Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Coexistence of Discoid Lupus Erythematosus and Paraneoplastic Pemphigus: A Case Report and Literature Review
Authors Saowaluksakul W , Seree-aphinan C , Rutnin S , Boonyawat K, Chanprapaph K
Received 9 September 2022
Accepted for publication 8 November 2022
Published 16 November 2022 Volume 2022:15 Pages 2477—2486
DOI https://doi.org/10.2147/CCID.S389341
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Wasin Saowaluksakul,1 Chutima Seree-aphinan,1 Suthinee Rutnin,1 Kochawan Boonyawat,2 Kumutnart Chanprapaph1
1Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; 2Division of Hematology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Kumutnart Chanprapaph, Division of Dermatology, Department of Medicine, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, 270 Rama VI Road, Ratchatewi, Bangkok, 10400, Thailand, Tel +66 2 201 1141, Fax +66 2 201 1211, Email [email protected]
Abstract: Pemphigus and lupus erythematosus are both B-cell-mediated autoimmune diseases, dependent on autoreactive CD4+ T lymphocytes to modulate autoimmune B-cell response. Many forms of pemphigus have been reported to occur in association with systemic lupus erythematosus (SLE) as well as other autoimmune diseases. However, it remains unclear whether this association occurs because of a shared immunopathogenesis or the coexistence may be coincidental. We hereby present a case report of discoid lupus erythematosus and paraneoplastic pemphigus associated with marginal zone lymphoma in a 54-year-old Thai man who had persistent oral erosions for 1 year together with generalized polymorphic cutaneous eruptions for 2 months. Simultaneous occurrence of paraneoplastic pemphigus and discoid lupus erythematosus without SLE has never been reported in the same individual. Hydroxychloroquine, immunosuppressive agents including prednisolone and azathioprine together with chemotherapy were given to treat these conditions.
Keywords: paraneoplastic pemphigus, discoid lupus erythematosus, cutaneous lupus erythematosus, autoimmune bullous disease, non-Hodgkin lymphoma, marginal zone lymphoma
Introduction
Pemphigus and lupus erythematosus (LE) are classified as B-cell-mediated autoimmune diseases, both depending on autoreactive CD4+ T lymphocytes to modulate autoimmune B-cell response.1 Many forms of pemphigus have been anecdotally reported to occur in association with LE as well as other autoimmune diseases, but the pathogenic mechanism remains unclear.2–18 Previous reports have demonstrated cases with pemphigus foliaceus (PF) or pemphigus vulgaris (PV) in association to discoid lupus erythematosus (DLE).11,12 To date, there has been only one reported case of paraneoplastic pemphigus (PNP) associated with SLE.3 To the best of our knowledge, we present the first case of DLE and PNP associated with marginal zone lymphoma. This is a 54-year-old Thai man who had persistent oral erosions for 1 year and generalized polymorphic cutaneous eruptions for 2 months. An additional literature review on cases with pemphigus and LE has been performed. The search of English-language publications was conducted using MEDLINE, EMBASE and Scopus as electronic databases. The citations were identified with the use of a combination of the following text words: “pemphigus”, “paraneoplastic pemphigus”, “lupus erythematosus”, “SLE”, “CLE”, and “case report” from inception of each database to August 2022. Review of articles also included the abstracts of all references retrieved from the search.
Case Presentation
A 54-year-old Thai man with no known underlying medical conditions visited our institution with a 1-year history of persistent multiple painful oral erosions. He also developed generalized pruritic skin lesions on the scalp, face, right ear, trunk, both upper and lower extremities for 2 months. He lost 8 kg of weight within 6 months due to poor intake, but denied of any constitutional or systemic symptoms. He had an 8-pack-year history of smoking without a significant history of sun exposure. The patient was otherwise healthy.
Physical examination revealed multiple painful erosive patches on the lower lip, gingiva, buccal mucosa, tongue, hard palate, and genitalia as well as various forms of cutaneous lesions. Firstly, there were multiple, well-defined, erythematous to purplish plaques, with overlying crusted erosions on the forehead. Secondly, there were multiple ill-defined, erythematous papules, some coalescing into plaques and overlying with group of erosions and scattered bullae on both upper extremities (Figure 1A–D). Thirdly, there was a well-demarcated, erythematous atrophic plaque with follicular plugging and peripheral hyperpigmentation on the right concha (Figure 2A). Lastly, there were multiple, well-defined, purplish plaques, with overlying whitish scales and few erosions on scalp, face, upper chest, upper back and both lower legs (Figure 2B). Dermoscopic examination revealed thick, whitish and yellowish scales, follicular keratotic plugs, erythematous background with peripheral purplish-brown area on the scalp lesion (Figure 2C).
