Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Coexistence of Riehl’s Melanosis, Lupus Erythematosus and Thyroiditis in a Patient
Authors Lai K, Zheng X, Wei S, Zhou H, Zeng X, Liang G, Zhang Z, Zhang W
Received 29 May 2022
Accepted for publication 19 August 2022
Published 8 September 2022 Volume 2022:15 Pages 1809—1813
DOI https://doi.org/10.2147/CCID.S376614
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Kuan Lai,1 Xinyao Zheng,1 Shanshan Wei,2 Huifeng Zhou,1 Xuedan Zeng,1 Guixin Liang,1 Zhiwen Zhang,1 Wenjing Zhang3
1Department of Dermatology, Nanfang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 2Department of Dermatology, Zhujiang Hospital, Southern Medical University, Guangzhou, People’s Republic of China; 3Department of Dermatology, The First Affiliated Hospital of Jinan University, Guangzhou, People’s Republic of China
Correspondence: Wenjing Zhang, Department of Dermatology, The First Affiliated Hospital of Jinan University, No. 613, West Huangpu Avenue, Tianhe District, Guangzhou, 510630, People’s Republic of China, Email [email protected]
Introduction: Riehl’s melanosis (RM) is an acquired hyperpigmentation disorder, presenting diffused and reticulate brownish-gray pigmentation, preferentially on the face and neck. RM overlaps with systemic lupus erythematosus (SLE) and Hashimoto’s thyroiditis has never been reported.
Case: We report a case of RM patient accompanied with SLE and Hashimoto’s thyroiditis of primary hypothyroidism. Progressing, diffuse, symmetric, and reticular hyperpigmentation was seen on the face, neck, and upper limbs, manifesting as typical melanosis. Skin microscopy showed diffuse black-pepper-like changes and telangiectasias. The diagnosis of SLE and primary hypothyroidism were confirmed by follow-up investigations. The hyperpigmentation turned notably lighter after 14 months of treatment with prednisone, hydroxychloroquine, and L-thyroxine.
Discussion: The exact pathogenesis of RM is unclear and exposure to coal tar dyes, ultraviolet, and fragrance fixatives in cosmetics are believed to be contributing factors, while some cases involve no triggers. It is not impossible that RM is a rare skin manifestation of SLE that has never been reported. The skin hyperpigmentation in this patient was not triggered by thyroid disease.
Conclusion: RM could be a skin manifestation of autoimmunity. Coexistence of RM, lupus erythematosus and thyroiditis in the same patient is rare and has never been reported.
Keywords: case report, melanosis, cutaneous lupus erythematosus, systemic lupus erythematosus, primary hypothyroidism
Introduction
Autoimmune diseases are a series of diseases caused by morbid immune reactions. Coexistence of autoimmune diseases is common. Systemic lupus erythematosus (SLE)-associated Hashimoto’s thyroiditis has been reported up to 12.6% of the cases.1 Riehl’s melanosis (RM) is an acquired hyperpigmentation disorder, presenting diffused and reticulate brownish-gray pigmentation, preferentially on the face and neck, and the histopathological features include basal cell liquefactive degeneration, dermal melanin incontinence and/or lymphohistiocytic infiltrate, and upper dermal melanophage infiltration.2 RM overlaps with SLE and Hashimoto’s thyroiditis has never been reported.
Case
History
A 30-year-old Chinese woman came to the dermatology clinic with a two-year history of progressing, diffuse, lattice-like hyperpigmentation of the face and neck. Significant sunlight, chemical materials, medicine, and cosmetic exposures were denied. She had no notable family history of similar conditions. On further questioning, she admitted that she had experienced hair loss and ankle joint pain for two years, and these had worsened in the last two months.
Physical Examination
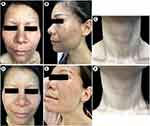
Diffuse, symmetric, and reticular gray brown hyperpigmentation was observed all over the face and neck, especially the cheeks and the area around the mouth (Figure 1A–C). Slight hyperpigmentation was seen on her upper limbs. No similar change can be seen on the rest of her skin and mucosa.
Investigation
Under skin microscopy, diffuse black-pepper-like changes separated by hair follicles with sweat glands and telangiectasias were seen (Figure 2A). The reflectance confocal microscopy showed abundant melanin in the epidermis and dermis, local liquefactive degeneration of basal layer cells, and the presence of melanophages in the dermis (Figure 2C and D). Histopathology of neck lesions (HE staining) showed punctiform epidermal atrophy, punctate liquefactive degeneration of basal layer cells, melanophages in the dermis, and a few lymphocytes infiltrating around the dermal adnexa (Figure 3A). Masson-Fontana silver staining showed amounts of melanin in the epidermis and dermis (Figure 3B). Laboratory investigations revealed the following: peripheral white blood cell (WBC) count 2.73 × 109/L (3.5–9.5 × 109/L), antinuclear antibody (ANA) (+++), anti-dsDNA antibody (dsDNA) (+), anti-Sjögren’s-syndrome-related antigen antibody A (+++), C3 0.48 g/L (0.90–1.80 g/L), C4 0.06 g/L (0.10–0.40 g/L), anti-thyroglobulin antibody (TGAb) >4000 IU/mL (0.00–115.00 IU/mL), and anti-thyroid peroxidase (ATPO) >600 IU/mL (0.00–34.00 IU/mL), thyroid stimulating hormone (TSH) 92 mIU/L (0.550–4.780 mIU/L), Thyronine-4 (T4) 2.5 μg/dl (4.5–10.9 μg/dl). Serum-free thyroxine (FT4), free triiodothyronine (FT3), thyronine-3 (T3), and adrenocorticotrophic hormone (ACTH) were all within normal range. Anti-TSH receptor antibody (TRab) was negative. Doppler ultrasonography of the thyroid showed hypothyroidism changes and a nodular thyroid cyst. Serum sex hormone, adrenal hormone, and the computed tomography scanning of the adrenal glands were all normal.
