Back to Journals » Clinical Interventions in Aging » Volume 19
Combination of Conventional EVD and Ommaya Drainage for Intraventricular Hemorrhage (IVH)
Authors Zhu T, Fu J, Zang D, Wang Z, Ye X, Wu X, Hu J
Received 23 August 2023
Accepted for publication 17 December 2023
Published 3 January 2024 Volume 2024:19 Pages 1—10
DOI https://doi.org/10.2147/CIA.S436522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Tongming Zhu,1,* Junyan Fu,2,* Di Zang,1,3,* Zhe Wang,1,4,* Xiangru Ye,1 Xuehai Wu,1 Jin Hu1
1Department of Neurosurgery, Fudan University Huashan Hospital, National Center for Neurological Disorders, National Key Laboratory for Medical Neurobiology, Shanghai Key Laboratory of Brain Function and Regeneration, Institutes of Brain Science, MOE Frontiers Center for Brain Science, Shanghai Medical College-Fudan University, Shanghai, People’s Republic of China; 2Department of Radiology, Fudan University Huashan Hospital, Shanghai, People’s Republic of China; 3Department of Neurosurgery, China-Japan Friendship Hospital, Beijing, People’s Republic of China; 4Department of Medical Imaging Technology, SJTU-Ruijin-UIH Institute for Medical Imaging Technology, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xuehai Wu; Jin Hu, Department of Neurosurgery, Fudan University Huashan Hospital, No. 12, Middle Wulumuqi Road, Shanghai, People’s Republic of China, Email [email protected]; [email protected]
Background: The effect of Ommaya reservoirs on the clinical outcomes of patients with intraventricular hemorrhage (IVH) remains unclear.
Objective: We aimed to determine the effect of combining the Ommaya reservoir and external ventricular drainage (EVD) therapy on IVH and explore better clinical indicators for Ommaya implantation.
Methods: A retrospective analysis was conducted on patients diagnosed with IVH who received EVD-Ommaya drainage between January 2013 and March 2021. The patient population was divided into two groups: the Ommaya-used group, comprising patients in whom the Ommaya drainage system was activated post-surgery, and the Ommaya-unused group, comprising patients in whom the system was not activated. The study analyzed clinical, imaging, and outcome data of the patient population.
Results: A total of 123 patients with IVH were included: 75 patients in the Ommaya-used group and 48 patients in the Ommaya-unused group. The patients in the Ommaya-used group showed a lower 3-month GOS than those in the Ommaya-unused group (p< 0.0001). The modified Graeb scale (mGS) in the Ommaya-unused group was significantly lower than that in the Ommaya-used group before the operation (p< 0.01) but not after surgery (p> 0.05). The GCS in the Ommaya-used group was significantly lower than that in the other group, and there was a close correlation between the GCS and 3-month GOS (p< 0.0001). The GCS score showed significance in predicting the use of Ommaya (p< 0.001).
Conclusion: The study demonstrated that combining EVD and Ommaya drainage was a safe and feasible treatment for IVH. Additionally, preoperative GCS was found to predict the use of Ommaya drainage in subsequent treatment, providing valuable information for pre-surgery decision-making.
Keywords: intraventricular hemorrhage, Ommaya, external ventricular drainage
Introduction
Spontaneous intracerebral hemorrhage (ICH) is a critical event that leads to a high mortality rate and permanent disability in survivors. It is more common in older adults, as aging increases the risk of ICH and worsens its outcomes. In parallel with the aging population, the global incidence of ICH has risen to about 3.5 million, posing a threat to the health of the elderly and a heavy burden to society. Age is highly associated with the development and clinical outcomes of ICH, whose risk ratio doubles with every 10 years of age.1 Intraventricular hemorrhage (IVH), a life-threatening complication of ICH that occurs when blood enters the ventricles, affects over 50% of ICH patients and leads to an even worse prognosis.
