Back to Journals » OncoTargets and Therapy » Volume 9
Combination of cold atmospheric plasma and iron nanoparticles in breast cancer: gene expression and apoptosis study
Authors Jalili A, Irani S , Mirfakhraie R
Received 2 September 2015
Accepted for publication 7 January 2016
Published 28 September 2016 Volume 2016:9 Pages 5911—5917
DOI https://doi.org/10.2147/OTT.S95644
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Faris Farassati
Azam Jalili,1 Shiva Irani,1 Reza Mirfakhraie2
1Department of Biology, Science and Research Branch, Islamic Azad University, 2Department of Medical Genetics, Shahid Beheshti University of Medical Sciences, Tehran, Iran
Background: Current cancer treatments have unexpected side effects of which the death of normal cells is one. In some cancers, iron nanoparticles (NPs) can be subjected to diagnosis and passive targeting treatment. Cold atmospheric plasma (CAP) has a proven induction of selective cell death ability. In this study, we have attempted to analyze the synergy between CAP and iron NPs in human breast adenocarcinoma cells (MCF-7).
Materials and methods: In vitro cytotoxicity of CAP treatment and NPs in cells measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and cell death was shown by 4',6-diamidino-2-phenylindole and annexin V staining. Fluctuations in BAX and BCL-2 gene expression were investigated by means of real-time polymerase chain reaction.
Results: MTT assay results showed that combination of plasma and iron NPs decreased the viability of cancer cells significantly (P<0.05). Real-time analysis showed that the combination therapy induced shifting the BAX/BCL-2 ratio in favor of apoptosis.
Conclusion: Our data indicate that synergy between CAP and iron NPs can be applied in breast cancer treatment selectively.
Keywords: breast cancer, cold atmospheric plasma, iron nanoparticles, BAX, BCL-2
Introduction
All current cancer treatments, such as chemotherapy, radiotherapy, hormonal therapy, immunotherapy, and surgery, are expensive and nonselective with side effects.1,2 Recently, the use of cold atmospheric plasma (CAP) and nanotechnology has been highly regarded.3,4 Plasma is an ionized gas that contains numerous positive and negative ions, electrons, free radicals, and reactive molecules.5 Nonthermal plasma known as CAP was derived from electric discharge of plasma.2,6 CAP is not thermodynamically stable as distinguished by high electron temperature but very low gas temperature.2 CAP is derived from inert gases such as argon and helium; when a gas such as oxygen is added to this combination, the active species including reactive oxygen species (ROS) and reactive nitrogen species increase. CAP has considerable characteristics of changing the intracellular biochemical signaling without electrical and thermal damages on the cells, which makes it adequately acceptable in biological and medicinal applications.7–10 Induction of growth arrest in cancer cells is one of the most important applications of CAP that more or less acts selectively on cancer cells.11 Recently, in cancer treatments, iron oxide nanoparticles (NPs) are also applied as a heating agent (or medium) in the presence of a magnetic field alternatively.1,12–17 Apoptosis as a cell death may be modulated by programmed control mechanisms and plays an important role in the development and differentiation of various organisms.18 BCL2 family proteins that are located on the outer membrane of mitochondria play anti- or pro-apoptotic roles in mitochondrial apoptosis pathways.19 Sensitivity of cells versus death signal is determined by the ratio between pro-apoptotic molecules (eg, BAX) and anti-apoptotic molecules (eg, BCL-2).20 Some novel studies have attempted to use NPs for enhancement of CAP effect. Shahmirani et al showed that the death of colon cancer cells which were treated by CAP for 180s and gold NPs increased significantly.2
The aim of this study was to investigate the synergistic effects of CAP and iron oxide (Fe2O3) NPs on cell viability, apoptosis, and messenger RNA (mRNA) expression levels of BAX and BCL-2 genes in breast cancer (MCF-7) human cell line.
