Back to Journals » International Journal of General Medicine » Volume 7
Cognition and dual-task performance in older adults with Parkinson's and Alzheimer's disease
Authors Christofoletti G, Pires de Andrade L, Beinotti F, Borges G
Received 8 April 2014
Accepted for publication 13 May 2014
Published 21 July 2014 Volume 2014:7 Pages 383—388
DOI https://doi.org/10.2147/IJGM.S65803
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Gustavo Christofoletti,1 Larissa Pires de Andrade,2 Fernanda Beinotti,3 Guilherme Borges3
1Federal University of Mato Grosso do Sul, Biological and Health Science Center, Campo Grande, MS, Brazil; 2State University of São Paulo, Bioscience Institute, Rio Claro, SP, Brazil; 3State University of Campinas, Faculty of Medical Sciences, Campinas, SP, Brazil
Background: Patients with neurodegenerative diseases usually experience significant functional deficits. Older adults with Parkinson's disease (PD) and Alzheimer's disease (AD) may suffer from both motor and cognitive impairments, making them especially vulnerable to poor dual-task performance.
Objective: To analyze the dual-task cost of walking in subjects with PD and AD exposed to motor and cognitive distracters.
Methods: A cross-sectional study was conducted involving 126 older adults comprising three groups: PD (n=43), AD (n=38), and control (n=45). The subjects were evaluated using the Timed Up and Go (TUG) test administered with motor and cognitive distracters. Mixed-design analysis of variance (ANOVA) with cognition as a covariant factor was used to test the possible main effects of dual-task on motion. A 5% threshold for significance was set, with a 95% confidence interval (CI). The partial eta square (n2p) analysis was included to estimate the magnitude of effect.
Results: Examining the effects for dual-task, ANOVA revealed the main effect for group×task interactions (F=13.09; P=0.001; n2p =0.178), for task (F=8.186; P=0.001; n2p =0.063) but not for group (F=2.954; P=0.056; n2p =0.047). Cognition applied as a covariant factor indicated interference on dual-tasks (F=30.43; P=0.001; n2p =0.201).
Conclusion: The findings of this study suggest that dual-task interference is a particularly noticeable problem in PD and AD, affecting subjects' ability to appropriately adapt to environmental challenges.
Keywords: Parkinson's disease, Alzheimer's disease, motion, task performance and analysis, cognition
Introduction
Advanced aging often degrades fine motor skills, a process attributed to age-associated alterations in several factors, including information processing, motor neuron organization, and neuromotor activity. One important consideration when examining the influence of aging on neural activity and motor performance is task dependency.1 During everyday activities, people often need to perform more than one task simultaneously. The capacity to add a second task while walking, for example, is highly advantageous because it allows communication between people, transportation of objects from one location to another, and monitoring the environment so that threats to balance can be avoided.2
Dual-task performance involves the execution of a primary task, which is the major focus of attention, and a secondary task conducted at the same time. The motor and cognitive systems act reciprocally to ensure successful motion. Limitations in dual-task performance are usually attributed to action selection.3,4 When two tasks must be done, response time on the second task is increased when the stimulus occurs within a few hundred milliseconds after the onset of the first task stimulus. Thus, the human capacity for processing information is limited. It is known that individuals retain a limited amount of information in their working memory, and when one attempts to do several tasks at the same time, performance deteriorates.5
Problems executing dual-tasks commonly lead to mobility impairments. Early publications involving movement disabilities barely mentioned cognitive functioning. It is only in the last decade that we have begun to realize the impact of cognitive functioning on locomotion. Because gait is no longer considered an automatic task, the role of cognitive functioning has been increasingly recognized. In particular, two closely related cognitive domains, namely executive function and attention, clearly influence human movement.6,7
Patients with neurodegenerative diseases usually have significant functional deficits. Older adults with Parkinson’s disease (PD) and Alzheimer’s disease (AD) may suffer from both motor and cognitive impairments, making them especially vulnerable to poor dual-task performance. Functional decline, a phenomenon expected in PD and AD, is characterized by reduced ability to carry out everyday activities.8–10 Typically thought to result from motor dysfunction, the challenges faced in these activities appear to also depend on cognitive status, especially executive processes.11,12
The meta-analysis conducted by Chu et al13 pointed to the need for more research to ascertain definite conclusions regarding the effect of task type and complexity. Although there are studies that already examined dual-task performance in participants with PD and AD, several unanswered questions remain, including whether all aspects of motion respond similarly to changes in attention and prioritization. Therefore, this study aimed to assess the dual-task cost of walking in subjects with PD and AD who were submitted to cognitive and motor distracters.