IVH can be either primary or secondary, depending on the source of bleeding. The volume of IVH is a significant predictor of poor outcome.2–4 Apart from that, rapid neurological deterioration can be a consequence of acute blockage of the cerebrospinal fluid (CSF) circulation, the mass effect caused by the hematoma, sudden increase in intracranial pressure, and reduced cerebral perfusion. Additionally, IVH causes mechanical disruption, ventricular wall distension, hypertensive hydrocephalus, and toxic metabolic damage to the ependyma.5 Given the significant impact of IVH on mortality and morbidity, diverse treatment strategies have been evaluated. The most common treatment of IVH is the emergency placement of an external ventricular drain (EVD) to alleviate hydrocephalus, reduce the hematoma, and normalize the intracranial pressure.6–8
The Ommaya reservoir, a ventricular access device for repetitive access to intrathecal space, was invented by Pakistani neurosurgeon Ayub Khan Ommaya in 1963.9,10 The safety and efficacy of a combined Ommaya reservoir and EVD as a modified approach in the treatment of ventricular hematoma have been preliminarily proven.11,12 The uses of the Ommaya Reservoir and EVD have both been proposed as major alternative treatment strategies for IVH in many centers.13–16 However, the therapeutic effect of Ommaya reservoirs for extensive use in IVH patients has not been reported in detail, and the benefit–risk balance of using Ommaya in such a disease of the elderly remains to be evaluated.
In this paper, we retrospectively analyzed 123 IVH patients who underwent combined Ommaya reservoir and EVD surgery at our hospital, dividing them into two groups based on whether Ommaya drainage was used post-surgery. The objective of this study is to investigate the correlation between clinical prognosis and the utilization of Ommaya, with the aim of providing guidance for the appropriate utilization of Ommaya in clinical practice.
Methods
We performed a retrospective analysis of a single-center cohort to investigate the effect of combined Ommaya reservoir and EVD surgery on IVH patients.
Patients
We reviewed all consecutive patients who were diagnosed with IVH, admitted to our neurosurgical intensive care unit at Huashan Hospital Affiliated to Fudan University between January 2013 and March 2021, and 123 patients were finally enrolled (Figure 1). The inclusion criteria were as follows: (1) age 18 years and above, (2) the origin of hemorrhage was located either at the basal ganglia, thalamus, or brain stem with relevant extension into any ventricle, (3) treated with combined Ommaya reservoir and EVD surgery, and (4) initial and follow-up computed tomography (CT) scans were available. The exclusion criteria were as follows: (1) age<18 years, (2) a clear secondary cause of ventricular hemorrhage (aneurysm rupture, arteriovenous malformation rupture or neoplasm, as well as traumatic hemorrhages), and (3) a hematoma removal was performed postoperatively for any reason. This study was approved by the institutional review board of Huashan Hospital, Fudan University (HIRB-2015-256). All participants provided written informed consent. The study was conducted in accordance with relevant guidelines and regulations.
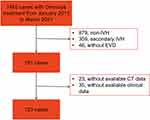 |
Figure 1 Flowchart of study enrollment. Abbreviations: IVH, intraventricular hemorrhage; EVD, external ventricular drainage. |
Grouping and Assessment
All IVH patients received combined EVDs and Ommaya reservoirs (Medtronic, Minneapolis, MN, USA) and were divided into Group A (Ommaya not used after surgery) and Group B (Ommaya used after surgery). The following information was extracted from the database: age, sex, the Glasgow Coma Scale (GCS) score at admission (before surgery), intraventricular urokinase, intracranial infection, hypertension, permanent ventriculoperitoneal shunt, length of hospital stay (LOS), length of EVD-drainage (LOE), length of Ommaya-drainage (LOO), in-hospital mortality, the modified Graeb Score (mGS, before surgery, 3 days and 7 days after surgery), and the Glasgow Outcome Scale (GOS) score (3 months postoperatively, if the patient was still alive).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 7 (GraphPad Software, San Diego, California, USA) and the open-source software Jamovi (version 2.3.2).
Descriptive statistics were used to describe the general information of all patients and were presented as the mean ± standard deviation if they fit the normal distribution and as the median and quantile intervals if the distributions are nonnormal.
