Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 12
Coil therapy for patients with severe emphysema and bilateral incomplete fissures – effectiveness and complications after 1-year follow-up: a single-center experience
Authors Kontogianni K, Gerovasili V, Gompelmann D, Schuhmann M, Hoffmann H, Heussel CP, Herth FJF , Eberhardt R
Received 20 July 2016
Accepted for publication 10 October 2016
Published 23 January 2017 Volume 2017:12 Pages 383—394
DOI https://doi.org/10.2147/COPD.S117655
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Konstantina Kontogianni,1,2 Vasiliki Gerovasili,3 Daniela Gompelmann,1,2 Maren Schuhmann,1,2 Hans Hoffmann,2,4 Claus Peter Heussel,2,5 Felix JF Herth,1,2 Ralf Eberhardt1,2
1Department of Pulmonology and Respiratory Care Medicine, Thoraxklinik at the University of Heidelberg, 2Translational Lung Research Center Heidelberg, Member of the German Center for Lung Research DZL, Heidelberg, Germany; 3Department of Respiratory Medicine, Harefield Hospital, Royal Brompton & Harefield NHS Foundation Trust, London, UK; 4Department of Thoracic Surgery, Thoraxklinik at the University of Heidelberg, 5Diagnostic and Interventional Radiology with Nuclear Medicine, Thoraxklinik at the University of Heidelberg, Heidelberg, Germany
Background: Lung volume reduction coil (LVRC) treatment is established in daily endoscopic lung volume reduction routine. The aim of this study was to evaluate the safety and efficacy of LVRC treatment.
Patients and methods: This was a retrospective analysis of 86 patients (male/female: 40/46, mean age: 64±7 years) with severe COPD and bilateral incomplete fissures. A total of 10 coils were unilaterally implanted in a single lobe, and 28 out of 86 patients were treated bilaterally. At 90-, 180-, and 365-day follow-up, changes in pulmonary function test (PFT), 6-minute walk test (6MWT) and modified Medical Research Council (mMRC) dyspnea scale, as well as possible complications, were recorded.
Results: At 90 days, the forced expiratory volume in 1 second did improve (P<0.001), but the improvement was not sustained at the 180- and 365-day follow-up (baseline: 0.71±0.21 vs 0.77±0.23 vs 0.73±0.22 vs 0.70±0.18 L). Both vital capacity and residual volume improved significantly (P<0.001) at the 90- and 180-day follow-up, but the improvement was lost after 365 days. Total lung capacity decreased at the 90-day follow-up but returned to baseline values at the 180- and 365-day follow-up. 6MWT (P=0.01) and mMRC (P=0.007) also improved at 90 and 180 days (Δ6MWT of 31±54 and 20±60 m, respectively), but the improvement was also lost at the 365-day follow-up. No significant further improvement was evident at any point in the follow-up after the second procedure. A total of 4 out of 86 patients passed away due to complications. Significant complications in the first 3 months and then at 12 months included the following: severe hemoptysis in 4 (3.5%) and 4 (3.5%) patients, pneumonia requiring hospitalization in 32 (28.1%) and 9 (7.9%) patients and pneumothorax in 7 (6.1%) and 2 (1.7%) patients, respectively. Milder adverse events included self-limited hemoptysis, pneumonias, or COPD exacerbations treated orally.
Conclusion: LVRC improved PFT, 6MWT and mMRC initially, but the improvement was lost after 365 days. Furthermore, we observed 4 deaths and significant severe complications, which need to be further elucidated.
Keywords: COPD, emphysema, bronchoscopy, coils, effectiveness, complications
Introduction
COPD is a highly prevalent disease,1 and epidemiological estimates predict a further increase in prevalence in the years to come.2 Emphysema is a key component of COPD and currently non-curable; it is characterized by lung tissue inelasticity, air trapping, and hyperinflation, causing progressively worsening dyspnea and exercise limitation that lead to impaired quality of life. Recent research efforts have been focusing on identifying clinical, physiologic, and radiologic COPD phenotypes that may be better responsive to specific treatments or interventions.
Invasive surgical procedures such as lung volume reduction surgery (LVRS) and lung transplantation are available, but for only a small subset of COPD patients.3 Patients with upper-lobe-predominant emphysema and low exercise capacity demonstrated an improved overall survival and better clinical and functional outcome after LVRS, a treatment nevertheless associated with significant morbidity and mortality.4,5 Furthermore, end-stage COPD accounts for 40% of all adult lung transplantations performed worldwide.6 Lung transplantation still remains a major operative treatment and is available only to a low number of patients, due to limited organ availability and access to specialized tertiary care centers.