Methods
Participants
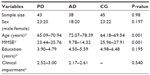
In this cross-sectional design study, 126 males and females aged 60–80 years old at entry, were recruited into three groups: PD, AD, and control group (CG). All patients enrolled in this study were selected from an outpatient clinic specializing in neurologic disorders.
We allowed for a 5% margin of error and a statistical power of 87% to calculate the necessary sample size. Based on this calculation, the minimal sample size should be 111 subjects (37 per group).
A research physician identified the subjects who met the following inclusion criteria: a) patients with idiopathic PD according to the UK Parkinson’s Disease Society brain bank clinical criteria,14 whose clinical profile indicated moderate impairment based on the Hoehn–Yarh scale15 and on the motor subscale of the Unified Parkinson’s Disease Rating Scale;16 b) subjects diagnosed with AD based on the NINCDS–ADRDA criteria17 and with a moderate clinical profile according to the clinical dementia rating;18 and c) control peers with no chronic or neurodegenerative diseases. All participants were independent in performing basic activities of daily living (mobility, dressing, eating, and bathing).
Patients with cognitive impairment other than AD, and those with movement disorders that were not compatible with a diagnosis of idiopathic PD, such as secondary Parkinsonism, were excluded. Prescriptions were reviewed for central nervous system-targeting medications and participants that were using sedatives, antidepressants, antipsychotics, and antiepileptics were disqualified. Subjects with congenital or acquired amaurosis and those with severe cardiovascular and musculoskeletal comorbidities (that preclude orthostatism and deambulation) were also excluded. All subjects were sedentary, not performing any physical activity superior to 3 metabolic equivalent of tasks.
Procedures
The participants were screened in a private and quiet evaluation room in the Neurologic Outpatient Clinic of the State University of Campinas. Two researchers were trained to administer the tests and questionnaires used in the study. Intra- and inter-examiner reliability to cognitive and functional tests were verified using the Kappa index in a pilot study with PD and AD patients, resulting in a substantial magnitude of agreement (0.81<k<1.0).19 Prior to testing, all subjects were interviewed about their medical history, and the research procedure was explained to them and their families.
Regarding the data appraisal, a semi-structured interview was used to collect demographic and clinical information. The subject’s cognitive function was assessed using the Mini-Mental State Examination.20 The Timed Up and Go test (TUG)21 was administered to assess mobility in patients during cognitive and motor dual-task performance.
The Mini-Mental State Examination is composed of questions grouped into seven categories, each designed to evaluate specific cognitive functions: time orientation, place orientation, three-word register, attention and calculation, immediate and delayed recall of three words, language, and visuoconstructive praxis. The scores vary from 0 to 30 points, and lower values represent a possible cognitive decline.22
The TUG is a mobility test that requires both static and dynamic balance activation. It measures the time required for a person to rise and stand up from a 17-inch chair, walk a distance of 9.84 feet, turn, walk back to the chair, and sit down. A longer time to complete the task indicates an increased risk of falls. Using TUG under a motor and a cognitive dual-task condition, patients were asked to: a) accomplish the task of holding a cup with 200 mL of water in the dominant hand; and b) accomplish the task of performing a numerical progressive odd count (ie, one, three, five, seven, nine, etc). Previous studies support the use of this instrument with dual-tasks in obtaining reliable outcome measures for use with people in PD and AD.23–25
The TUG tests were administered after a full explanation and once the patients understood the procedure, with one practice trial incorporated for each condition. The application order was normal test, TUG with motor distracter, and TUG with cognitive distracter, with a 5 minute rest between tests. The subjects were advised to observe the task priority (walking without spilling the contents of the cup and walking and maintaining the accuracy of the count) but as fast as possible, safely, and with no help from any assistive devices (crutch, walking cane, or other). Prior to the cognitive test, the authors assessed the patient’s capacity for counting to see if there was a shift in the prioritization of task performance.
All assessments were conducted in the morning to standardize comparisons between the patients. Subjects with PD and AD were tested while on their medications.
Statistical analysis
The data were first analyzed using descriptive statistics. Shapiro–Wilk and Levene’s tests were applied to determine the parametric character of the data.
Sociodemographic and clinical profiles of the groups were calculated using one-way analysis of variance (ANOVA) and the chi-square test (χ2). To estimate the main effects of dual tasks on motion (ie, differences among tasks and clinical conditions), we applied mixed-design ANOVA with “task” and “group” as factors. Covariant factors were used in the cases with baseline differences and t-tests with Bonferroni corrections for multiple testing were applied. A 5% threshold for significance was set for these analyses (P<0.05) with a 95% confidence interval (CI). The partial eta square (n2p) analysis was included to estimate the magnitude of effect.