Differences between the used and unused Ommaya groups were determined using the Mann–Whitney U-test (α = 0.05) after testing the normality and equality of variance. An unpaired t-test was applied to test the age differences between the used and unused groups. We also compared the categorical variables (such as hypertension versus no hypertension, pneumonia, versus no pneumonia, etc.) between the used and unused Ommaya groups by performing contingency table analyses and estimating the P values by Fisher’s exact test.
To estimate the relationship between the independent clinical factors (such as age, inpatient time, GCS, etc.) and clinical outcomes, a multiple linear regression analysis was performed using the least-squares method with the GOS score taken as the dependent variable (Y). The two-sided test level was α = 0.05, and p < 0.05 was considered to be statistically significant. All significance was accepted at the 0.05 level of probability.
We also apply a binomial logistic regression model to predict or infer the probability of using Ommaya. Significant preoperative clinical factors (GCS and mGS) were included as predictors in the model. Omnibus likelihood ratio tests were used to test the significant contributions of the predictors in the model. All significance was accepted at the 0.05 level of probability.
Results
Patient Characteristics
Table 1 summarizes the relevant characteristics of the study cohort. Figure 1 shows the flowchart of study enrollment. A total of 123 patients were enrolled finally, and there were 75 patients (52 males, 23 females) in the Ommaya-used group and 48 patients (31 males, 17 females) in the Ommaya-unused group. The GCS in the Ommaya-used group (8.920 ± 2.954) was significantly lower than that in the Ommaya-unused group (11.920 ± 1.724) (Figure 2A, p<0.0001). The average LOS was 16.46 (SD = 9.177) days in the unused group and 25.65 (SD = 12.57) days in the used group, which was a significant difference between the two groups. There was no increase in the number of patients with intracranial infections in the Ommaya-used group (11/75) compared to the Ommaya-unused group (6/48) (p>0.05). The age of the patients was 54.64 ± 13.41 versus 52.40 ± 15.04 between the respective groups. There were 22 patients in the Ommaya-used group and 9 patients in the Ommaya-unused group had permanent VP shunts placed. No significant differences were found in age, sex, hypertension, LOE, or permanent shunting between the Ommaya-used and Ommaya-unused groups (p>0.05). In this retrospective study, there were no reports of occlusion during Ommaya use, which may be due to the low incidence or neglect of recording.
 |
Table 1 Demographic, Clinical, and Neuroradiological Characteristics of Patients |
Imaging Evaluation
Figure 2 and Table 1 summarize the performances between the groups regarding the different clinical scores recorded at different times during the course. The mGS before the operation in the Ommaya-unused group (14.21 ± 3.831) was significantly lower than that in the Ommaya-used group (16.31 ± 5.342) (Figure 2D, p<0.01). Significant differences were also found in the 3-day mGS (11.17 ± 4.091 vs 13.48 ± 5.356) and 7-day mGS (6.688 ± 3.909 vs 9.027 ± 5.128) postoperatively between the groups, and the Ommaya-used group had higher scores (Figure 2E and F, p<0.05). However, no significance difference was found between the baseline mGS and the mGS 7 days later (Figure 2C), although marginal differences were found when comparing the mGS before drainage, and 3-day and 7-day mGS separately. Typical CT imaging of IVH patients before surgery and 3d, and 7d after surgery in Ommaya-used group and Ommaya-unused group are shown in Figure 3.
There were more patients in the Ommaya-used group who underwent intraventricular urokinase therapy (44/75) than that in the Ommaya-unused group (5/48), and this was a significant difference (p<0.0001).