Materials and methods
Materials
Plasma source
The plasma jet device primarily consisted of an insulating shield made of pyrex tube through which the inert gas such as helium (99.99%) was injected to carry a reactive gas (5% oxygen) (Figure 1).
  | Figure 1 Experimental setup of CAP. |
RNA extraction solution easy-BLUE (Total RNA Extraction Solution) was purchased from Intron Biotechnology (Gyeonggi-do, Republic of Korea), according to the manufacturer’s protocol (INTRON, cat17061). Complementary DNA (cDNA) synthesis was performed using Revert Aid TM First-Strand cDNA Synthesis Kit (ABI, cat4368814) according to the manufacturer’s instruction (Thermo Fisher Scientific, Waltham, MA, USA). Real-time polymerase chain reaction (RT-PCR) kit was supplied by Takara (Otsu, Japan). The human breast cancer cell lines (MCF-7) and human fibroblast (HF) primary cells were provided by the National Cell Bank Pasteur Institute (Tehran, Iran). Dulbecco’s Modified Eagle’s Medium (DMEM), fetal bovine serum (FBS), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (MTT), and 4′,6-diamidino-2-phenylindole (DAPI) were provided by Thermo Fisher Scientific.
Synthesis and characterization of iron NPs
Iron NPs were prepared by citrate reduction of FeCl3·3H2O following the methods of Nigam et al.20 First, 4.4 g of FeCl3 and 1.7 g of FeCl2 were mixed in 80 mL of water. After 30 minutes with fixed temperature at 70°C, 20 mL of ammonia solution was added immediately to the mixture and kept for another 30 minutes. Next, 4 mL of aqueous solution of citric acid (0.5 g/mL) was added, and the temperature was slowly increased consequently up to 90°C under reflux for 60 minutes; when the mixture degree reached room temperature, the black colored precipitates were rinsed with water. Iron NPs used in medical applications have an average diameter of 50 nm and a negative surface charge. The iron NP sample was diluted before measurement by a light scattering instrument (ZetasizerNanoZS; Malvern Instruments, Malvern, UK).21
Cell culture and in vitro cytotoxicity study
Human breast cancer cells (MCF-7) and HF cells were obtained from the cell bank of Pasteur Institute of Iran. The MCF-7 cells and HF cells were cultured separately in high-glucose DMEM (Thermo Fisher Scientific) with 10% (v/v) FBS and 1% penicillin at 37°C in a 5% CO2 humidified atmosphere. Cell culture medium was refreshed at every 48 hours. When the cells arrived at an acceptable confluence, they were removed from the culture flasks by treatment with 0.25% trypsin/ethylenediaminetetraacetic acid solution. In this study, 1×104 cells per well in 96-well plates were seeded for 24 hours. For plasma treatment, the nozzle of plasma was set 1.5 cm above the cell culture medium, and the cells were exposed to plasma radiation for 15 seconds, 30 seconds, and 45 seconds and then incubated for 24 hours.
The tests were carried out with three replicates, and an untreated well served as control.
The MCF-7 cells were treated with iron NPs at ten variable concentrations of 1.48–750 ppm, and the HF cells were tested with four variable concentrations. The least number of living cells that had been observed at 23 ppm, 45 ppm, 93 ppm, 187 ppm, and 375 ppm in cancer cells were treated with helium/oxygen plasma at variable times of 15 seconds, 30 seconds, and 45 seconds. Each well of 96-well cluster dishes was placed right under the nozzle and the experiment was carried out at room temperature.
To determine the viability of MCF-7 and HF cells, MTT assay was employed based on the mitochondrial conversion of the tetrazolium salt, MTT. The cells were trypsinized and resuspended in DMEM–10% FBS, counted, and seeded at a concentration of 1×104 cells for MCF-7 and HF cells per 96 wells. Then the cultured and attached cells were treated with plasma and iron NPs for different times and continuously cultured for 24 hours. After 24-hour incubation, the medium was removed from each well and 100 μL of the culture medium comprising 20 μL of MTT reagent (5 mg/mL) was added to each well and incubated for 4 hours at 37°C. After this incubation period, 100 μL of dimethyl sulfoxide was added for dissolving the formazan, and the optical density of each well was measured at 570 nm using an ELISA plate reader (ELx800; BioTek Instruments, Inc., Winooski, VT, USA).
