting of intensity-modulated radiation therapy (IMRT) and helical tomotherapy or radiotherapy combined with platinum-based chemotherapy remains the standard treatment for NPC. In reality, the majority of patients are initially diagnosed with locoregionally advanced nasopharyngeal carcinoma or even metastatic nasopharyngeal carcinoma. Radiotherapy1–4 is effective against primary lesion and lymph node lesions of NPC due to its obvious short-term effects. However, this treatment modality is associated with poor long-term efficacy and a relatively high occurrence rate of severe adverse reactions. For patients treated with radiotherapy alone, the 5-year survival rate is merely 20%. This low survival is attributed to the high rate of local recurrence and distant metastasis. Compared with radiotherapy, chemotherapy5,6 is a form of systemic therapy. Currently, the recommended treatment for patients with advanced NPC is concurrent chemoradiotherapy.7 However, it lacks optimal bioavailability; its considerable therapeutic benefits are always accompanied by unavoidable increased adverse effects. Hence, a new systemic comprehensive treatment with efficient and tolerable agents is essential.8–11
In recent years, the pursuit of optimal bioavailability contributed to the innovation and exploration of targeted biotherapy. Based on the development of targeted biotherapy and high expression12–16 of EGFR, anti-epidermal growth factor receptor monoclonal antibody (anti-EGFR MoAb) including cetuximab (CTX) is considered as a potential addition to the standard concurrent chemoradiotherapy regimen for NPC.17 This new systemic comprehensive treatment may improve the anti-tumor efficacy, while maintaining low toxicity. At present, more and more clinical trials18–30 have tried the additional treatment of CTX to promote the efficacy of treatment performed as better objective response rate (ORR) and prolonged survival. However, there are no definite comprehensive conclusions regarding the potential benefits of combination treatment with CTX in NPC patients.
Accordingly, we conducted a meta-analysis using pooled data to evaluate the efficacy and safety of the combination treatment with cetuxiamb compared to conventional treatment in NPC patients . In addition, we evaluated the special adverse effects associated with CTX, for example, skin toxicity (ST),31,32 which manifests as rashes and appears frequently during the treatment of CTX, to explore the relationship between ST and the outcome of combination treatment with cetuxiamb.
Materials and methods
Literature search
We performed systematic electronic searches for relevant articles in PubMed, Embase, Cochrane library, China National Knowledge Infrastructure, and WanFang Data published until December 31, 2017. The following keywords related to skin toxicity (“skin toxicity”, “skin rash”, “ST”), cetuximab (“cetuximab”, “CTX”, “anti-EGFR”, “targeted therapy”), and nasopharyngeal carcinoma (“nasopharyngeal carcinoma”, “nasopharynx cancer”, “NPC”) were used to retrieve articles and abstracts. Articles published in English and Chinese languages were included, and relevant references from these searched studies were also analyzed. No limitation was used during the literature search. Ethics Committee approval was waived because no human participants or animals were involved in this study.
Study selection and inclusion criteria
The studies that met the following inclusion criteria were included in this meta-analysis: 1) prospective or retrospective clinical studies focusing on the treatment of NPC patients with CTX; 2) studies that assessed the outcomes such as efficacy (survival and tumor response) or adverse reactions from additional CTX treatment; 3) studies that described in detail the overall survival (OS), progression-free survival (PFS), disease-free survival (DFS), distant metastasis-free survival (DMFS), complete response (CR), partial response (PR), stable disease (SD), progressive disease (PD), or adverse reactions in NPC patients with CTX treatment; and 4) studies with more comprehensive analysis so as to avoid duplication of data.
Data extraction
The collected data from each article were extracted by two authors independently as follows: 1) major characteristics, such as year of publication, first author, country of origin, number of overall patients, clinical stage, therapy strategy, study design; 2) information regarding clinical outcomes, such as CR, PR, SD, PD, OS, PFS, DFS, DMFS, and HR with its corresponding 95% CI that was used as the expression for survival comparison and the unprovided HR (HR were not shown in some articles) and its 95% CI were extracted from Kaplan–Meier curves; and 3) adverse reactions resulting from corresponding treatment, especially ST.
Quality assessment
The Newcastle–Ottawa Scale (NOS) was used to assess the quality of nonrandomized studies including cohort studies. The highest score for the three aspects of methodological assessment (selection, comparability, and outcome) were 4, 2, and 3, respectively. Studies were considered as high-quality studies if NOS score ≥6. Meanwhile, the risks of bias in randomized controlled trials were assessed by Cochrane Collaboration’s tool. According to random sequence generation, allocation concealment, blinding of participants and outcome assessment, incomplete outcome data, selective reporting, and other biases, the risk was evaluated as high, low, or unclear. Two investigators evaluated the included studies independently and sequent disagreements were resolved by discussion with a third investigator. Eventually, a total of 23 studies34–56 were included in the analysis.