Ethical approval was obtained from a local research ethics committee, and this study complied with the Declaration of Helsinki. Informed consent forms were signed by all subjects participating in this study. For AD subjects, the patient’s next of kin (usually the main informal caregiver) was approached to give informed consent.
Results
The sample of 126 subjects comprised three groups. Table 1 summarizes the subjects’ sociodemographic and clinical characteristics. There were no statistical differences between the groups in sample size (χ2=619; P=0.734), sex distribution (χ2=7.33; P=0.197), clinical impairment (χ2=7.63; P=0.540) and education level (χ2=27.446; P=0.195). One-way ANOVA test indicated differences between the groups for age (F=9.45; P=0.001), with differences between AD and PD (95% CI =−12.14 to −2.28; P=0.002), AD and CG (95% CI =3.49 to 13.24; P=0.001), but not PD and CG (95% CI =−3.56 to +5.87; P=0.830). Similarly, the one-way ANOVA test pointed to differences between the groups for cognition (F=11.875; P=0.001), with a significant difference between AD and PD (95% CI =9.96 to 15.14; P=0.001) and AD and CG (95% CI =12.31 to 17.44; P=0.001). There was no difference for cognition between PD and CG (95% CI =−4.80 to 0.15; P=0.073).
Dual-task performances
Figure 1 show participants’ performance in each dual-task activity. Analyzing the effects for dual-task performance in the three groups, mixed-design ANOVA pointed to a main effect for group×task interaction (F=13.09; P=0.001; n2p =0.178), for task (F=8,186; P=0.001; n2p =0.063) but not for group (F=2.954; P=0.056; n2p =0.047).
  | Figure 1 Timed Up and Go test applied in each task. |
Paired analyses indicated that subjects with PD presented with the most difficulty under the motor dual-task conditions (95% CI: 23.39–36.85), followed by the cognitive dual task (95% CI: 20.31–33.44) and the single-task conditions (95% CI: 18.69–30.86). Important differences were found in all pairwise comparisons in the AD group, such that patients with AD experienced the most difficulty on the cognitive dual task (95% CI: 54.32–78.25), followed by the motor dual task (95% CI: 46.87–67.75), and single task (95% CI: 42.79–62.38). The dual-task conditions did not affect motion patterns in the control group during the single task (95% CI: 18.69–30.86), motor dual task (95% CI: 23.38–36.85), or cognitive dual task (95% CI: 20.31–33.44).
As we identified baseline differences for age, a covariant factor was used to analyze the effect of this variable with regards to the results. Applying mixed-design ANOVA with aging as a covariant, we found that subjects’ age did not affect their performance on the tasks (F=0.759; P=0.469; n2p =0.006). Similarly, a covariant factor was used to see the effect of cognition on dual tasks. The analysis showed the main effect for cognition×task interaction (F=30.43; P=0.001; n2p =0.20), indicating that this variable interfered on motion.
Discussion
Falling is the leading cause of fatal and nonfatal injuries in the older adult population and is a significant public health concern.26 Results from our study support the concept that older adults with a moderate impairment of PD and AD experience marked deterioration when required to perform either a motor or a cognitive secondary task under a sit-to-walk condition. As observed, the time required showed significant variation according to the tasks performed by the subjects. Identification of potentially modifiable risk factors is important for developing fall prevention strategies and interventions.
A general consensus holds that neurodegenerative disorders compromise behavioral motor function due to encephalic disturbances. The influence of alterations in cognition or motor control on performing dual tasks can be an indicator of the functional status of a patient during illness or rehabilitation. In the literature, such alteration is usually regarded as motor-cognitive interference.27
The present findings demonstrate that the effects of dual tasks are feature specific (ie, there are differences between PD and AD patients). Thus, we speculate that in certain patient groups (eg, post-stroke or other neurological conditions) the ability to maintain stability during dual-task performance might also be impaired.
Motion deficits are one of the most common negative consequences of PD. Patients with PD share many fall risk factors with the general older adult population, and while clearly not identical, the profiles of motor and cognitive deficits overlap. Our findings are consistent with those reported by Wild et al,28 where subjects with PD gave priority to the test itself while their cognitive and motor dual-task performance suffered.