Real-time quantitative PCR analysis: RNA extraction
The MCF-7 breast cancer and HF cells at a density of 1×105 cells/mL were seeded into 6-well plates and incubated for 24 hours. Afterward, the cells were treated by plasma, iron oxide NPs, and plasma–iron NPs. Then, the cells were cultured for 24 hours, and the total cellular RNA was extracted from treated and untreated cells using Trizol reagent (easy-BLUE Total RNA Extraction Solution) purchased from Intron Biotechnology, Korea, according to the manufacturer’s protocol (INTRON, cat17061). cDNA synthesis was performed using Revert Aid TM First-Strand cDNA Synthesis Kit (ABI, cat4368814) according to the manufacturer’s instruction (Thermo Fisher Scientific).
Primer design
Oligonucleotide primers were designed using Primer premier v6 software (Thermo Fisher Scientific) and further analyzed by Gene Runner software. The sequence of primers for the PCR amplification of BCL2 transcripts was (F: 5′-CGGAGGCTGGGATGCCTTTGT-3′) and (R: 5′-CAAGCTCCCACCAGGGCCAAA-3′). Gene expression levels for each sample were normalized to the expression levels of housekeeping gene encoding beta-2-microglobulin (β2M) within a given sample. β2M amplification was performed using specific primers (F: 5′-AGATGAGTATGCCTGCCGTG-3′) and (R: 5′-GCGGCATCTTCAAACCTCCA-3′).
Real-time PCR
This reaction was performed by Rotor-Gene 6000 (Corbett Research, Sydney, Australia) using SYBR GREEN® (nonspecific DNA-binding factors). To amplify the desired fragments, the following thermal cycling was used: −95°C for 30 seconds and then 45 cycles of 95°C for 5 seconds and 60°C for 30 seconds. The following temperature program was also applied for amplification: each RT-PCR was performed in duplicates with cDNA of three different cell cultures. The changes of gene expression of BAX and BCL-2 compared with β2M were normalized by LinReg software (Linreg version 2012.1, Amsterdam, the Netherlands). Expression levels of BAX and BCL2 were determined by using the REST program (Relative Expression Software Tool).22
Nuclear morphological analysis
The morphology of treated cancer cells was assessed by fluorescence microscopy after 24 hours of treatment. After implantation of the cells on the cover slip in a 6-well plate and after 24 hours of cell attachment, treated by plasma, iron oxide NPs, and plasma–iron NPs, the cells were washed twice in phosphate-buffered saline (PBS) 10× for 10 minutes. The cells were exposed to paraformaldehyde (4%) for 10 minutes and washed again in PBS 10× for 10 minutes. Then, the cells were treated with Triton-X 100 for 5 minutes, washed in PBS 1%, stained with DAPI for 5 minutes, and then washed twice in PBS 1% for 10 minutes. The cells were maintained in the dark before observation under a fluorescence microscope (Bell, INV-100FL).22
Apoptosis study
For apoptosis study by flow cytometry, MCF-7 cells were seeded at 1×105 for 24 hours. Then, the cells were treated by CAP for 30 seconds, iron NPs only, and combination of CAP/iron NPs. After 24 hours of incubation, the treated cells were trypsinized, washed with PBS, and suspended with binding buffer. Two microliters of annexin-V were added and incubated for 10 minutes in the dark. Stained cells were analyzed by flow cytometry (BD Biosciences, San Jose, CA, USA).
Statistical analysis
MTT and RT-PCR data analyses were carried out by one-way analysis of variance using SPSS 18, with P-value <0.05. For the RT-PCR data, the Ct and the average ratio of gene expression and correlation coefficient (R2) were determined before statistical calculation.
Results
Cell viability
The application of different concentrations of iron NPs and plasma treatment for various times showed a cytotoxicity effect on cancer cells after 24 hours. The MTT results showed a distinctive decrease in the number of viable MCF-7 cells, which were treated by plasma at 30 seconds (P<0.05) and 45 seconds (P<0.001) (Figure 2).
The plasma treatment of HF cells did not indicate significant reduction in cell proliferation after 24 hours (P>0.05) (Figure 3). The MTT assay demonstrated that treatment with iron NPs at a concentration of 187.5 ppm had toxic effect on MCF-7 cells (P<0.05) (Figure 4A) and no toxic effect on HF cells (P>0.05) (Figure 4B). The results showed that treatment of MCF-7 cells with iron NPs in 187.5 ppm and then by CAP has considerable reduction in viable cells compared with single treatment with iron NPs or plasma (Figure 4C).