Statistical analysis
Referring to PRISMA guideline (PRISMA 2009 checklist),33 OS, PFS, DFS, and DMFS expressed as HR and corresponding 95% CI were considered to be the primary endpoints, and ORR expressed as risk ratio (RR) and corresponding 95% CI were the secondary endpoints. Meanwhile, the risk of occurrence of adverse reactions (ST) was expressed as risk ratio (RR) and its 95% CI as well. Pooled data were calculated with Revman 5.3 software (Cochrane Center) and Stata 14.0 (Stata Corp., College Station, TX, USA). The effect model was chosen according to heterogeneity. If the heterogeneity was not significant (P>0.1, I2<50.0%), then a fixed-effect model was performed, whereas if the heterogeneity was significant, then a random-effect model was used. The results of meta-analysis were presented as forest plots and P-value <0.05 was considered significant. Sensitivity analysis was performed by sequentially excluding individual studies to evaluate the stability of results. Publication bias was presented as funnel plots and detected by Begg’s test and Egger’s test.
Results
Description of studies
After excluding 401 irrelevant studies, 105 studies were chosen, from which 36 duplicate studies were excluded subsequently (Figure 1). Finally, 23 studies34–56 presenting the clinical outcome of additional CTX treatment were included in the analysis.
  | Figure 1 Flow diagram of selection process of studies. |
The general characteristics of the included studies with a total of 3,177 patients are summarized in Table 1. The studies were published from 2013 to 2017, and the number of samples ranged from 22 to 791. All included studies were from China, which were consistent with the finding of the WHO that 80% of NPCs occur in China.57 Of the 23 studies conducted, 16 studies focused on the effect of CTX combined with concurrent chemoradiotherapy, five studies on IMRT only, and two studies on chemotherapy only. Moreover, a majority of patients were treated with platinum-based chemotherapy, containing cisplatin, nedaplatin, carboplatin, and lobaplatin. A total of 17 studies37,39–42,44–48,50–56 and nine studies34,35,38,41,43,49 assessed the outcomes of response rates and survival rates, respectively. Twenty-one studies34–44,46–55 described a series of adverse reactions due to the treatment and 1134,37,38,40,44,46,48–50,52,54 of these studies assessed the ST associated with CTX.
Quality of assessment
Risk of bias was used to assess the quality of 13 randomized trials.36,37,40,42,44,46–48,51–54,56 Of those, four studies had low risk of bias and nine had unclear risk (Figures S1 and S2). The bias mostly resulted from allocation concealment, blinding of participants and personnel, and outcome assessment. In addition, the NOS scores of ten nonrandomized studies34,35,38,39,41,43,45,49,50,55 were all ≥6 (Table 2), thereby indicating that the overall quality of the cohort studies was high.
  | Table 2 Methodological quality assessment of included studies by Newcastle–Ottawa Scale |
Survival: comparison between combination treatment with CTX and conventional treatment (Figure 2A and B)
In studies with CTX treatment, available data on OS,34,35,38,39,41–43,49,53 PFS,34,39,43 DMFS,35,36,38,43,49 and DFS35,36,38,49 were provided. By comparing experimental group with control group (Figure 2A), the pooled HR for OS was 0.70 (95% CI: 0.55–0.89, P=0.003), and no publication bias was detected using Begg’s test (P=0.835) and Egger’s test (P=0.817) (Figure S3). The pooled HR for PFS was 0.63 (95% CI: 0.39–1.02, P=0.06), for DMFS was 0.57 (95% CI: 0.41–0.81, P=0.001), and for DFS was 0.70 (95% CI: 0.52–0.94, P=0.02). Moreover, to stress the efficacy of additional CTX and avoid the difference in treatments, studies that focused on concurrent chemoradiotherapy (2D-CRT or IMRT combined with cisplatin) with or without CTX were selected. As a result (Figure 2B), the pooled HR for OS was 0.64 (95% CI: 0.49–0.84, P=0.001), for PFS was 0.56 (95% CI: 0.33–0.96, P=0.04), for DMFS was 0.48 (95% CI: 0.32–0.73, P=0.0007), and for DFS was 0.62 (95% CI: 0.42–0.91, P=0.02). Analysis of the comprehensive outcome of survival suggested that patients treated with CTX could benefit from the combination CTX treatment with longer OS, decreased risk of metastasis, and relapse.