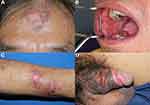 |
Figure 1 Clinical manifestations of PNP on the forehead (A), oral mucosa (B), left forearm (C) and genitalia (D). |
 |
Figure 2 Clinical manifestations of DLE on the right concha (A) and upper back (B); Dermoscopic findings of DLE on the scalp (C). |
Skin biopsies were taken from two sites: the forehead and upper back. Histopathological examination from the forehead revealed suprabasal separation with acantholytic keratinocytes and focal lichenoid lymphocytic infiltration with melanophages (Figure 3) suggestive of paraneoplastic pemphigus (PNP). Direct immunofluorescence (DIF) showed intercellular deposition of IgG (Figure 4A) and C3. There was also granular deposition of IgG, IgM and C3 at perieccrine areas (Figure 4B). Indirect immunofluorescence using rat bladder substrate was positive for intercellular deposition as well. Moreover, histopathological examination from the upper back revealed focal lichenoid, superficial and deep perivascular lymphocytic infiltration in association with irregular epidermal hyperplasia. There was also perifollicular interface changes with scattered basal necrotic keratinocytes, compatible with hypertrophic discoid lupus erythematosus (DLE) (Figure 5).
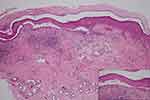 |
Figure 3 Histopathological examination of PNP showing suprabasal separation with lichenoid lymphocytic infiltration (H&E, ×10); acantholytic keratinocytes with tombstone appearance (inset, ×40). |
 |
Figure 4 Direct immunofluorescence of PNP demonstrating intercellular deposition with IgG (A); perieccrine granular deposition of C3 (B). |
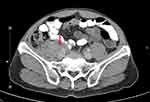
Laboratory tests, composing of complete blood count, renal function, liver function test, and urinalysis, were within normal limits. Anti-nuclear antibody (ANA) was also negative. Enzyme-linked immunosorbent assay (ELISA) for anti-desmoglein-1 autoantibody was positive (153.33; reference range 0–20 U/mL) at the time of the initial diagnosis. However, ELISA for anti-desmoglein-3, anti-BP180, anti-BP230, and anti-type VII collagen autoantibody were negative. To evaluate for associated tumor, computed tomography (CT) scan of the chest and whole abdomen with intravenous contrast media was performed and showed an infiltrative soft-tissue mass surrounding the periaortic and aortocaval regions with extension to involve the right psoas muscle (Figure 6). CT-guided biopsy was done for histopathology and immunohistochemistry which confirmed the diagnosis of marginal zone lymphoma.
Based on the clinical manifestation and investigation findings, this patient had coexistence of DLE and PNP associated with marginal zone lymphoma. The patient was initially treated with prednisolone (20 mg/day), azathioprine 50 mg/day for PNP together with hydroxychloroquine 200 mg/day for DLE. After the diagnosis of marginal zone lymphoma was made, chemotherapy (CVP regimen) with of cyclophosphamide, vincristine and prednisolone plus rituximab was administered.
Discussion
DLE is the most common clinical subtype of CCLE. It is categorized as localized (above the neck) or generalized (above and below the neck, typically over the extensor forearms and hands) form.19 Approximately 80% of DLE patients appear as localized form, while less than 20% of DLE patients have generalized skin lesions which are more likely to develop features of clinically significant systemic lupus erythematosus (SLE).19,20 Hypertrophic DLE is regarded as a rare variant of DLE, representing 2% of all lesions of CCLE, and is characterized histologically by irregular epidermal hyperplasia associated with features of classic CCLE, including interface changes.21,22
PNP is a rare mucocutaneous autoimmune disease associated with neoplasm.23 Tumors associated with PNP are either benign or malignant neoplasms which can be hematologic or non-hematologic in origin.24 PNP mostly affects adults between 45 and 70 years of age with no gender differences, but it may also be found in children and adolescents.25–27 Intractable stomatitis and polymorphous cutaneous eruptions, including blisters and lichenoid dermatitis, are characteristic clinical features caused by both humoral and cell-mediated immunity, respectively.28 Pathologic findings are presence as acantholytic blisters and interface dermatitis, which sometimes coexist in the same lesion.28,29 Immunofluorescence is a useful technique in the diagnosis of PNP. In particular, indirect immunofluorescence using rat bladder is a highly specific method to differentiate PNP from other pemphigus groups that do not harbor anti-plakin autoantibodies.28,30,31