Real-time PCR results
The expression of BCL2 and BAX mRNAs in MCF-7 cultured cells is shown in Figure 5. BCL-2 mRNA expression was suppressed in response to CAP treatment for 30 seconds and 45 seconds, whereas BAX mRNA expression was not altered.
In contrast, by combination therapy with iron NPs for 30 seconds at 187 ppm on MCF-7 cells, BCL2 gene was noticeably downregulated in its expression level and the BAX gene was upregulated significantly.
Apoptosis morphological changes
Nuclear morphological changes of MCF-7 cells after treatment with plasma for 30 seconds and 187.5 ppm and plasma–iron NPs were assessed to determine apoptotic process (Figure 6). The treated cells showed DNA fragmentation, a typical morphological feature of apoptosis.
Apoptosis study
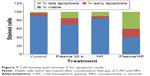
To confirm our results, we examined the effects of CAP and iron NPs on apoptosis in MCF-7 cells by annexin-V–fluorescein isothiocyanate. Results are shown in Figure 7. Exposure to 30-second CAP and iron NPs together led to higher annexin-V labeling than that in untreated cells, suggesting that cell death induced by CAP and iron NPs was due to apoptosis.
Discussion
The main aim of this study was to evaluate cytotoxicity, apoptotic gene expression, apoptosis, and passive targeting in breast cancer cells (MCF-7) treated with CAP and iron NPs. In recent years, CAP has been used in a broad range of potential applications such as selective and target cancer therapy.23 Our findings establish the efficacy of CAP in the treatment of breast cancer cells (MCF-7). CAP decreases metabolic activity and induces apoptosis in MCF-7 selectively. Kim et al11 induced apoptosis in melanoma cells by cold plasma treatment. Plasma as an ionized gas contains ions and electrons, free radicals, reactive molecules, and photons.24 Hydroxyl radicals, anion superoxides, alkoxyls, and nitric oxides are radical species, and the nonradical species, for example, hydrogen peroxides, ozone, and singlet oxygen, are all reactive species that are generated by plasma.2
Cancer cells are totally sensitive to ROS that are produced by CAP. Recent studies, based on current hypotheses, indicate the important effects of reactive oxygen in plasma mechanism.6,22,25–28
Cancer therapy by applying plasma, which is generated just by helium gas, is not efficient as the percentage of active production is not sufficiently high for activation of apoptosis pathway; therefore, oxygen gas is added to helium gas for increasing the percentage of ROS of plasma.11–30
Our studies have shown that iron NPs of 50 nm and negative zeta potential have variable effects on MCF-7 and HF cells. This study indicated that iron NPs have dose-dependent and selective effects on cancer and normal cells. Thus, in low dose, they have proliferation effect on MCF-7 cells, but at high concentration, apoptosis effects as in HF cells show no toxicity effect.
As spherical iron NPs are suitable for monolayer cell culture and can easily enter the cells, iron NPs were employed in this study. NPs with this size selection were unable to pass the nuclear membrane, and thus they accumulated around the nucleus in the cytoplasm.2 Iron NPs have been shown to increase intracellular ROS,21–29 which indicates that iron NPs (50 nm) induced apoptosis in MCF-7 cells through an unknown mechanism. Shahmirani et al showed that the cell death increased in colon cancer cells by combined treatment of plasma and gold NPs that confirm our results. The exact molecular mechanism involved in inducing apoptosis in cells by exposure to plasma is unclear.11 Previous studies revealed that the expression level of BCL-2 gene serves as a diagnostic biomarker in breast cancer. Gao et al31 have shown high expression level of BCL-2 gene in MCF-7 breast cancer cells at G1 phase, while BAX gene is not changed. In this study, the expression level of antiapoptotic BCL-2 gene was significantly decreased in MCF-7 cells treated with CAP at 30 seconds, while the expression level of the proapoptotic BAX gene did not change. Therefore, we may conclude that plasma induces apoptosis through shifting the BAX/BCL-2 ratio in favor of apoptosis. Yan et al19 demonstrated that the plasma induces concomitant apoptosis in HepG2 cells by increasing the BAX/BCL-2 ratio, which is accompanied by the upregulation of p53 tumor suppressor. In addition, the expression level of BAX gene was not changed in MCF-7 cells treated with iron NPs at 187.5 ppm. However, BCL-2 expression level was decreased in iron NP-treated MCF-7 cells, which suggests that these NPs induce apoptosis through shifting the BAX/BCL-2 ratio in favor of apoptosis.