Response rate: comparison between combination treatment with CTX and conventional treatment (Figure 3A and B)
Data on ORR, including CR and PR, were extracted from 17 studies37,39–42,44–48,50–56 involving a total of 1,242 NPC patients. In patients who underwent several treatment options with CTX (concurrent chemoradiotherapy with or without CTX, IMRT with or without CTX, and chemotherapy with or without CTX), the experimental group showed a significantly improved response rate (RR: 1.39, 95% CI: 1.29–1.50, P<0.00001) when compared with the control group (Figure 3A). To eliminate the synergistic efficiency obtained from chemotherapy and avoid differences in treatments, different treatment options with CTX were analyzed to further evaluate the efficacy of the additional CTX treatment. The results (Figure 3B) showed that the addition of CTX to concurrent chemoradiotherapy (RR: 1.39, 95% CI: 1.25–1.54, P<0.00001), IMRT (RR: 1.45, 95% CI: 1.24–1.69, P<0.00001), and chemotherapy (RR: 2.48, 95% CI: 1.00–6.17, P=0.05) all achieved a better response rate. No significant publication bias (Figure S4) was observed in Begg’s test (P=0.127) and Egger’s test (P=0.251).
Adverse reactions (except ST): comparison between combination treatment with CTX and conventional treatment (Figure 4A and B)
A total of 21 studies,34–44,46–55 including the studies on CTX treatment, provided data on a series of adverse reactions, including hematologic reactions (leukopenia, thrombocytopenia, anemia, hepatotoxicity, and nephrotoxicity) and non-hematologic reactions (mucositis, vomiting, nausea, diarrhea, weight loss, and radiothermitis). The pooled results showed (Figure 4A) that there were no notable differences in the rate of occurrence of thrombocytopenia (RR: 0.98, 95% CI: 0.5–1.94, P=0.96), anemia (RR: 0.72, 95% CI: 0.29–1.78, P=0.48), hepatotoxicity (RR: 1.13, 95% CI: 0.63–2.04, P=0.68), nephrotoxicity (RR: 0.66, 95% CI: 0.24–1.80, P=0.41), vomiting (RR: 0.83, 95% CI: 0.57–1.20, P=0.31), nausea (RR: 0.81, 95% CI: 0.63–1.04, P=0.10), weight loss (RR: 1.01, 95% CI: 0.66–1.54, P=0.98), and radiothermitis (RR: 0.84, 95% CI: 0.58–1.21, P=0.36) between the experimental and control groups. However, the rate of occurrence of leukopenia in the experimental group was significantly lower compared to that in the control group (RR: 0.81, 95% CI: 0.69–0.96, P=0.01), and the rates of occurrence of mucositis and diarrhea in the experimental group were higher compared to that in the control group (RR: 1.33, 95% CI: 1.12–1.58, P=0.001; RR: 1.56, 95% CI: 1.30–1.87, P<0.00001). In addition, studies that focused on the treatment of concurrent chemoradiotherapy with or without CTX (Figure 4B) showed that the combination of CTX and chemoradiotherapy did not increase the rate of occurrence of leukopenia (RR: 0.98, 95% CI: 0.84–1.14, P=0.80), thrombocytopenia (RR: 0.69, 95% CI: 0.44–1.08, P=0.10), anemia (RR: 0.55, 95% CI: 0.19–1.60, P=0.27), nephrotoxicity (RR: 0.91, 95% CI: 0.51–1.65, P=0.76), mucositis (RR: 1.16, 95% CI: 0.98–1.38, P=0.08), vomiting (RR: 1.20, 95% CI: 0.91–1.60, P=0.20), nausea (RR: 0.90, 95% CI: 0.78–1.04, P=0.15), and radiothermitis (RR: 0.84, 95% CI: 0.58–1.21, P=0.36) when compared with studies on chemoradiotherapy alone. Besides, higher rates of occurrence of hepatotoxicity, diarrhea, and weight loss were observed in group that underwent combination treatment with CTX (RR: 1.77, 95% CI: 1.06–2.95, P=0.03; RR: 1.52, 95% CI: 1.26–1.85, P<0.0001; RR: 1.13, 95% CI: 1.01–1.27, P=0.04).