Executive function is defined as a set of cognitive skills required for planning, monitoring, and executing a sequence of goal-directed complex actions.27 Attention, problem solving, working memory, and judgment may be considered specific examples of executive function. The term encompasses various processes related to how an organism becomes receptive to stimuli and how it begins to process internal or external excitation. Previous articles have shown significant associations between executive abilities and self-reported measures of physical functioning, particularly for day-to-day activities.27,29
Corroborating with the Pettersson et al30 findings, in our study participants with AD were slower and had difficulties in dual-task performance requiring concurrently performing a cognitive task while walking. In fact, most changes commonly seen in AD are related to metabolic changes in cells in the associative cortex and in the hippocampal complex.31 Reductions in glucose use and perfusion occur in the inferior parietal lobules, posterior superior temporal sulcus, precuneus, posterior cingulate cortex, and orbitofrontal cortex. According to Marshall et al,32 the cingulate gyrus and the orbitofrontal cortex are vital for these cognitive processes and may be an explanation for the difficulty of patients with AD in performing dual tasks.
The change in cognitive modulation and the decision-making challenges at the end of the TUG test (ie, to return and sit in the chair while keeping the progressive numerical count) is likely to be influenced by neural apoptosis commonly seen in the prefrontal cortex of AD patients, which is linked to difficulty in performing complex tasks. Although one may argue that it does not seem clear whether differences are due to executive deficits, working memory pitfalls or neurological impairment, one has to consider the study developed by Yogev-Seligmann et al33 where patients subjected to dual-task activities find themselves having to prioritize one action, which leads to simultaneous activation of the prefrontal cortex and anterior cingulate gyrus – both areas commonly affected in AD patients.
After a cerebral lesion, motor–cognitive interference can occur, making previously automatic activities require a control process that increases attention demands. A major goal for people with PD and AD is to help them perform both basic and instrumental daily activities with a low risk of falling. Important strategies also involve the practice of systematic exercise programs and to teach patients to avoid simultaneous tasks whenever possible so as to prevent diversion of attention from taking long strides.34
The manner in which instructions are phrased has an important role in the way individuals perceive an activity. The translation or understanding of instructions could be a determining factor in performing the task. Thus, we used simple and easily understood tests, although we could not measure if participants prioritized one task over the other.
While we believe this study does have merits, the limitations should be pointed out. One limitation is that we used cross-section data that do not allow us to assess causal relationships. Longitudinal research aimed at assessing the predictive value of cognitive functions on motion is needed to better understand the mechanisms whereby the central nervous system affects physical function in later years, and to identify possible target areas for interventions.
Another limitation is that the number of patients recruited was relatively small, forcing us to only examine patients with moderate clinical profiles. This may limit the ability to generalize our findings to the whole PD or AD population. Thus, further studies should investigate a larger and more heterogeneous sample, perhaps supplemented with more detailed cognitive testing.
Furthermore, the groups were different with regards to age. However, as evidenced by the magnitude of effect (n2p =0.006), the age difference did not exert significant interference in the results.
In summary, findings of this study suggest that the dual-task cost of walking is a particularly noticeable problem in PD and AD, affecting subjects’ ability to appropriately adapt to environmental challenges. These results further highlight the importance of cognition for the performance of certain complex tasks. Even when the locomotor apparatus is preserved, cognitive deficits alter motion, which is primarily evident in the performance of non-automatic activities.
Additional studies should investigate a larger and more heterogeneous sample to ensure that the results are applicable to the general populations of PD and AD patients.
Acknowledgment
The authors would like to thank the National Council for Scientific and Technological Development (CNPq), Brazil.
Disclosure
The authors report no conflicts of interest in this work.