Plasma destroys the cell membrane by modifying its permeability. Apoptosis is the last step of DNA damage, which results from cell membrane damage.29 The death mechanism of cancer cells is strictly related to ROS concentrations.32 In total, the cells are very different in their responses to plasma treatment. Cancer cells are more activated in metabolic system than others and are under increased oxidative stress, and hence cancer cells are demolished considerably in response to plasma.
Conclusion
Investigating MCF-7 cells revealed increasing levels of cell death through an unknown mechanism by treating them with iron NPs at a concentration of 187 ppm and size of 50 nm and irradiated by plasma for 30 seconds. Cell death occurs as a consequence of increasing the production of ROS, which may temporarily enhance the permeability of cell membrane. By combining plasma and NPs, several benefits such as anticancer effect and diagnosis without any side effects on normal cells were observed.
Disclosure
The authors report no conflicts of interest in this work.
References
Aljarrah K, Mhaidat NM, Al-Akhras MA, et al. Magnetic nanoparticles sensitize MCF-7 breast cancer cells to doxorubicin-induced apoptosis. World J Surg Oncol. 2012;10:62. | ||
Shahmirani Z, Irani S, Atyabi SM, Mirpour S. Effect of cold atmospheric pressure plasma and gold nanoparticles on cell viability. Arch Med Sci. 2015;11(6):1286–1295. | ||
Yousfi M, Merbahi N, Pathak A, Eichwald O. Low-temperature plasmas at atmospheric pressure: toward new pharmaceutical treatments in medicine. Fundam Clin Pharmacol. 2014;28(2):123–135. | ||
Cheng X, Murphy W, Recek N, et al. Synergistic effect of gold nanoparticles and cold plasma on glioblastoma cancer therapy. J Phys D Appl Phys. 2014;47(33):335402. | ||
Kieft I, Broers J, Caubet-Hilloutou V, Slaaf D, Ramaekers F, Stoffels E. Electric discharge plasmas influence attachment of cultured CHO K1 cells. Bioelectromagnetics. 2004;25(5):362–368. | ||
Kim SJ, Chung T, Bae S, Leem S. Induction of apoptosis in human breast cancer cells by a pulsed atmospheric pressure plasma jet. Appl Phys Lett. 2010;97(2):023702. | ||
Stoffels E, Kieft I, Sladek R, Van den Bedem L, Van der Laan E, Steinbuch M. Plasma needle for in vivo medical treatment: recent developments and perspectives. Plasma Sources Sci Technol. 2006;15(4):S169. | ||
Fridman G, Shereshevsky A, Jost MM, et al. Floating electrode dielectric barrier discharge plasma in air promoting apoptotic behavior in melanoma skin cancer cell lines. Plasma Chem Plasma Process. 2007;27(2):163–176. | ||
Fridman G, Peddinghaus M, Balasubramanian M, et al. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem Plasma Process. 2006;26(4):425–442. | ||
Kalghatgi S, Dobrynin D, Fridman G, et al. Plasma assisted decontamination of biological and chemical agents. In: Güçeri S, Fridman A, Gibson K, Haas C, editors. Applications of Non Thermal Atmospheric Pressure Plasma in Medicine. Netherlands: Springer; 2008:173–181. | ||
Kim C-H, Bahn JH, Lee S-H, et al. Induction of cell growth arrest by atmospheric non-thermal plasma in colorectal cancer cells. J Biotechnol. 2010;150(4):530–538. | ||
Apopa PL, Qian Y, Shao R, et al. Iron oxide nanoparticles induce human microvascular endothelial cell permeability through reactive oxygen species production and microtubule remodeling. Part Fibre Toxicol. 2009;6:1. | ||
Corot C, Robert P, Idée J-M, Port M. Recent advances in iron oxide nanocrystal technology for medical imaging. Adv Drug Deliv Rev. 2006;58(14):1471–1504. | ||