ST: comparison between combination treatment with CTX and conventional treatment (Figure 5A and B)
We collected data regarding ST, which presented as rashes, from 11 studies that focused on CTX treatment,34,37,38,40,44,46,48–50,52,54 and a higher occurrence rate of ST was observed in the experimental group (RR: 2.46, 95% CI: 1.81–3.34, P<0.00001) (Figure 5A). Moreover, studies on patients who underwent treatment with concurrent chemoradiotherapy with or without CTX also showed that addition of CTX increased the occurrence rate of ST compared to the studies on those who underwent treatment with concurrent chemoradiotherapy (RR: 1.84, 95% CI: 1.02–3.31, P=0.04) (Figure 5B). Based on these pooled data, it can be concluded that ST is more likely to occur in patients who undergo combined treatment with CTX. In other words, patients treated with CTX could obtain more benefit from additional treatment, but with more occurrences of ST. The publication bias was analyzed by Begg’s test (P=0.755) and Egger’s test (P=0.512) and is shown in funnel plots (Figure S5).
Discussion
Following the widespread use of anti-EGFR MoAb, CTX and nimotuzumab are the preferred treatment of choice for NPC. Due to the high occurrence of NPC in China, several studies have tried to examine the efficacy of CTX combined with nimotuzumab, and comprehensive statistics articles have already evaluated the efficacy of nimotuzumab combined with chemoradiotherapy.58 Our study is the first to make a definite comprehensive conclusion with regard to the potential benefits of combination treatment with CTX in NPC patients.
This systematic literature retrieval and meta-analysis of the relevant articles aimed to assess the therapeutic effect of combination treatment with CTX compared to the conventional treatment for NPC. The comprehensive analysis suggested that patients who accepted additional CTX treatment could obtain more benefits, such as increased response rate and prolonged survival, from combination treatment. Besides, the two treatment methods did not show any significant difference in the rates of occurrence of most adverse reactions. It seemed that the treatment plan of CTX is more feasible and efficient for NPC with similar adverse reactions when compared with conventional treatment.
With regard to clinical outcome of efficacy (survival and response rate), the pooled HR for OS, DMFS, and DFS in survival and the pooled RR for ORR are significant for CTX treatment compared with conventional treatment (P<0.05). When studies with treatment of concurrent chemoradiotherapy with or without CTX were selected, the superiority of combination CTX treatment compared to chemoradiotherapy for NPC patients was found for OS, PFS, DMFS, and DFS in survival and ORR (P<0.05). Nevertheless, the studies included were still insufficient, especially the studies that reported data about PFS, DMFS, and DFS (n≤5). Further, the indicator of survival would be more accurate with increasing relevant research.
With regard to adverse reactions, addition of CTX did not increase the risk of occurrence of adverse reactions, thereby indicating the safety of additional CTX treatment. We emphasized the assessment of ST in our study, which seemed to be a potential on-treatment marker for anti-EGFR MoAb treatment.59 Currently, anti-EGFR MoAb is widely used in the clinical treatment and has provided new treatment options for a variety of malignant tumors. CTX served as a kind of anti-EGFR MoAb when used in the treatment of colorectal cancer, lung cancer, and NPC. Compared with conventional therapy for NPC, which barely showed any significant efficacy, CTX has provided a new direction in the treatment of NPC. In clinical application, the selection of an anti-EGFR MoAb is normally directed by molecular markers (EGFR, K-ras, and so on) before targeted treatment. However, the molecular markers do not ensure an absolutely effective anti-EGFR treatment due to the complexity and variability that arise during the treatment. Therefore, the observation of an on-treatment clinical marker is of equal importance. ST is considered to be an adverse effect that arises due to EGFR inhibition, and it seems to be a potential predictor of better response to personalized anti-EGFR treatment. In one of the studies included in this meta-analysis,34 the results of univariate analysis showed that CTX-treated patients with grade 3–4 rashes presented with better OS outcomes compared to those with grade 0–2 rashes. Therefore, ST has been concluded to be a potential predictor, and based on this on-treatment marker, suitable clinical decision about anti-EGFR strategies during the treatment of CTX could be made. Future work should focus on whether ST could predict the absolute benefit of the addition of CTX in the treatment of NPC. Besides, future studies should also focus on how to use this on-treatment marker in clinical decision-making and determine the predict value of ST in additional CTX treatment of NPC.
Furthermore, this study has some limitations in spite of the confirmation of our statistical results with the sensitivity analysis. Firstly, the included studies were of diverse quality with different clinical settings and standard treatment plans. Although the studies investigating CCRT with or without cetuximab were selected to pool the corresponding data, this may have led to significant heterogeneity and influenced the interpretation of the results. Secondly, some subanalyses involved only a small number of studies, so the analysis results from these studies were unstable. With more relevant studies in the future, the accuracy of the results would increase. Thirdly, only articles in English and Chinese were included, and all the available information were from China, which led to potential publication bias.