An effort to find alternatives to surgery or transplantation brought up a number of minimally invasive interventional strategies. Endoscopic lung volume reduction (ELVR) by means of one-way endobronchial valve placement has been the most widespread and extensively investigated strategy so far.7–9 Endobronchial valves showed clinically significant improvements in selected patients with upper- and lower-lobe-predominant emphysema and intact fissures.7,10–12 The frequent presence of incomplete fissures as a parameter for collateral ventilation presents a limiting factor in valve therapy and indicates the need for ELVR treatments that work independently of collateral ventilation. Treatment by implanting coils overcomes this limiting factor and might serve as an alternative choice. Several studies in the past couple of years have addressed the lung volume reduction coils (LVRCs).
The aim of this retrospective analysis was to evaluate the safety and efficacy of LVRC treatment in a single-center setting in a larger group of patients with severe heterogeneous emphysema and bilateral incomplete fissures who were treated between September 2011 and December 2015.
The primary efficacy end points were changes at 6 and 12 months in forced expiratory volume in 1 second (FEV1) and 6-minute walk test (6MWT). Secondary end points included comparison between baseline and 6 and 12 months of the remaining values of pulmonary function test (PFT) and dyspnea perception score. The safety outcomes included all nonserious and serious adverse events.
Patients and methods
This was a single-center, retrospective analysis of prospective sampled data of a patient cohort, which was treated with LVRCs outside of clinical trials. Patients with severe heterogeneous emphysema and bilateral incomplete interlobar fissures were included after providing written informed consent. The study protocol was approved by the ethics committee of the University of Heidelberg (ethics number S-609/2012).
ELVR treatment is part of the standard clinical care of patients with severe COPD in our hospital. Inclusion criteria for LVRC were predominantly unilateral heterogeneous emphysema and bilaterally incomplete fissures and previously documented criteria for ELVR, ie, FEV1 <40%, residual volume (RV) >180% predicted and total lung capacity (TLC) >100% predicted.7–9
A total of 86 patients (male/female: 40/46, mean age: 64±7 years) were included in this retrospective analysis between September 2011 and December 2015. PFT, exercise capacity test, dyspnea score, and radiological tests were performed prior to and following the coil therapy. Before the intervention, we obtained the medical history of the patients and documented their medication, and the patients filled in the modified Medical Research Council (mMRC) dyspnea questionnaire.13,14 A PFT with post-bronchodilator spirometry, body plethysmography and measurement of diffusion capacity and the 6MWT15 were also performed, all according to the current American Thoracic Society/European Respiratory Society guidelines.16,17 Image data from inspiratory and expiratory thin-section chest computed tomography (CT) scan and echocardiogram were also collected.
All the patients underwent a bronchoscopy under general anesthesia with a combination of rigid and flexible bronchoscopy as per hospital standards. A total of 10 coils were placed in a single lobe and in a single session under fluoroscopic guidance into the subsegmental bronchi of the lobe most affected by emphysema according to prior CT evaluation. Twenty eight patients received additional treatment of a contralateral lobe in a second bronchoscopy.
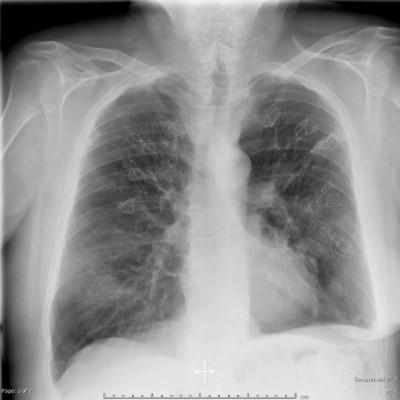
The LVRCs (PneumRx Inc., Mountain View, CA, USA) are made from preformed nitinol wire with shape memory. They are bronchoscopically delivered straight via the working channel of a flexible bronchoscope and recover to a non-straight, predetermined shape upon deployment. LVRCs were available in 3 different sizes (100, 125, and 150 mm) to accommodate the varying airway lengths. Further details of the coil design, function, and insertion technique have been described previously.18 Figure 1 demonstrates a patient’s chest X-ray after bilateral coil insertion.