Jedlovszky-Hajdú AL, Bombelli FB, Monopoli MP, Tombácz E, Dawson KA. Surface coatings shape the protein corona of SPIONs with relevance to their application in vivo. Langmuir. 2012;28(42):14983–14991. | ||
El-Dakdouki MH, Zhu DC, El-Boubbou K, et al. Development of multifunctional hyaluronan-coated nanoparticles for imaging and drug delivery to cancer cells. Biomacromolecules. 2012;13(4):1144–1151. | ||
Pal A, Singh A, Nag TC, Chattopadhyay P, Mathur R, Jain S. Iron oxide nanoparticles and magnetic field exposure promote functional recovery by attenuating free radical-induced damage in rats with spinal cord transection. Int J Nanomedicine. 2013;8:2259–2272. | ||
Ahamed M, Alhadlaq HA, Khan MM, Akhtar MJ. Selective killing of cancer cells by iron oxide nanoparticles mediated through reactive oxygen species via p53 pathway. J Nanopart Res. 2013;15(1):1–11. | ||
Ahn HJ, Kim KI, Kim G, Moon E, Yang SS, Lee J-S. Atmospheric-pressure plasma jet induces apoptosis involving mitochondria via generation of free radicals. PLoS One. 2011;6(11):e28154. | ||
Yan X, Zou F, Zhao S, et al. On the mechanism of plasma inducing cell apoptosis. IEEE Trans Plasma Sci. 2010;38(9):2451–2457. | ||
Nigam S, Barick K, Bahadur D. Development of citrate-stabilized Fe 3 O 4 nanoparticles: conjugation and release of doxorubicin for therapeutic applications. J Magn Magn Mater. 2011;323(2):237–243. | ||
Chen Q, Espey MG, Sun AY, et al. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc Natl Acad Sci U S A. 2007;104(21):8749–8754. | ||
Keidar M, Walk R, Shashurin A, et al. Cold plasma selectivity and the possibility of a paradigm shift in cancer therapy. Br J Cancer. 2011;105(9):1295–1301. | ||
Walk RM, Snyder JA, Srinivasan P, et al. Cold atmospheric plasma for the ablative treatment of neuroblastoma. J Pediatr Surg. 2013;48(1):67–73. | ||
Georgescu N, Lupu AR. Tumoral and normal cells treatment with high-voltage pulsed cold atmospheric plasma jets. IEEE Trans Plasma Sci. 2010;38(8):1949–1955. | ||
Kim JY, Wei Y, Li J, et al. Single-cell-level microplasma cancer therapy. Small. 2011;7(16):2291–2295. | ||
Lupu A-R, Georgescu N. Cold atmospheric plasma jet effects on V79-4 cells. Roum Arch Microbiol Immunol. 2010;69(2):67–74. | ||
Zhang X, Li M, Zhou R, Feng K, Yang S. Ablation of liver cancer cells in vitro by a plasma needle. Appl Phys Lett. 2008;93(2):021502. | ||
Kim G, Kim W, Kim K, Lee J. DNA damage and mitochondria dysfunction in cell apoptosis induced by nonthermal air plasma. Appl Phys Lett. 2010;96(2):021502. | ||
Joh HM, Kim SJ, Chung T, Leem S. Comparison of the characteristics of atmospheric pressure plasma jets using different working gases and applications to plasma-cancer cell interactions. AIP Adv. 2013;3(9):092128. | ||
Li J, Cheung H-Y, Zhang Z, Chan GK, Fong W-F. Andrographolide induces cell cycle arrest at G2/M phase and cell death in HepG2 cells via alteration of reactive oxygen species. Eur J Pharmacol. 2007;568(1):31–44. | ||
Gao G, Dou QP. G1 phase-dependent expression of Bcl-2 mRNA and protein correlates with chemoresistance of human cancer cells. Molecular Pharmacology. 2000;58(5):1001–1010. | ||
Joh HM, Kim SJ, Chung T, Leem S. Reactive oxygen species-related plasma effects on the apoptosis of human bladder cancer cells in atmospheric pressure pulsed plasma jets. Appl Phys Lett. 2012;101(5):053703. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.