Conclusion
The anti-EGFR MoAb, CTX, showed improved efficacy in combination treatment compared with conventional treatment. In other words, NPC patients could obtain definite benefits from additional treatment with CTX. Moreover, ST, a significant adverse effect associated with CTX treatment, may serve as an on-treatment marker to guide treatment with CTX against NPC.
Acknowledgment
This work was supported by the Natural Science Foundation of Jiangsu Province (No. BK20160195), Wuxi Young Medical Talents (No. QNRC054) and Youth Foundation of Wuxi Health and Family Planning Commission (No. Q201820).
Disclosure
The authors report no conflicts of interest in this work.
References
Qiu WZ, Peng XS, Xia HQ, Huang PY, Guo X, Cao KJ. A retrospective study comparing the outcomes and toxicities of intensity-modulated radiotherapy versus two-dimensional conventional radiotherapy for the treatment of children and adolescent nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2017;143:1563–1572. doi:10.1007/s00432-017-2401-y | ||
Ren G, Du L, Ma L, et al. Clinical observation of 73 nasopharyngeal carcinoma patients treated by helical tomotherapy: the China experience. Technol Cancer Res Treat. 2011;10:259–266. doi:10.7785/tcrt.2012.500201 | ||
Yang Q, Zou X, You R, et al. Proposal for a new risk classification system for nasopharyngeal carcinoma patients with post-radiation nasopharyngeal necrosis. Oral Oncol. 2017;67:83–88. doi:10.1016/j.oraloncology.2017.02.012 | ||
Lin J, Lv X, Niu M, et al. Radiation-induced abnormal cortical thickness in patients with nasopharyngeal carcinoma after radiotherapy. Neuroimage Clin. 2017;14:610–621. doi:10.1016/j.nicl.2017.02.025 | ||
Forastiere AA. Chemotherapy in the treatment of locally advanced head and neck cancer. J Surg Oncol. 2008;97:701–707. doi:10.1002/jso.21012 | ||
OuYang PY, Zhang LN, Xiao Y, et al. Validation of published nomograms and accordingly individualized induction chemotherapy in nasopharyngeal carcinoma. Oral Oncol. 2017;67:37–45. doi:10.1016/j.oraloncology.2017.01.009 | ||
Koukourakis MI, Tsoutsou PG, Karpouzis A, et al. Radiochemotherapy with cetuximab, cisplatin, and amifostine for locally advanced head and neck cancer: a feasibility study. Int J Radiat Oncol Biol Phys. 2010;77:9–15. doi:10.1016/j.ijrobp.2009.04.060 | ||
Yang ES, Murphy BM, Chung CH, et al. Evolution of clinical trials in head and neck cancer. Crit Rev Oncol Hematol. 2009;71:29–42. doi:10.1016/j.critrevonc.2008.09.015 | ||
Razak AR, Siu LL, Le TC. Molecular targeted therapies in all histologies of head and neck cancers: an update. Curr Opin Oncol. 2010;22:212–220. doi:10.1097/CCO.0b013e328338001f | ||
Chan SL, Ma BB. Novel systemic therapeutic for nasopharyngeal carcinoma. Expert Opin Ther Targets. 2012;16(Suppl 1):S63–S68. doi:10.1517/14728222.2011.635646 | ||
Ward MC, Reddy CA, Adelstein DJ, Koyfman SA. Use of systemic therapy with definitive radiotherapy for elderly patients with head and neck cancer: a National Cancer Data Base analysis. Cancer. 2016. doi:10.1002/cncr.30214 | ||
Ma X, Huang J, Wu X, et al. Epidermal growth factor receptor could play a prognostic role to predict the outcome of nasopharyngeal carcinoma: A meta-analysis. Cancer Biomark. 2014;14:267–277. doi:10.3233/CBM-140401 | ||
Sun W, Long G, Wang J, Mei Q, Liu D, Hu G. Prognostic role of epidermal growth factor receptor in nasopharyngeal carcinoma: a meta-analysis. Head Neck. 2014;36:1508–1516. doi:10.1002/hed.23481 | ||
Ma BB, Poon TC, To KF, et al. Prognostic significance of tumor angiogenesis, Ki 67, p53 oncoprotein, epidermal growth factor receptor and HER2 receptor protein expression in undifferentiated nasopharyngeal carcinoma–a prospective study. Head Neck. 2003;25:864–872. doi:10.1002/hed.10307 | ||
Cao XJ, Hao JF, Yang XH, et al. Prognostic value of expression of EGFR and nm23 for locoregionally advanced nasopharyngeal carcinoma. Med Oncol. 2012;29:263–271. doi:10.1007/s12032-010-9782-y | ||