Clinical Outcome
To perform the multiple linear regression analysis, the postoperative GOS was used as the dependent variable, and relevant clinical factors were included as independent variables after being selected based on variance inflation factors (Table 2). The patients in the Ommaya-used group showed a lower 3-month GOS (3.107 ± 0.9526) than those in the Ommaya-unused group (3.917 ± 0.9639), which showed a significant difference (Figure 2B, p<0.0001). The multiple linear regression revealed that patients with a longer LOS and LOO had significantly worse outcomes (p<0.01). In the multiple linear regression, there was a close correlation between the GCS and 3-month GOS (p<0.0001).
 |
Table 2 Linear Regression Analysis Between Independent Clinical Factors and Clinical Outcomes |
In the binomial logistic regression analysis (Figure 4), the preoperative GCS was supposed to be the main significant predictor in the model (p<0.001), while the preoperative mGS was found to be marginally significant (p = 0.086). The model showed a satisfying performance in predicting the usage of Ommaya reservoir. The model achieved 0.74 accuracy, 0.79 specificity, 0.67 sensitivity, and 0.82 AUC when the cutoff value was set to 0.5 (Figure 2).
In the multiple linear regression, all variance inflation factors were <5, and there was no multicollinearity among all the independent variables. Residuals of the model passed the normality test (D’Agostino-Pearson omnibus test, p=0.67). The results showed that the GCS, LOO, age, and LOS were the main statistically significant factors in the regression analysis, accounting for 63.3% of the variation in the GOS score. The preoperative GCS score had the largest weight in the regression equation, which indicates its prominent influence on the clinical outcome.
Discussion
As the population ages, the incidence of ICH and IVH is on a rise. However, the management of IVH is still a challenging problem. IVH is a serious event that causes sudden neurological deterioration, coma, and cerebral hernia. Mortality rates range from 50% to 80%, and most survivors (more than 70%) are left permanently affected with varying degrees of disability. One consensus is that a significant accumulation of CSF and enlargement of the brain’s ventricles are associated with poorer outcomes, and individuals who have these complications have the highest death rates. Historically, the placement of an EVD has been the most prevalent method for managing IVH as it facilitates CSF clearance until a decision regarding the necessity of permanent shunt placement is made.17 Despite some authors reporting satisfactory outcomes with the use of endoscopic suction-irrigation techniques, limitations associated with this treatment have been well-documented in literature.18–20 These limitations include difficulty in mobilizing solid clots that are adhered to the ventricular walls using the small instruments available through the endoscope’s working channel. Additionally, endoscopic equipment is relatively expensive and may not be accessible in all medical institutions.
In recent decades, the combination of an Ommaya reservoir and conventional EVD with urokinase (UK) in IVH patients has been widely applied at multiple medical centers.21–25 Some studies have suggested that this approach can shorten the duration of conventional EVD, increase IVH clearance, reduce the incidence and mortality of hydrocephalus, and result in good clinical outcomes without increasing the risk of ventriculitis infection.26 However, these studies have small sample sizes, and a further validation is needed. In some cases, Ommaya device is not used after implantation, only aiming to prevent the uncertain risk of hydrocephalus, which may bring little treatment benefit compared with the high operation risks especially for elderly patients. Given the potential abuse of Ommaya reservoir, we consider it substantial to examine whether the Ommaya system has been used appropriately, whether there is abuse, and whether there are certain indicators that can predict Ommaya use.
Reducing the number of patients who need permanent CSF diversion may potentially decrease the amount of shunt complications, the incidence of poor neurological outcomes and overall health-care costs.27–29 In our study, 25% of the IVH patients ultimately underwent permanent hydrocephalus shunt surgery, and this finding is basically consistent with previous literature reports. The early removal of conventional EVD followed by the use of an Ommaya reservoir for CSF drainage likely reduces the need for a permanent VP shunt.30,31 However, our study found that Ommaya usage in patients with IVH was not associated with early removal of EVD and a lower risk of shunt dependency. Notably, deferred removal of EVD potentially increases the risk of ventriculitis.32,33 Ommaya drainage was expected to reduce the incidence of intracranial infection,34 but the Ommaya-use group did not show a difference in infection in our study, which indicated that prolonged drainage time may not increase the risk of ventriculitis when using the Ommaya reservoir. More intraventricular UK injections were performed in the Ommaya-use group, which did not increase the infection risk when using the clinical procedure in our study.