  | Figure 1 X-ray after bilateral coil implantation. |
Our local radiologists visually assessed each patient’s baseline multislice CT (MSCT), and each fissure with a defect of >10% was considered incomplete.19 No Chartis analysis was performed in these cases.20 The heterogeneity of emphysema was visually determined by non-enhanced thin-section CT scan. Lobar-specific emphysema amount (emphysema index) was quantified using an automated in-house software tool (YACTA), which enhances the emphysema distribution and quantifies the individual distribution of emphysema. Morphological destruction and regional lung perfusion were matched with perfusion scintigraphy.21,22 At baseline, the emphysema distribution was visualized as color coded in the CT, and the total lung volume and emphysema index were quantified with YACTA analysis of the CT data.23 YACTA is a CT software program that fully automatically detects the lung tissue (<−500 Hounsfield units [HU]) based on threshold values and an anatomical knowledge-based algorithm after exclusion of the tracheobronchial tree. For the detection of emphysema, an upper threshold of −950 HU is used23–25 with a correction between −910 and −950 HU for noise reduction. This procedure was followed by a second YACTA analysis of the CT performed 90 days after the intervention to assess possible lung volume reduction and possible changes in emphysema index and emphysema volume.
According to our standard protocol, the patients received intravenous antibiotic prophylaxis (second-generation cephalosporin) during the procedure, followed by a second dose 8 hours later and completed by an equivalent oral regime for a total of 7 days. Patients remained in the hospital under observation for 3–4 days on average and received a chest radiograph immediately after the procedure and the following day.
After the procedure, patients were followed up at 3, 6, and 12 months. At the follow-up visits, a general health assessment, PFT with post-bronchodilator spirometry, body plethysmography and measurement of diffusion capacity, the 6MWT, and the mMRC dyspnea questionnaire were conducted and reported. Radiological monitoring was performed with a chest radiograph at 30, 90, 180, and 365 days and with an MSCT scan at 90 days.
Possible complications such as hemoptysis, COPD exacerbations, pneumonias, chest discomfort, pneumothorax, and death were also recorded.
Statistical analysis
All continuous variables are presented as mean ± SD. Normality of distribution was checked by employing Shapiro–Wilk test. Two-way repeated-measures ANOVA was used to assess differences at different time points. When the sphericity assumption did not hold, the multivariate results were used. For pairwise comparisons of adjacent time points, Bonferroni-adjusted P-values were reported. For ordinal values, Friedman test was used to assess for multiple comparisons. To account for within-patient changes over time, linear fixed-effects models were fitted. Data were assessed for linear and quadratic trends over time. A quadratic trend is indicative of nonlinear variation, such as a change of direction. P-values of <0.05 were considered statistically significant.
Results
Efficacy outcomes
A total of 86 patients with heterogeneous emphysema were enrolled. Baseline characteristics of the patients are shown in Table 1.
All patients were initially treated unilaterally, with 28 patients receiving a bilateral coil supplementation after a period of minimum 3 months following the first procedure. A total of 114 procedures were performed. Approximately 47% of the procedures involved the right upper lobe; 25%, the left upper lobe; 13%, the right lower lobe; and 15%, the left lower lobe. No periprocedural technical events occurred, and none of the coils placed needed to be replaced or removed.
Patients treated unilaterally were assessed initially at 90 days post-procedure and were then reassessed at 180 and 365 days; Figure 2 shows a flowchart of patients’ follow-up. All patients treated bilaterally were initially followed up for at least 3 months after the first procedure, and afterward we proceeded with the second procedure. The time point of the second procedure (10±7 months after the first implantation) was individually decided based on the progress of respiratory symptoms and potential loss of benefit gained after the initial treatment. A total of 10 patients underwent the second treatment in the period between the 90- and 180-day follow-up visit, 8 patients in the period between the 180- and 365-day follow-up visit, and the remaining 10 patients were treated more than a year after the initial coil therapy. Accordingly, they were also assessed at 90 days after the second procedure and were then reassessed at 180 and 365 days (Figure 2).
  | Figure 2 Patient flowchart. |
Post-bronchodilator FEV1 measurement
FEV1 improved significantly at 90 days postintervention; however, this improvement was lost at the 180- and 365-day follow-up (Table 2). Compared to baseline, ΔFEV1 at 90 days after LVRC treatment increased by 0.05±0.13 L. Thus, FEV1 improved >12% (minimal clinically important difference, MCID)26 in 30 patients (38%) at 90 days.