Zhang P, Wu SK, Wang Y, et al. p53, MDM2, eIF4E and EGFR expression in nasopharyngeal carcinoma and their correlation with clinicopathological characteristics and prognosis: A retrospective study. Oncol Lett. 2015;9:113–118. doi:10.3892/ol.2014.2631 | ||
Yuan C, Xu XH, Chen Z. Combination treatment with antiEGFR monoclonal antibodies in advanced nasopharyngeal carcinoma: a meta-analysis. J BUON. 2015;20:1510–1517. | ||
Ma BB, Kam MK, Leung SF, et al. A phase II study of concurrent cetuximab-cisplatin and intensity-modulated radiotherapy in locoregionally advanced nasopharyngeal carcinoma. Ann Oncol. 2012;23:1287–1292. doi:10.1093/annonc/mdr401 | ||
Zhang X, Du L, Zhao F, Wang Q, Yang S, Ma L. A phase II clinical trial of concurrent helical tomotherapy plus cetuximab followed by adjuvant chemotherapy with cisplatin and docetaxel for locally advanced nasopharyngeal carcinoma. Int J Biol Sci. 2016;12:446–453. doi:10.7150/ijbs.12937 | ||
He X, Xu J, Guo W, Jiang X, Wang X, Zong D. Cetuximab in combination with chemoradiation after induction chemotherapy of locoregionally advanced nasopharyngeal carcinoma: preliminary results. Future Oncol. 2013;9:1459–1467. doi:10.2217/fon.13.151 | ||
Xu T, Ou X, Shen C, Hu C. Cetuximab in combination with chemoradiotherapy in the treatment of recurrent and/or metastatic nasopharyngeal carcinoma. Anticancer Drugs. 2016;27:66–70. doi:10.1097/CAD.0000000000000294 | ||
Riaz N, Sherman E, Koutcher L, et al. Concurrent chemoradiotherapy with cisplatin versus cetuximab for squamous cell carcinoma of the head and neck. Am J Clin Oncol. 2016;39:27–31. doi:10.1097/COC.0000000000000006 | ||
Shapiro LQ, Sherman EJ, Riaz N, et al. Efficacy of concurrent cetuximab vs. 5-fluorouracil/carboplatin or high-dose cisplatin with intensity-modulated radiation therapy (IMRT) for locally-advanced head and neck cancer (LAHNSCC). Oral Oncol. 2014;50:947–955. doi:10.1016/j.oraloncology.2014.07.001 | ||
Niu X, Hu C, Kong L. Experience with combination of cetuximab plus intensity-modulated radiotherapy with or without chemotherapy for locoregionally advanced nasopharyngeal carcinoma. J Cancer Res Clin Oncol. 2013;139:1063–1071. doi:10.1007/s00432-013-1419-z | ||
Licitra L, Bossi P, Locati LD, Bergamini C. Is restoring platinum sensitivity the best goal for cetuximab in recurrent/metastatic nasopharyngeal cancer? J Clin Oncol. 2005;23:7757–7759; author reply 7758–7759. doi:10.1200/JCO.2005.02.7854 | ||
Chan AT, Hsu MM, Goh BC, et al. Multicenter, phase II study of cetuximab in combination with carboplatin in patients with recurrent or metastatic nasopharyngeal carcinoma. J Clin Oncol. 2005;23:3568–3576. doi:10.1200/JCO.2005.02.147 | ||
Suntharalingam M, Kwok Y, Goloubeva O, et al. Phase II study evaluating the addition of cetuximab to the concurrent delivery of weekly carboplatin, paclitaxel, and daily radiotherapy for patients with locally advanced squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys. 2012;82:1845–1850. | ||
Jensen AD, Krauss J, Potthoff K, et al. Phase II study of induction chemotherapy with TPF followed by radioimmunotherapy with Cetuximab and intensity-modulated radiotherapy (IMRT) in combination with a carbon ion boost for locally advanced tumours of the oro-, hypopharynx and larynx–TPF-C-HIT. BMC Cancer. 2011;11:182. doi:10.1186/1471-2407-11-182 | ||
Yoshino T, Hasegawa Y, Takahashi S, et al. Platinum-based chemotherapy plus cetuximab for the first-line treatment of Japanese patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck: results of a phase II trial. Jpn J Clin Oncol. 2013;43:524–531. doi:10.1093/jjco/hyt034 | ||
Riaz N, Sherman EJ, Fury M, Lee N. Should cetuximab replace Cisplatin for definitive chemoradiotherapy in locally advanced head and neck cancer. J Clin Oncol. 2013;31:287–288. doi:10.1200/JCO.2012.46.9049 | ||