Diagnosis
Diagnoses of RM accompanied with SLE and Hashimoto’s thyroiditis of primary hypothyroidism were made via the clinical features and laboratory investigations.
Treatment
Prednisone (20 mg/day), hydroxychloroquine (400 mg/day), and L-thyroxine (100 μg/day) were administered orally. The prednisone was withdrawn gradually over the course of 7 months.
Follow-Up
Significant improvement was observed after 14 months of treatment. The hyperpigmentation has turned much lighter (Figures 1D–F and 2B). Under reflectance confocal microscopy, the melanin in the epidermis was found to have decreased significantly, the local liquefactive degeneration of basal layer cells showed improvement, and the melanophages in dermis was found to have decreased in number (Figure 2E). WBC, C3, C4, and T4 returned to normal. The TSH decreased to 5.667 mIU/L.
Discussion
The exact pathogenesis of RM is unclear and exposure to coal tar dyes, ultraviolet (UV), and fragrance fixatives in cosmetics are believed to be contributing factors, while some cases involve no triggers.2 In this patient, clinical and histological features confirmed the diagnosis of RM. Significant sunlight, chemical materials, medicine, and cosmetic exposures were denied. The hyperpigmentation is not likely caused by contact elements. Hyperpigmentation after inflammation was excluded for those without a history or accompanied with erythema and edema. Liquefactive degeneration of basal layer cells can be seen in cutaneous lupus erythematosus (CLE) as well, but increased melanin in the epidermis and melanophages in the dermis are rare. Pigment changes in CLE are usually post-inflammatory hyperpigmentation and are not common. Acute cutaneous lupus erythematosus (ACLE) usually leaves transient pigmentary changes, especially in dark-skinned people.3 The concurrent onset of hyperpigmentation and symptoms of SLE in this patient, as well as their improvement during treatment for SLE suggest an association. It is not impossible that RM is a rare skin manifestation of SLE that has never been reported. Recently, Calleja et al4 and Azrielant et al5 reported six and three LE patients who presented facial pigmentation, respectively. The hyperpigmentation of these patients is mostly isolated, limited in the face, and accompanied with erythema, which is different from the lesion of the present patient and not consistent with the diagnosis of melanosis. Miyoshi et al6 and Takeo et al7 have reported several patients with Sjogren’s syndrome who had Riehl’s melanosis-like eruption associated with anti-SSA (Ro) positive. In this case, anti-SSA was positive as well, implying this autoantibody plays a role in the development of hyperpigmentation in autoimmune diseases.
Song8 reported a case in which a 42-year-old Chinese woman with Graves’ disease developed general hyperpigmentation. T4, ACTH, and TRab were elevated. The elevation of ACTH and TRab was found to stimulate the production of cAMP, promoting the proliferation and differentiation of melanocytes, resulting in hyperpigmentation of skin. In the present case, FT4 and ACTH were within normal range, and the TRab was negative. Serum adrenal hormones, sex hormones, and blood glucose were all within normal ranges, and the results of adrenal computed tomography scanning were negative, and type II polyglandular autoimmune syndrome was excluded, which could cause hyperpigmentation as skin damage.9 This suggests that skin hyperpigmentation in this patient was not triggered by thyroid disease.
Conclusion
The exact pathogenesis of RM is not clear, and it could be a skin manifestation of autoimmunity. RM overlaps with SLE and Hashimoto’s thyroiditis has never been reported before.
Ethical Statement
Patient’s consent and institutional approval had been obtained for the purpose of image publication.
Consent Statement
The authors certify that they have obtained all appropriate patient consent forms. The patient signed a consent form for the publication of the case details and images.
Funding
This work was supported by the National Natural Science Foundation of China (No.82073440).
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Posselt RT, Coelho VN, Skare TL. Hashimoto thyroiditis, anti-thyroid antibodies and systemic lupus erythematosus. Int J Rheum Dis. 2018;21:186–193.
2. Kim SM, Lee ES, Sohn S, et al. Histopathological features of Riehl melanosis. Am J Dermatopathol. 2020;42:117–121.
3. Werth VP. Clinical manifestations of cutaneous lupus erythematosus. Autoimmun Rev. 2005;4:296–302.
4. Calleja AA, Aragón MR, Prieto BM, et al. Generalized facial pigmentation: an uncommon presentation of cutaneous lupus erythematosus. Indian J Dermatol Venereol Leprol. 2020;86(4):431–435.
5. Azrielant S, Ellenbogen E, Peled A, et al. Diffuse facial hyperpigmentation as a presenting sign of lupus erythematosus: three cases and review of the literature. Case Rep Dermatol. 2021;13(2):263–270.
6. Miyoshi K, Kodama H. Riehl’s melanosis-like eruption associated with Sjögren’s syndrome. J Dermatol. 1997;24:784–786.
7. Takeo N, Sakai T, Saito-Shono T, et al. Three cases of pigmented cosmetic dermatitis-like eruptions associated with primary Sjögren’s syndrome or anti-SSA antibody. J Dermatol. 2016;43(8):947–950.
8. Song X, Shen Y, Zhou Y, et al. General hyperpigmentation induced by Grave’s disease: a case report. Medicine. 2018;97:e13279.
9. Vryonidou A, Paschou SA, Dimitropoulou F, et al. Cardiac tamponade in a patient with autoimmune polyglandular syndrome type 2. Endocrinol Diabetes Metab Case Rep. 2017;2017:17–0097.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.