Fitted models were consequently used to account for trends in within-patient changes over time confirming a quadratic relationship between outcome variables and time. Intercepts and coefficients are characteristics of the models. Intercepts indicate the value of each parameter at baseline, and coefficients are an indication of the rate of change of the outcome variable as time passes on a monthly basis. Accordingly, the model for FEV1 predicts an increase in FEV1 up to 90 days postintervention followed by leveling off and a subsequent decrease (Table 3; Figure 3).
No difference was noted in FEV1 after the second procedure at the 90-, 180-, and 365-day follow-up (Table 4; Figure 4).
Remaining post-bronchodilator PFT values
Vital capacity (VC) and RV improved significantly at 90 days postintervention, and the improvement was sustained at 180-day follow-up, but not at the 365-day follow-up. TLC decreased at the 90-day follow-up but returned to baseline values at the 180- and 365-day follow-up. RV/TLC was significantly lower at 90 and 180 days post-intervention (Table 2).
Fitted models showed similar trends for RV and VC, namely an improvement up to 90 days postintervention followed by leveling off and subsequent decrease (Table 3; Figure 3).
Following the second procedure, VC tended to increase at the 90-day follow-up (P=0.068). No other improvement in PFT values was evident after the second procedure (Table 4; Figure 4).
Exercise capacity
6MWT improved significantly after 90 days, and the improvement was sustained at the 180-day follow-up (Table 2). Compared to baseline values at 90 and 180 days after LVRC treatment, there was a Δ6MWT of 31±54 and 20±60 m, respectively. A total of 42 of 71 (59%) patients improved by >26 m (MCID) at 90 days while 21 patients (30%) improved by >54 m.27,28
A fitted model was consequently used to account for trends in within-patient changes over time also confirming a quadratic relationship between outcome variables and time. The model for 6MWT predicts an increase in 6MWT up to 90 days postintervention followed by leveling off and a subsequent decrease (Table 3; Figure 3).
No difference was noted in 6MWT after the second procedure at the 90-, 180-, and 365-day follow-up (Table 4; Figure 4).
mMRC dyspnea score
mMRC improved at the 90- and 180-day follow-up, but the improvement was not sustained at the 365-day follow-up. No improvement in mMRC score was evident after the second procedure.
Prognostic markers
Univariate linear regression showed that lower 6MWT was associated with greater improvement in 6MWT at 90-day follow-up (β, −0.27; 95% CI, −0.40 to −0.14; P<0.0001) and at 180-day follow-up (β, −0.23; 95% CI, −0.40 to −0.07; P=0.006). Univariate analysis for all other clinical (age, gender, FEV1 at baseline, VC at baseline, TLC at baseline), procedural (lobe treated, complications) or radiologic (YACTA analysis) parameters did not show statistically significant linear associations.
Safety outcomes
In a total of 114 procedures, no periprocedural deaths occurred, and 4 patients died within the first 3 months after the treatment (mortality rate, 3.5%). All the 4 patients who died suffered from severe pneumonia of the treated lung that was followed by sepsis and finally death; 2 of them also developed abscesses surrounding some of the coils as was evident on CT (Figures 5 and 6); 3 out of the 4 patients who died developed the fatal complication after bilateral treatment. A summary of all respiratory adverse events is listed in Table 5.
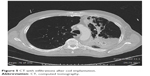  | Figure 5 CT with infiltrations after coil implantation. |
  | Figure 6 Lung autopsy after coil implantation. |
  | Table 5 Adverse events in a total of 114 procedures |
The most frequent severe complications observed within the first 90 days included pneumonias requiring hospitalization and treatment with intravenous antibiotics (28%), followed by pneumothorax with chest tube insertion (6%). Significant, persistent hemoptysis was documented in 4 cases (3.5%) and hypercapnia and hypoxemia in 4.4% and 1.8% respectively. At the 1-year follow-up, 4 additional cases of severe hemoptysis were reported (3.5%); 3 of them required surgical intervention to control the bleeding. Furthermore, 2 cases of pneumothoraces were reported, 1 of which required surgery and showed a perforation of 1 coil into the pleural space. Finally, respiratory failure and/or hypercapnia requiring noninvasive ventilation due to respiratory acidosis occurred in 4 patients (2.6% and 0.87%, respectively).
At 90 days after the procedure, the most frequent nonserious adverse event observed were mild self-limiting hemoptysis (22%), followed by lower respiratory tract infections treated with oral antibiotics (21%) and COPD exacerbations treated only with oral steroids (18.5%). Pleural pain/discomfort on the treated side was also reported (5.2%), and in 1 case (0.87%) infiltrates were documented which responded only to long-term cortisone therapy. At 365 days, lower respiratory tract infection treated with oral antibiotics was the most prominent complication (25.4%) documented, followed by COPD exacerbations treated with oral steroids (12.3%).