Bibault JE, Morelle M, Perrier L, et al. Toxicity and efficacy of cetuximab associated with several modalities of IMRT for locally advanced head and neck cancer. Cancer Radiother. 2016;20:357–361. doi:10.1016/j.canrad.2016.05.009 | ||
Feng HX, Guo SP, Li GR, et al. Toxicity of concurrent chemoradiotherapy with cetuximab for locoregionally advanced nasopharyngeal carcinoma. Med Oncol. 2014;31:170. doi:10.1007/s12032-014-0374-0 | ||
Page MJ, Moher D. Evaluations of the uptake and impact of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) Statement and extensions: a scoping review. Syst Rev. 2017;6:263. doi:10.1186/s13643-017-0663-8 | ||
Wu X, Huang J, Liu L, et al. Cetuximab concurrent with IMRT versus cisplatin concurrent with IMRT in locally advanced nasopharyngeal carcinoma: A retrospective matched case-control study. Medicine (Baltimore). 2016;95(39):e4926. doi:10.1097/MD.0000000000004864 | ||
Xia W-X, Liang H, Lv X, et al. Combining cetuximab with chemoradiotherapy in patients with locally advanced nasopharyngeal carcinoma: a propensity score analysis. Oral Oncol. 2017;67:167–174. doi:10.1016/j.oraloncology.2017.02.026 | ||
Xu T, Liu Y, Dou S, Li F, Guan X, Zhu G. Weekly cetuximab concurrent with IMRT aggravated radiation-induced oral mucositis in locally advanced nasopharyngeal carcinoma: results of a randomized phase II study. Oral Oncol. 2015;51:875–879. doi:10.1016/j.oraloncology.2015.06.008 | ||
Zhou XS. The effect of cetuximab combined with radiotherapy in the treatment of advanced nasopharyngeal carcinoma. J North Pharm. 2017;14(5):42–43. Chinese. | ||
You R, Hua YJ, Liu YP, et al. Concurrent chemoradiotherapy with or without anti-EGFR-targeted treatment for stage II–IVb nasopharyngeal carcinoma: retrospective analysis with a large cohort and long follow-up. Theranostics. 2017;7:2314–2324. doi:10.7150/thno.19710 | ||
Wang XX. Clinical asesment of the radiotherapy and chemotherapy combined with cetuximab in the treatment of nasopharyngeal carcinoma. J Clin Otorhinolaryngol Head Neck Surg. 2016;30(15):1229–1231. Chinese. | ||
Sun JH, Li J. Clinical efficacy of rituximab combined with chemotherapy in the treatment of patients with locally avanced nasopharyngeal carcinoma. China J Pharm Econ. 2016;7:90–92. Chinese. | ||
Zeng T, Zhang YL, Li JJ. Analysis of the efficacy of cetuximab combined with chemotherapy and radiotherapy in the treatment of advanced nasopharyngeal carcinoma. Chin J Front Med Sci. 2016;8(4):45–48. Chinese. | ||
Cao XQ, Liao Y. Clinical study of cetuximab combined with radiotherapy on patients with advanced nasopharyngeal carcinoma. Chin J Clin Pharmacol. 2016;32(2):120–122. Chinese. | ||
Li Y, Chen QY, Tang LQ, et al. Concurrent chemoradiotherapy with or without cetuximab for stage II to IVb nasopharyngeal carcinoma: a case-control study. BMC Cancer. 2017;17:567. doi:10.1186/s12885-017-3552-6 | ||
Yang ZY, Wan H, Chen CX, Yuan H, Wang P, Liu J. Study on the efficacy of cetuximab in combination with cisplatin chemotherapy and intensity modulated radiotherapy in treatment of patients with nasopharyngeal carcinoma. J Clin Exp Med. 2016;15(1):45–48. Chinese. | ||
Fu MH. The effect of cetuximab combined with chemoradiotherapy on prognosis of patients with advanced nasopharyngeal carcinoma. Anti Infect Pharm. 2015;12(1):121–122. Chinese. | ||
Zhao ZQ, OuYang XN, Yu ZY, Li J, Wang H, Yin LH. Curative effects of cetuximab combined with intensity modulated radiation therapy and concurrent chemotherapy on advanced nasopharyngeal carcinoma. Med J Nation Defening Forces Southwest China. 2015;25(7):750–753. Chinese. | ||
Yao XQ, Yang CL, Yang G, Yang YB. The effect of cetuximab combined with gemcitabine in the treatment of the advanced nasopharyngeal carcinoma followed by Paclitaxel. Anhui Med Pharm J. 2015;19(7):1391–1392. Chinese. | ||