According to literature review of DIF in dermatologic diseases, perieccrine and apocrine immunofluorescence findings may add diagnostic sensitivity in DIF evaluation, particularly of LE and subepidermal immunobullous disease, in which findings at the epidermis or dermo-epidermal junction are uninterpretable.32 However, the presence of immunoreactants in adnexal structures, such as IgG and C3 deposition in the intercellular space of hair follicles or sweat ducts, can be found in PNP.33 DIF report of our case showed not only epidermal intercellular deposition that can be found in PNP but also periadnexal granular deposition that was a characteristic of CCLE. In addition, clinical manifestation of the right ear, compatible with concha sign and dermoscopic findings of the scalp lesion supported the diagnosis of DLE as well.34,35
The presence of any autoimmune diseases increases the probability of additional ones occurring during the disease’s course because they can share common immunopathogenic mechanisms and risk factors which explain why several diseases can coexist.36 CLE-alone is also associated with a risk of non-SLE autoimmune diseases.37 To date, many forms of pemphigus have been reported to occur in association with both CLE and SLE.2–18 Table 1 summarizes the data on patients with pemphigus in association with lupus erythematosus (LE) and other autoimmune diseases. In general, pemphigus and SLE are both B-cell-mediated autoimmune diseases, dependent on autoreactive CD4+ T lymphocytes to modulate B-cell response.1 Two novel regulatory genes, interferon regulatory factor 8 (IRF8) and signal transducer and activation transcription 1 (STAT1), have been identified as genetic markers that are significantly associated with pemphigus and SLE.38 Nevertheless, there is still a shortage of knowledge about autoimmune diseases that appear concurrently with CLE. The pathogenetic function of autoantibodies and B cells in CCLE, particularly in solitary DLE, is lacking.39 There is, however, strong evidence for a function of cytotoxic T cell-mediated immune reaction,40–42 whereas the pathogenesis of PNP is driven by both humoral and cell-mediated immune responses.28 In cell-mediated immunity, autoreactive CD8+ T cells contributing to the formation of lichenoid dermatitis and CD56+ cells are detected within the dermo-epidermal junction of PNP lesions.43–45 Variable genetic factors and genetic susceptibility polymorphisms have been observed in PNP and DLE.24,40 Nonetheless, the coexistence of PNP and DLE in the same patient has never been reported, so it remains to be determined whether this association occurs because of a shared immunopathogenesis as mentioned previously or merely a coincidental finding.
 |
Table 1 Data on Patients with Pemphigus in Association with Lupus Erythematosus and Other Autoimmune Diseases |
Prognosis of PNP is poor and mortality is high, with 1-year, 2-year, and 5-year overall survival rate of 49%, 41% and 38%, respectively, depending on the underlying cause.46
There is currently no standard treatment for PNP due to the absence of randomized controlled trials.24 However, it is vital to define and treat the associated neoplasm.47 In patients with benign and operable tumor, a surgical cure is often the best chance of inducing remission.48 Nonetheless, in PNP with malignant neoplasms, reducing the tumor burden may not lead to disease control.46 The most widely used treatment for mucocutaneous lesions is systemic corticosteroid with or without other immunosuppressive agents including cyclosporine, cyclophosphamide, azathioprine and mycophenolate mofetil.46,49 Other treatments that have shown promising effects are intravenous immunoglobulin (IVIg), plasmapheresis, rituximab, ibrutinib, alemtuzumab and tocilizumab.28,50–56 In addition to photoprotection and smoking cessation, the first-line treatment of DLE is topical or intralesional corticosteroids, topical calcineurin inhibitor and systemic antimalarial therapy for individuals who do not respond to topical and intralesional treatments, or with extensive disease.57 Nevertheless, the therapeutic options having benefit for both PNP and DLE consist of mycophenolate mofetil, IVIg and rituximab.28,57 For marginal zone lymphoma, there are many different chemotherapy regimens, dependent on individual fitness of the patients, including rituximab/bendamustine, rituximab/cyclophosphamide/doxorubicin/vincristine/prednisone (R-CHOP), rituximab/cyclophosphamide/vincristine/prednisone (R-CVP) or rituximab/fludarabine.58 In our case, we advised for photoprotection, smoking cessation and prescribed systemic corticosteroid, azathioprine and systemic antimalarial drug to control mucocutaneous lesions of PNP and DLE. R-CVP regimen was administered for marginal zone lymphoma with significant clinical improvement (Figure 7).
 |
Figure 7 Improvement of PNP lesions of the oral mucosa (A) and DLE lesions of the upper back (B) after treatment. |
Conclusion
We report a case of co-occurrence of discoid lupus erythematosus and paraneoplastic pemphigus associated with marginal zone lymphoma in a middle-aged Thai man. Owing to the rarity of the coexistence of these two conditions, definitive explanation to pathogenesis of the association is lacking. Further studies are warranted.