Discussion
The main findings of this retrospective study are that LVRC therapy resulted in an initial, short-term, significant improvement in lung function, exercise capacity, and perception of dyspnea in a cohort of patients with severe COPD, hyperinflation, heterogeneous emphysema, and bilateral incomplete fissures; ~1/3 of patients also improved by the MCID. This improvement was, however, lost 1 year posttreatment. Additional treatment of the contralateral side did not add any further benefit for the patients. Furthermore, we did record a significant increase in complications involving the complementary treatment.
While selecting our patients, we excluded patients with severe COPD from coil treatment who showed complete fissures and absence of collateral ventilation, since those patients were first considered for ELVR therapy with endobronchial valve implantation in our institute. ELVR by means of removable endobronchial valve placement is probably the most widely used method, and its significant benefits have been shown in patients with emphysema, in whom collateral ventilation can be excluded.29
This was a single-center, retrospective analysis with real-life clinical decision making; so CT assessments and emphysema allocation were conducted by the investigator using the dedicated in-house software analysis system (YACTA) and perfusion scintigraphy, without central reading center or proprietary software analyses. Patients with homogeneous emphysema were excluded because of a lack of evidence regarding coil treatment in this subgroup of emphysema patients. In the pilot trial of coil therapy in 2010,18 results showed that the efficacy of coil therapy seemed to be better in patients with heterogeneous emphysema compared to patients with homogeneous emphysema. Based on this knowledge, in the following time period, particularly patients with heterogeneous emphysema received coil therapy. Thus, the patients enrolled in our trial and treated between 2011 and 2015 had a heterogeneous emphysema distribution. The first trial that demonstrated the efficacy of coil therapy in patients with homogeneous emphysema was published in 2014 by Klooster et al.30 In this retrospective trial, coil treatment led to improvement of clinical measures in 10 patients. The first mention of efficacy of coil treatment in a large cohort of patients with homogenous emphysema was in the recently published RENEW trial in 2016.31
One of the main pathophysiological causes for dyspnea and exercise limitation in COPD patients with severe emphysema is the static and dynamic hyperinflation of the lungs, which renders the respiratory muscles inefficient.32,33 The alveolar destruction caused mainly by cigarette smoking leads to impairment in gas exchange and elastic recoil of the lung, thus causing air trapping with an increase in RV and hyperinflation. Because of this hyperinflation, the respiratory muscles are forced to function at a mechanical disadvantage leading to decreased compliance of the chest wall and increase in work of breathing. As a consequence, patients experience chronic shortness of breath and limited exercise capacity and thus a gradually declining quality of life. The pathophysiological mechanism involved in coil treatment appears to be the improvement in lung mechanics due to the compression of destroyed lung parenchyma by the inserted coils, resulting in volume reduction as well as an improvement in elastic recoil. This results in a reduction of dynamic hyperinflation during exercise and also improves the exercise capacity. The long-term benefits of this treatment are currently being investigated worldwide.
In our study, we observed only an initial improvement of the lung function parameters, exercise tolerance, and perception of dyspnea. FEV1 improved significantly at 90 days postintervention; however, this improvement was lost at the 180- and 365-day follow-up. The same applied also for TLC. VC, RV, and mMRC improved significantly at 90 days postintervention and sustained the improvement until the 180-day follow-up but not at the 365-day follow-up. Compared to baseline values, the FEV1 improved >12% (MCID) in 30 patients (responder rate, 38%) at 90 days. An early improvement in FEV1, other PFT parameters, and mMRC is also in accordance with previously published data.34,35 In the study by Slebos et al,34 ΔFEV1 ΔFVC, ΔRV were significantly higher at 3 and 6 months compared to the baseline value. In the recently published randomized superiority trial from the REVOLENS group comparing coils with usual care,36 improvements from baseline were significant at 6 months and also at 12 months in the coil group compared to the usual care group, even though a small decline of the values obtained 6 months after treatment can be observed 1 year after the implantation. 6MWT improved significantly at 90 days, and the improvement was sustained but with a tendency to decline at the 180-day follow-up. A responder rate of 59% was observed; these patients improved the 6MWT by >26 m (MICD) at 90 days follow-up.27,28 The initial improvement in 6MWT is also in accordance with previous studies.34,35 In the study by Slebos et al,34 the improvement in 6MWT was sustained up to 6 months after the intervention, even though the 6-month values tended to be lower than those at 3 months. Shah et al,35 reported the results at 90 days after bilateral LVRC treatment for 46 patients included in a randomized controlled study and demonstrated a significant improvement not only in 6MWT but also in quality of life and lung function. We observed, in our study, that the initial increase in exercise capacity was not sustained at 1-year follow-up and the distance recorded in the 6MWT was approaching the baseline values 1 year after treatment. Similar findings regarding 6MWT have been reported in the REVOLENS trial;36 improvements from baseline in 6MWT were significant at 6 months in the coil group compared to the usual care group only when analyzed as percent change but not when analyzed by distance walked; at 12 months, improvements from baseline were not significant any more in the coil group.