Wang DX. Clinical analysis of cetuximab combined with chemoradiotherapy for advanced nasopharyngeal carcinoma. Mod Diagn Treat. 2015;26(11):2465–2466. Chinese. | ||
You R, Sun R, Hua YJ, et al. Cetuximab or nimotuzumab plus intensity-modulated radiotherapy versus cisplatin plus intensity-modulated radiotherapy for stage II-IVb nasopharyngeal carcinoma. Int J Cancer. 2017;141:1265–1276. doi:10.1002/ijc.30819 | ||
Zhou LS. The curative effect of cetuximab combined with chemoradiotherapy for 63 patients with advanced nasopharyngeal carcinoma. China Prac Med. 2013;8(31):131–132. Chinese. | ||
Zheng ZH, Li SE, Luo WJ. Analysis on the curative effect of cetuximab combined with concurrent cisplatin chemotherapy and intensity modulated radiation therapy for locally advanced nasopharyngeal carcinoma. China Foreign Med Treat. 2013;34:3–4. Chinese. | ||
Tang DH, Zhang YX, Cai J. The clinical efficacy of cetuximab combined with radiotherapy and chemotherapy in the treatment of locally advanced nasopharyngeal carcinoma. Anti-Tumor Pharm. 2013;3(2):119–121+129. Chinese. | ||
Fu XL, Meng MS, Chen XF, Li YX, Wu WY. RT combined with cetuximab in treating advanced nasopharyngeal carcinoma. China Pharm. 2015;24(18):41–43. Chinese. | ||
Wu CZ. Curative effects of cetuximab combined with concurrent chemo-and radiotherapy on recurrent nasopharyngeal carcinoma. Med J Nation Defening Forces Southwest China. 2013;23(5):509–512. Chinese. | ||
Gao HB, Zheng DY. The application of cetuximab in patients with local advanced nasopharyngeal carcinoma. Guangdong Med J. 2013;34(14):2244–2246. Chinese. | ||
Peng ZY, Zheng FB, Wen H. Clinical efficacy of cetuximab combined with IMRT in treatment of patients with locally advanced nasopharyngeal carcinoma. Chin J Clin Oncol Rehabil. 2013;20(9):993–995. Chinese. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi:10.3322/caac.21338 | ||
Li ZZ, Li YY, Yan SP, et al. Nimotuzumab combined with concurrent chemoradiotherapy benefits patients with advanced nasopharyngeal carcinoma. Onco Targets Ther. 2017;10:5445–5458. doi:10.2147/OTT.S141538 | ||
Hu J, Zhang Z, Zheng R, et al. On-treatment markers as predictors to guide anti-EGFR MoAb treatment in metastatic colorectal cancer: a systematic review with meta-analysis. Cancer Chemother Pharmacol. 2017;79:275–285. doi:10.1007/s00280-016-3196-2 |
Supplementary materials
Item 8 – Presents full electronic search strategy for at least one database, including any limits used, such that it could be repeated.
For example, search strategy for PubMed, keywords related to skin toxicity (“skin toxicity”, “skin rash”), cetuximab (“cetuximab”, “CTX”, “anti-EGFR”, “targeted therapy”), and nasopharyngeal carcinoma (“nasopharyngeal carcinoma”, “nasopharynx cancer”, “NPC”) were used to retrieve articles and abstracts in PubMed. The search builder was ((cetuximab [Mesh Terms]) OR (cetuximab [Title/Abstract]) OR (CTX [Title/Abstract]) OR (anti-EGFR [Title/Abstract]) OR (targeted therapy [Title/Abstract])) AND ((nasopharyngeal carcinoma [Mesh Terms]) OR (nasopharyngeal carcinoma [Title/Abstract]) OR (nasopharynx cancer [Title/Abstract]) OR (NPC [Title/Abstract])) AND ((skin toxicity [Mesh Terms]) OR (skin toxicity [Title/Abstract]) OR (skin rash [Title/Abstract])). No limitation was used during the literature search.
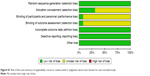  | Figure S1 Risk of bias and summary of applicability concerns: review authors’ judgments about each domain for each included study. |
  | Figure S2 Risk of bias and graph of applicability concerns: review authors’ judgments about each domain presented as percentages across included studies. |
  | Figure S3 Funnel plot with pseudo 95% CI of publication bias. |
  | Figure S4 Funnel plot with pseudo 95% CI of publication bias. |
  | Figure S5 Funnel plot with pseudo 95% CI of publication bias. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