It is well established that a large volume of IVH is strongly associated with poor neurological outcomes.35,36 Previous studies have shown that administering thrombolytic agents directly into ventricles can effectively accelerate the clearance of IVH and improve drainage.37 In our study, patients in the Ommaya-use group suffered more severe IVH attacks, and the mGS score of the Ommaya-use group was always higher than that of the Ommaya-unused group before and after surgery, but the gap between them tended to decrease after surgery. The difference in mGS between the groups was statistically significant before the operation (p<0.01), but not after the operation (p>0.05), indicating that the Ommaya drainage was effective in reducing the amount of blood in the ventricles. However, the mGS score did not show a significant correlation with the 3-month GOS (p>0.05), suggesting that the amount of blood in the ventricles was not a good predictor of the functional outcome of the patients. Therefore, the mGS score could be used as a measure of the severity of IVH and the efficacy of the Ommaya drainage, but not as a clinical indicator for selecting the patients who might benefit from the Ommaya drainage, or for assessing their prognosis. A higher ratio of UK injection in the Ommaya-treated group reflected the practicality and effectiveness of the Ommaya reservoir. The combination of Ommaya and EVD with UK hastened the clearance of intraventricular hematomas, but the efficiency of this technique needs further evaluation through additional prospective studies.
Patients in the group that did not use the Ommaya drainage system had better clinical outcomes, as evidenced by higher scores on the 3-month Glasgow Outcome Scale. These results could not negate the role of Ommaya drainage because the Ommaya drainage system is often used in patients with more severe IVH and lower Glasgow Coma Scale scores. Results from the study suggest that patients with lower preoperative GCS scores and longer lengths of stay or lengths of operation have worse clinical outcomes, which is consistent with expectations. Logistic regression analysis indicated that IVH patients with lower GCS scores, rather than modified Graeb scale scores, are more likely to have the Ommaya drainage system implanted. However, it is important to note that the Ommaya reservoir is an intracranial implant that carries not only a short-term risk of bleeding but also a long-term risk of infection and can have a significant impact on patients’ psychological well-being. Therefore, finding a preoperative indicator that can predict the need for an Ommaya reservoir in IVH patients would be beneficial in order to avoid unnecessary use of the system. We prove that the GCS is expected to be a predictive indicator for single-center data and rigorous statistical analysis, but the specific value has yet to be determined. In summary, the difference in GCS between the groups was statistically significant (p<0.001), indicating that patients who received Ommaya drainage had a lower level of consciousness than those who did not. This might imply that the Ommaya drainage was more likely to be used in patients with more severe IVH and worse neurological status, who might have a higher risk of complications and poor outcomes. The GCS score also showed a strong correlation with the 3-month GOS (p<0.0001), suggesting that the level of consciousness was a good predictor of the functional outcome of the patients. Therefore, the GCS score could be used as a clinical indicator for selecting the patients who might benefit from the Ommaya drainage, as well as for assessing their prognosis. Our study compared EVD alone and EVD plus Ommaya drainage for IVH treatment. We found no significant benefits of Ommaya drainage in functional outcomes, mortality, or shunt dependency. Therefore, we do not recommend routine use of Ommaya reservoirs in IVH patients, as they may increase cost, complexity, and infection risk. However, Ommaya drainage was associated with lower 3-month GOS, indicating higher IVH severity and worse neurological status in Ommaya patients. Thus, Ommaya reservoirs may still help some patients with severe IVH and low consciousness, who may have higher complications and poor outcomes. The decision to use Ommaya reservoirs should consider patient characteristics and preferences, and Ommaya drainage availability and feasibility. We also found that preoperative GCS score was a better predictor of Ommaya drainage use and 3-month GOS than mGS score. This suggests that consciousness level is a more reliable and relevant indicator for selecting Ommaya patients and assessing their prognosis. By using GCS score as a clinical indicator, clinicians could avoid unnecessary Ommaya implantations in patients with high consciousness and good prognosis, and prioritize Ommaya implantations in patients with low consciousness and bad prognosis. This could improve efficiency, safety, and quality of care for IVH patients, and reduce cost and burden of the health system. Our study provides valuable information for pre-surgery decision-making and post-surgery management of IVH patients and contributes to the improvement of clinical practice and patient care.