Identifying pre-interventional prognostic markers of positive post-procedural outcomes is highly desirable, especially as approximately one-third of our patients were responders. A lower baseline 6MWT was associated with a larger improvement in 6MWT at 90- and 180-day follow-up. All other clinical, procedural, or radiologic parameters did not show statistically significant linear associations in our study. The recently published RENEW study from Sciurba et al31 has shown a positive response stratified by the degree of air trapping (RV >225% vs RV <225%). In our study, only 13 patients (15%) had a baseline RV of <225%, which might explain the lack of association.
Furthermore, the patients who underwent a bilateral treatment in our institute did not show further benefit from the second treatment neither with regard to lung function nor to exercise tolerance or dyspnea perception. However, due to the small number of patients, definite conclusions could not be drawn and this should be addressed in further studies.
This study provides the first long-term data analysis demonstrating a loss of response at 12 months after the treatment. This unanticipated finding could be at least partially explained by the fact that the majority of our patients received unilateral treatment only. In most of the abovementioned studies, the patients underwent a second procedure with treatment of a contralateral lobe. It is also worth mentioning that the contralateral implantation in these studies took place within the first 4–12 weeks after the first procedure. Interestingly, our patients did not benefit further after the bilateral treatment. A recent study by Hartman et al,37 where patients were followed up for a total of 3 years after LVRC treatment, showed, when compared to baseline, an increase in mMRC, 6MWT, and lung function parameters except FEV1 at 1-year follow-up, but at 2-year follow-up the number of parameters that still remained improved declined, and in the 3-year follow-up only the mMRC remained better than at baseline. The authors explained this fact with the progression of the disease.
The results of the current study are in concordance with a previous study of our group,38 where we also observed a significant improvement of all parameters in the short term and a tendency to decrease at 6-month follow-up. These long-term follow-ups reveal values further dropping to levels close to or at baseline.
Concerning the complications, we have reported a mortality of 3.5% in a total of 114 procedures (n=4) in our institute and a significant number of mild and serious adverse events. Taking into account that the number of coils inserted and that the procedure itself are standardized among the different institutes, it would appear that we need to further elucidate possible aggravating factors and reduce the number of adverse events.
All these findings highlight the importance of identification of possible pre-procedural characteristics that could correlate with better and most importantly longer lasting post-procedural outcome benefit. The results of a large (n=315) randomized controlled trial (NCT01608490) with 5-year follow-up are anticipated and might help to give additional insight into the long-term effectiveness and safety of coil treatment.36 The advantage of this trial, however, is an independent analysis of a cohort treated with LVRC in a clinical routine.
Limitations
This study has some limitations. 1) This is a retrospective analysis of a patient cohort selected in a single center. 2) It is the lack of a control group and also the fact that this is an unblinded study, which cannot exclude the possibility of inducing placebo effects. Finally, the results of the longitudinal analysis should be interpreted with caution due to the monocentric and limited sample size, especially with regard to the results after bilateral treatment. The timing of the second intervention was also not standardized.
Conclusion
One-year follow-up showed that LVRC treatment in patients with advanced COPD, heterogeneous emphysema, and incomplete fissures demonstrated an initial improvement in lung function, exercise capacity, and perception of dyspnea for more than one-third of patients. The benefits seem to level off at 6 months and then to further decline and reach baseline values within a year after treatment. Bilateral treatment did not show to add to the initial improvements. Furthermore, mortality and several severe and milder adverse events seem to have a bearing on the safety profile of this ELVR technique in our institute. Further multicenter studies are needed to assess the long-term effects of LVRC and help to identify possible pre-procedural characteristics of patients, which could correlate with better and longer lasting outcomes and minimize possible complications from the therapy.