References
Johnson AN, Shinohara M. Corticomuscular coherence with and without additional task in the elderly. J Appl Physiol (1985). 2012;112(6):970–981. | |
Schaefer S, Schumacher V. The interplay between cognitive and motor functioning in healthy older adults: findings from dual-task studies and suggestions for intervention. Gerontology. 2011;57(3):239–246. | |
Yogev-Seligmann G, Hausdorff JM, Giladi N. Do we always prioritize balance when walking? Towards an integrated model of task prioritization. Mov Disord. 2012;27(6):765–770. | |
Rémy F, Wenderoth N, Lipkens K, Swinnen SP. Dual-task interference during initial learning of a new motor task results from competition for the same brain areas. Neuropsychologia. 2010;48(9):2517–2527. | |
Piai V, Roelofs A. Working memory capacity and dual-task interference in picture naming. Acta Psychol (Amst). 2013;142(3):332–342. | |
Yogev-Seligmann G, Rotem-Galili Y, Mirelman A, Dickstein R, Giladi N, Hausdorff JM. How does explicit priorization alter walking during dual-task performance? Effects of age and sex on gait speed and variability. Phys Ther. 2010;90(2):177–186. | |
Buracchio TJ, Mattek NC, Dodge HH, et al. Executive function predicts risk of falls in older adults without balance impairment. BMC Geriatr. 2011;11:74. | |
Christofoletti G, Oliani MM, Bucken-Gobbi LT, Gobbi S, Beinotti F, Stella F. Physical activity attenuates neuropsychiatric disturbances and caregiver burden in patients with dementia. Clinics (Sao Paulo). 2011;66(4):613–618. | |
Vidoni ED, Thomas GP, Honea RA, Loskutova N, Burns JM. Evidence of altered corticomotor system connectivity in early-stage Alzheimer’s disease. J Neurol Phys Ther. 2012;36(1):8–16. | |
Brauer SG, Woollacott MH, Lamont R, et al. Single and dual task gait training in people with Parkinson’s disease: a protocol for a randomised controlled trial. BMC Neurol. 2011;11:90. | |
Christofoletti G, Carregaro RL, Oliani MM, Stella F, Bucken-Gobbi LT, Gobbi S. Locomoção, distúrbios neuropsiquiátricos e alterações do sono de pacientes com demência e seus cuidadores. Fisioter Mov. 2013;26(1):47–53. | |
Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson’s disease: a review. J Neuropsychol. 2013;7(2):193–224. | |
Chu YH, Tang PF, Peng YC, Chen HY. Meta-analysis of type and complexity of a secondary task during walking on the prediction of elderly falls. Geriatr Gerontol Int. 2013;13(2):289–297. | |
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55(3):181–184. | |
Hoehn MH, Yahr MD. Parkinsonism: onset, progression, and mortality. 1967. Neurology. 2001;57(10 Suppl 3):S11–S26. | |
Fahn S, Elton RL. Unified Parkinson’s Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. New York: MacMillan; 1987:153–163. | |
McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of this NINCDS-ADRDA work group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34(7):934–944. | |
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. | |
Sim J, Wright CC. The kappa statistic in reliability studies: use, interpretation, and sample size requirements. Phys Ther. 2005;85(3):257–268. | |
Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. | |
Podsiadlo D, Richardson S. The timed “Up and Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. | |
Brucki SM, Nitrini R, Caramelli P, Bertolucci PH, Okamoto IH. Suggestions for utilization of the mini-mental state examination in Brazil. Arq Neuropsiquiatr. 2003;61(3B):777–781. | |
Hofheinz M, Schusterschitz C. Dual task interference in estimating the risk of falls and measuring change: a comparative, psychometric study of four measurements. Clin Rehabil. 2010;24(9):831–842. | |
Huang SL, Hsieh CL, Wu RM, Tai CH, Lin CH, Lu WS. Minimal detectable change of the timed “up and go” test and the dynamic gait index in people with Parkinson disease. Phys Ther. 2011;91(1):114–121. | |
Ries JD, Echternach JL, Nof L, Gagnon Blodgett M. Test-retest reliability and minimal detectable change scores for the timed “up and go” test, the six-minute walk test, and gait speed in people with Alzheimer disease. Phys Ther. 2009;89(6):569–579. | |
Siracuse JJ, Odell DD, Gondek SP, et al. Health care and socioeconomic impact of falls in the elderly. Am J Surg. 2012;203(3):335–338. | |
Martyr A, Clare L. Executive function and activities of daily living in Alzheimer’s disease: a correlational meta-analysis. Dement Geriatr Cogn Disord. 2012;33(2–3):189–203. | |
Wild LB, de Lima DB, Balardin JB, et al. Characterization of cognitive and motor performance during dual-tasking in healthy older adults and patients with Parkinson’s disease. J Neurol. 2013;260(2):580–589. | |
Aretouli E, Brandt J. Everyday functioning in mild cognitive impairment and its relationship with executive cognition. Int J Geriatr Psychiatry. 2010;25(3):224–233. | |
Pettersson AF, Olsson E, Wahlund LO. Motor function in subjects with mild cognitive impairment and early Alzheimer’s disease. Dement Geriatr Cogn Disord. 2005;19(5–6):299–304. | |
Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86(3):216–244. | |
Marshall GA, Monserratt L, Harwood D, Mandelkern M, Cummings JL, Sultzer DL. Positron emission tomography metabolic correlates of apathy in Alzheimer’s disease. Arch Neurol. 2007;64(7):1015–1020. | |
Yogev-Seligmann G, Hausdorff JM, Gilali N. The role of executive function and attention in gait. Mov Disord. 2008;23(3):329–342. | |
de Andrade LP, Gobbi LT, Coelho FG, Christofoletti G, Costa JL, Stella F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer’s disease: a controlled trial. J Am Geriatr Soc. 2013;61(11):1919–1926. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.