In many centers, the use of traditional EVD is decreasing in favor of EVD devices with intracranial pressure monitoring capabilities. Multicenter data are needed to determine whether the use of new devices can help improve the prognosis of IVH patients. Further exploration is required to determine whether specific thresholds of intracranial pressure have a role in predicting the use of the Ommaya system.
This study has several limitations, mainly its retrospective and non-experimental design. There was no patient randomization, and the surgical choice was based on experience from a single clinical center, which may not be representative of other settings. Some patients did not receive Ommaya device utilization even though they had it implanted, which may have affected the results. Another limitation is that it covered a long period (9 years), which may have introduced confounding factors due to the rapid evolution of neurosurgical techniques and ICU care standards. This study also had a small sample size, which may have reduced its statistical power and generalizability. We strongly recommend further studies to determine the optimal treatment strategy for IVH patients, which should include treatment randomization, larger sample size, and neurological outcome measures.
Conclusion
The study suggested that combining EVD and Ommaya drainage may be a safe and feasible treatment for IVH, but it did not show any significant benefit in terms of shunt dependency, functional outcome, or mortality compared to EVD alone. Therefore, our study suggests that the routine use of Ommaya reservoirs in IVH patients is not warranted and that the decision-making regarding the use of Ommaya reservoirs should be based on the individual characteristics and preferences of the patients. However, patients who used Ommaya reservoir had worse admission status and larger IVH, which may have confounded the results. Further studies with larger and matched cohorts are needed to confirm the efficacy and optimal timing of Ommaya reservoir use for IVH management.
Data Sharing Statement
The data analyzed during the current study are available from the corresponding author Jin Hu on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Ethics Approval and Consent to Participate
The study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1975. This study was approved by the institutional review board of Huashan Hospital, Fudan University (HIRB-2015-256). All participants provided written informed consent. The study was conducted in accordance with relevant guidelines and regulations.
Funding
This work was supported by grants [No. 2022YFE0141300, No. 82271224, 2018YFA0107900, 92168103, 32171417, 2019CXJQ01], from National Key Research and Development Program of China, National Natural Science Foundation of China, Ministry of Science and Technology of China, National Nature Science Foundation and Shanghai Municipal Government, Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai. Shanghai hospital development center, technology promotion, and management optimization in general hospitals [SHDC22023224, SHDC22022210], National High Level Hospital Clinical Research Funding [No. 2023-NHLHCRF-BQ-43].
Disclosure
The authors confirm that there are no competing interests.
References
1. Poon MT, Bell SM, Al-Shahi Salman R. Epidemiology of intracerebral haemorrhage. Front Neurol Neurosci. 2015;37:1–12. doi:10.1159/000437109
2. Gluski J, Garling RJ, Kappel A, et al. Factors impacting hydrocephalus incidence in intracerebral hemorrhage: a retrospective analysis. World Neurosurg. 2021;148:e381–e389. doi:10.1016/j.wneu.2020.12.164
3. Stretz C, Sheikh Z, Maciel CB, et al. Seizures, periodic and rhythmic patterns in primary intraventricular hemorrhage. Ann Clin Transl Neurol. 2018;5(9):1104–1111. doi:10.1002/acn3.627
4. Reger RM, Meinicke A, Härtig W, et al. Changes in CSF surface tension in relation to surfactant proteins in children with intraventricular hemorrhage. Brain Sci. 2022;12(11):1440. doi:10.3390/brainsci12111440
5. Hughes JD, Puffer R, Rabinstein AA. Risk factors for hydrocephalus requiring external ventricular drainage in patients with intraventricular hemorrhage. J Neurosurg. 2015;123(6):1439–1446. doi:10.3171/2015.1.JNS142391
6. Chung DY, Olson DM, John S, et al. Evidence-based management of external ventricular drains. Curr Neurol Neurosci Rep. 2019;19(12):94. doi:10.1007/s11910-019-1009-9
7. Gilard V, Djoubairou BO, Lepetit A, et al. Small versus large catheters for ventriculostomy in the management of intraventricular hemorrhage. World Neurosurg. 2017;97:117–122. doi:10.1016/j.wneu.2016.09.105
8. Thien A, Soh S, Lock C, et al. The national neuroscience institute external ventricular drain study: a pragmatic multisite risk-stratification pathway to reduce ventriculostomy-related infection. World Neurosurg. 2020;135:e126–e136. doi:10.1016/j.wneu.2019.11.070