Disclosure
DG received lecture and travel fees from Pulmonx, Novartis, Astra Zeneca, Mundipharma, Berlin Chemie and Grifols. MS received travel subsistence or speaker fees from Olympus, PneumRx, Pulmonx, Boston Scientific, AstraZeneca, GlaxoSmithKline, Novartis, Boehringer, and Teva. CPH has stock ownership in medical industry, Stada, and GSK, and patents, method, and device for representing the microstructure of the lungs; IPC8 Class: AA61B5055FI, PAN: 20080208038, Inventors: W Schreiber, U Wolf, AW Scholz, CP Heussel; Consultation or other fees Schering-Plough 2009, 2010, Pfizer 2008–2014, Basilea 2008, 2009, 2010, Boehringer Ingelheim 2010–2014, Novartis 2010, 2012, Roche 2010, Astellas 2011, 2012, Gilead 2011–2014, MSD 2011–2013, Lilly 2011, Intermune 2013–2014, Fresenius 2013, 2014; Expert testimony no; Research funding Siemens 2012–2014, Pfizer 2012–2014, MeVis 2012, 2013, Boehringer Ingelheim 2015; Lecture fees Gilead 2008–2014, Essex 2008, 2009, 2010, Schering-Plough 2008, 2009, 2010, AstraZeneca 2008–2012, Lilly 2008, 2009, 2012, Roche 2008, 2009, MSD 2009–2014, Pfizer 2010–2014, Bracco 2010, 2011, MEDA Pharma 2011, Intermune 2011–2014, Chiesi 2012, Siemens 2012, Covidien 2012, Pierre Fabre 2012, Boehringer Ingelheim 2012, 2013, 2014, Grifols 2012, Novartis 2013, 2014; and had no relationship with tobacco industry. FJFH received consultant and lecture fees from Astra, Allmirall, Berlin Chemie, Boehringer, Roche, GSK, Pulmonx, PneumRx, Boston Scientific, Medupdate, Grifols, CSL Behring, Omniamed, Lilly, Novartis, Teva, Uptake, and Vital Air. RE received lecture and travel fees from Pulmonx and Olympus. KK, VG, and HH report no conflicts of interest in this work.
References
Rabe KF, Hurd S, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176(6):532–555. | ||
Pfeifer M. Chronic critically ill patients from a pneumonological perspective. Med Klin Intensivmed Notfmed. 2013;108(4):279–284. | ||
Martinez FJ, Chang A. Surgical therapy for chronic obstructive pulmonary disease. Semin Respir Crit Care Med. 2005;26(2):167–191. | ||
Fishman A, Martinez F, Naunheim K, et al. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe emphysema. N Engl J Med. 2003;348(21):2059–2073. | ||
Naunheim KS, Wood DE, Mohsenifar Z, et al. Long-term follow-up of patients receiving lung-volume-reduction surgery vs medical therapy for severe emphysema by the National Emphysema Treatment Trial Research Group. Ann Thorac Surg. 2006;82(2):431–443. | ||
Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report – 2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):965–978. | ||
Sciurba FC, Ernst A, Herth FJ, et al; VENT Study Research Group. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med. 2010;363(13):1233–1244. | ||
Springmeyer SC, Bolliger CT, Waddell TK, Gonzalez X, Wood DE; IBV Valve Pilot Trials Research Teams. Treatment of heterogeneous emphysema using the spiration IBV valves. Thorac Surg Clin. 2009;19(2):247–253. | ||
Sterman DH, Mehta AC, Wood DE, et al; IBV Valve US Pilot Trial Research Team. A multicenter pilot study of a bronchial valve for the treatment of severe emphysema. Respiration. 2010;79(3):222–233. | ||
Eberhardt R, Herth FJ, Radhakrishnan S, Gompelmann D. Comparing clinical outcomes in upper versus lower lobe endobronchial valve treatment in severe emphysema. Respiration. 2015;90(4):314–320. | ||
Koenigkam-Santos M, Puderbach M, Gompelmann D, et al. Incomplete fissures in severe emphysematous patients evaluated with MDCT: incidence and interobserver agreement among radiologists and pneumologists. Eur J Radiol. 2012;81(12):4161–4166. | ||
Herth FJ, Noppen M, Valipour A, et al. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012;39(6):1334–1342. | ||
Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest. 1988;93(3):580–586. | ||
Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581–586. | ||
Troosters T, Vilaro J, Rabinovich R, et al. Physiological responses to the 6-min walk test in patients with chronic obstructive pulmonary disease. Eur Respir J. 2002;20(3):564–569. | ||
Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. | ||
Wanger J, Clausen JL, Coates A, et al. Standardisation of the measurement of lung volumes. Eur Respir J. 2005;26(3):511–522. | ||
Herth FJ, Eberhardt R, Gompelmann D, Slebos DJ, Ernst A. Bronchoscopic lung volume reduction with a dedicated coil: a clinical pilot study. Ther Adv Respir Dis. 2010;4(4):225–231. | ||
Koenigkam-Santos M, de Paula WD, Owsijewitsch M, et al. Incomplete pulmonary fissures evaluated by volumetric thin-section CT: semi-quantitative evaluation for small fissure gaps identification, description of prevalence and severity of fissural defects. Eur J Radiol. 2013;82(12):2365–2370. | ||
Herth FJ, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J. 2013;41(2):302–308. | ||
Thurnheer R, Engel H, Weder W, et al. Role of lung perfusion scintigraphy in relation to chest computed tomography and pulmonary function in the evaluation of candidates for lung volume reduction surgery. Am J Respir Crit Care Med. 1999;159(1):301–310. | ||
Lim HJ, Weinheimer O, Wielpütz MO, et al. Fully automated pulmonary lobar segmentation: influence of different prototype software programs onto quantitative evaluation of chronic obstructive lung disease. PLoS One. 2016;11(3):e0151498. | ||
Heussel CP, Achenbach T, Buschsieweke C, et al. Quantification of pulmonary emphysema in multislice-CT using different software tools. Rofo. 2006;178(10):987–998. | ||
Achenbach T, Buschsieweke C, Gerhards A, Gast K, Heussel CP, Thelen M. Does HRCT emphysema index represent the entire lung? Rofo. 2005;177(5):655–659. | ||
Newell JD, Hogg JC, Snider GL. Report of a workshop: quantitative computed tomography scanning in longitudinal studies of emphysema. Eur Respir J. 2004;23(5):769–775. | ||
Donohue JF. Minimal clinically important differences in COPD lung function. COPD. 2005;2(1):111–124. | ||
Puhan MA, Chandra D, Mosenifar Z, et al. The minimal important difference of exercise tests in severe COPD. Eur Respir J. 2011;37(4):784–790. | ||
Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91(2):221–225. | ||
Herth FJ, Noppen M, Valipour A, et al; International VENT Study Group. Efficacy predictors of lung volume reduction with Zephyr valves in a European cohort. Eur Respir J. 2012;39(6):1334–1342. | ||
Klooster K, Ten Hacken NH, Franz I, Kerstjens HA, van Rikxoort EM, Slebos DJ. Lung volume reduction coil treatment in chronic obstructive pulmonary disease patients with homogeneous emphysema: a prospective feasibility trial. Respiration. 2014;88(2):116–125. | ||
Sciurba FC, Criner GJ, Strange C, et al; RENEW Study Research Group. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA. 2016;315(20):2178–2189. | ||
Ofir D, Laveneziana P, Webb KA, Lam YM, O’Donnell DE. Mechanisms of dyspnea during cycle exercise in symptomatic patients with GOLD stage I chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2008;177(6):622–629. | ||
O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(5):770–777. | ||
Slebos DJ, Klooster K, Ernst A, Herth FJ, Kerstjens HA. Bronchoscopic lung volume reduction coil treatment of patients with severe heterogeneous emphysema. Chest. 2012;142(3):574–582. | ||
Shah PL, Zoumot Z, Singh S, et al; RESET trial Study Group. Endobronchial coils for the treatment of severe emphysema with hyperinflation (RESET): a randomised controlled trial. Lancet Respir Med. 2013;1(3):233–240. | ||
Deslée G, Mal H, Dutau H, et al. Lung volume reduction coil treatment vs usual care in patients with severe emphysema: the REVOLENS randomized clinical trial. JAMA. 2016;315(2):175–184. | ||
Hartman JE, Klooster K, Gortzak K, ten Hacken NH, Slebos DJ. Long-term follow-up after bronchoscopic lung volume reduction treatment with coils in patients with severe emphysema. Respirology. 2015;20(2):319–326. | ||
Kontogianni K, Gerovasili V, Gompelmann D, et al. Effectiveness of endobronchial coil treatment for lung volume reduction in patients with severe heterogeneous emphysema and bilateral incomplete fissures: a six-month follow-up. Respiration. 2014;88(1):52–60. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