9. Zubair A, De Jesus O. Ommaya Reservoir. Treasure Island (FL): StatPearls; 2022.
10. Dossani RH, Kalakoti P, Thakur JD, et al. Ayub Khan Ommaya (1930–2008): legacy and contributions to neurosurgery. Neurosurgery. 2017;80(2):324–330. doi:10.1093/neuros/nyw031
11. Kumble S, Zink EK, Burch M, et al. Physiological effects of early incremental mobilization of a patient with acute intracerebral and intraventricular hemorrhage requiring dual external ventricular drainage. Neurocrit Care. 2017;27(1):115–119. doi:10.1007/s12028-017-0376-9
12. Yang XT, Feng D-F, Zhao L, et al. Application of the Ommaya reservoir in managing ventricular hemorrhage. World Neurosurg. 2016;89:93–100. doi:10.1016/j.wneu.2015.12.040
13. Panigrahi M. External ventricular drain-related complications - whether continuous CSF drainage via Ommaya reservoir is the answer? Neurol India. 2021;69(4):1096. doi:10.4103/0028-3886.325316
14. Saez-Alegre M, Martín R, Palpán A, et al. Development of machine learning-based predictor algorithm for conversion of an Ommaya reservoir to a permanent cerebrospinal fluid shunt in preterm posthemorrhagic hydrocephalus. World Neurosurg. 2022;162:e264–e272. doi:10.1016/j.wneu.2022.02.120
15. Zheng WJ, Li L-M, Hu Z-H, et al. Bilateral external ventricular drains increase ventriculostomy-associated cerebrospinal fluid infection in low modified graeb score intraventricular hemorrhage. World Neurosurg. 2018;116:e550–e555. doi:10.1016/j.wneu.2018.05.030
16. Singh H, Patir R, Vaishya S, et al. External ventricular drain related complications-whether continuous csf drainage via ommaya reservoir is the answer? Neurol India. 2020;68(2):458–461. doi:10.4103/0028-3886.284354
17. Lovasik BP, McCracken DJ, McCracken CE, et al. The effect of external ventricular drain use in intracerebral hemorrhage. World Neurosurg. 2016;94:309–318. doi:10.1016/j.wneu.2016.07.022
18. Feletti A, Basaldella L, Fiorindi A. How I do it: flexible endoscopic aspiration of intraventricular hemorrhage. Acta Neurochir. 2020;162(12):3141–3146. doi:10.1007/s00701-020-04499-z
19. Song P, Duan F-L, Cai Q, et al. Endoscopic surgery versus external ventricular drainage surgery for severe intraventricular hemorrhage. Curr Med Sci. 2018;38(5):880–887. doi:10.1007/s11596-018-1957-3
20. Zhu J, Tang C, Cong Z, et al. Endoscopic intraventricular hematoma evacuation surgery versus external ventricular drainage for the treatment of patients with moderate to severe intraventricular hemorrhage: a multicenter, randomized, controlled trial. Trials. 2020;21(1):640. doi:10.1186/s13063-020-04560-3
21. Abdelmalik PA, Ziai WC. Spontaneous intraventricular hemorrhage: when should intraventricular tPA be considered? Semin Respir Crit Care Med. 2017;38(6):745–759. doi:10.1055/s-0037-1607991
22. Fu C, Liu L, Chen B, et al. Risk factors for poor outcome in hypertensive intraventricular hemorrhage treated by external ventricular drainage with intraventricular fibrinolysis. World Neurosurg. 2017;102:240–245. doi:10.1016/j.wneu.2017.03.029
23. Unal TC, Gulsever CI, Sahin D, et al. How I do it: ultrasound-guided placement of ommaya reservoir in a patient with small ventricles and cavum septum pellucidum. Acta Neurochir. 2021;163(3):721–724. doi:10.1007/s00701-021-04719-0
24. van Solinge TS, Muskens IS, Kavouridis VK, et al. Fibrinolytics and intraventricular hemorrhage: a systematic review and meta-analysis. Neurocrit Care. 2020;32(1):262–271. doi:10.1007/s12028-019-00786-5
25. Wang D, Liu J, Norton C, et al. Local fibrinolytic therapy for intraventricular hemorrhage: a meta-analysis of randomized controlled trials. World Neurosurg. 2017;107:1016–1024 e1. doi:10.1016/j.wneu.2017.07.135
26. Staub-Bartelt F, van Lieshout JH, Beez T, et al. Evaluation of volumetric change of intracerebral hemorrhage in patients treated with thrombolysis for intraventricular hemorrhage. Neurocrit Care. 2021;34(2):529–536. doi:10.1007/s12028-020-01054-7
27. Kuo LT, Lu H-Y, Tsai J-C, et al. Prediction of shunt dependency after intracerebral hemorrhage and intraventricular hemorrhage. Neurocrit Care. 2018;29(2):233–240. doi:10.1007/s12028-018-0532-x
28. Murthy SB, Awad I, Harnof S, et al. Permanent CSF shunting after intraventricular hemorrhage in the CLEAR III trial. Neurology. 2017;89(4):355–362. doi:10.1212/WNL.0000000000004155
29. Palpan Flores A, Saceda Gutiérrez J, Brin Reyes JR, et al. Risk factors associated with conversion of an Ommaya reservoir to a permanent cerebrospinal fluid shunt in preterm posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 2020:1–8. doi:10.3171/2019.11.PEDS19320
30. Covrig RC, Schellinger PD, Glahn J, et al. Shunt dependence after intraventricular hemorrhage and intraventricular fibrinolysis with uPA versus rt-PA. J Neurol Surg a Cent Eur Neurosurg. 2022;84(03):255–260.
31. Trobs RB, Sander V. Posthemorrhagic hydrocephalus in extremely low birth weight infants: ommaya reservoir vs. ventriculoperitoneal shunt. Childs Nerv Syst. 2015;31(8):1261–1266. doi:10.1007/s00381-015-2754-y
32. Krenzlin H, Frenz C, Schmitt J, et al. High CSF thrombin concentration and activity is associated with an unfavorable outcome in patients with intracerebral hemorrhage. PLoS One. 2020;15(11):e0241565. doi:10.1371/journal.pone.0241565
33. Yasar S, Kirik A. Older patients with intraventricular hemorrhage are prone to infection after external ventricular drainage. Ulus Travma Acil Cerrahi Derg. 2020;26(6):870–874. doi:10.14744/tjtes.2020.06159
34. Badholm M, Blixt J, Glimåker M, et al. Cerebrospinal fluid cell count variability is a major confounding factor in external ventricular drain-associated infection surveillance diagnostics: a prospective observational study. Crit Care. 2021;25(1):291. doi:10.1186/s13054-021-03715-1
35. Hussain SS, Raza A, Shahid S, et al. Postoperative reduction of intraventricular hemorrhage volume: single- versus dual-catheter drainage. J Neurol Surg a Cent Eur Neurosurg. 2018;79(4):279–284. doi:10.1055/s-0037-1617757
36. Maki Y, Ishibashi R, Yasuda T, et al. Correlation of scoring systems with the requirement of an external ventricular drain in intraventricular hemorrhage. World Neurosurg. 2022;163:e532–e538. doi:10.1016/j.wneu.2022.04.023
37. Mei L, Fengqun M, Qian H, et al. Exploration of efficacy and safety of interventions for intraventricular hemorrhage: a network meta-analysis. World Neurosurg. 2020;136:382–389 e6. doi:10.1016/j.wneu.2019.10.177
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