Ethics Approval and Consent to Participate
This article was performed in accordance with the principles of Declaration of Helsinki. Ethical review and approval were not required to publish the case details in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the patient for publication of this case report and any accompanying images as per our standard institutional rules.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rutnin S, Chanprapaph K. Vesiculobullous diseases in relation to lupus erythematosus. Clin Cosmet Investig Dermatol. 2019;12:653–667. doi:10.2147/CCID.S220906
2. Malik M, Ahmed AR. Concurrence of systemic lupus erythematosus and pemphigus: coincidence or correlation? Dermatology. 2007;214:231–239. doi:10.1159/000099588
3. Mascaró JM, Ferrando J, Solé MT, et al. Paraneoplastic pemphigus: a case of long-term survival associated with systemic lupus erythematosus and polymyositis. Dermatology. 1999;199:63–66. doi:10.1159/000018182
4. Nanda A, Kapoor MM, Dvorak R, Al-Sabah H, Alsaleh QA. Coexistence of pemphigus vulgaris with systemic lupus erythematosus. Int J Dermatol. 2004;43:393–394. doi:10.1111/j.1365-4632.2004.02105.x
5. Somorin AO, Agbakwu SN, Nwaefuna A. Systemic lupus erythematosus and pemphigus vulgaris preceded by depressive psychosis. Cent Afr J Med. 1981;27:12–14.
6. Kuchabal DS, Kuchabal SD, Pandit AM, Hashi HK. Pemphigus vulgaris associated with systemic lupus erythematosus. Int J Dermatol. 1998;37:636–638. doi:10.1046/j.1365-4362.1998.00418.x
7. Fong PH, Chan HL. Systemic lupus erythematosus with pemphigus vulgaris. Arch Dermatol. 1985;121:26–27. doi:10.1001/archderm.1985.01660010030014
8. Hidalgo-Tenorio C, Sabio-Sánchez JM, Tercedor-Sánchez J, León-Ruíz L, Pérez-Alvarez F, Jiménez-Alonso J. Pemphigus vulgaris and systemic lupus erythematosus in a 46-y-old man. Lupus. 2001;10:824–826. doi:10.1177/096120330101001112
9. Calebotta A, Cirocco A, Giansante E, Reyes O. Systemic lupus erythematosus and pemphigus vulgaris: association or coincidence. Lupus. 2004;13:951–953. doi:10.1191/0961203304lu1073cr
10. Malik M, Ahmed AR. Dual diagnosis of pemphigus vulgaris and connective tissue disease. J Am Acad Dermatol. 2006;55:699–704. doi:10.1016/j.jaad.2006.04.052
11. Thongprasom K, Prasongtanskul S, Fongkhum A, Iamaroon A. Pemphigus, discoid lupus erythematosus, and dermatomyositis during an 8-year follow-up period: a case report. J Oral Sci. 2013;55:255–258. doi:10.2334/josnusd.55.255
12. Bilgic Temel A, Ergun E, Poot AM, et al. A rare case with prominent features of both discoid lupus erythematosus and pemphigus foliaceus. J Eur Acad Dermatol Venereol. 2019;33:e5–e7. doi:10.1111/jdv.15099
13. Ng PP, Ng SK, Chng HH. Pemphigus foliaceus and oral lichen planus in a patient with systemic lupus erythematosus and thymoma. Clin Exp Dermatol. 1998;23:181–184. doi:10.1046/j.1365-2230.1998.00367.x
14. Sawamura S, Kajihara I, Makino K, et al. Systemic lupus erythematosus associated with myasthenia gravis, pemphigus foliaceus and chronic thyroiditis after thymectomy. Australas J Dermatol. 2017;58:e120–e122 . doi:10.1111/ajd.12510
15. Ngo AW, Straka C, Fretzin D. Pemphigus erythematosus: a unique association with systemic lupus erythematosus. Cutis. 1986;38:160–163.
16. Itoh K, Umehara F, Iwasaki H, Kanda A, Arimura K. Zenshin-sei eritematōdesu to tenpōsō o gappei shita jūshōkinmuryokushō no 1-rei [A case of myasthenia gravis associated with systemic lupus erythematosus and pemphigus erythematosus]. Rinsho Shinkeigaku. 1997;37:111–114. Japanese.
17. Ascherman DP, Katz P. Systemic lupus erythematosus, pemphigus erythematosus, and thymoma in the same patient. J Clin Rheumatol. 1996;2:152–155. doi:10.1097/00124743-199606000-00008
18. Marinovic B, Basta-Juzbasic A, Bukvic-Mokos Z, Leovic R, Loncaric D. Coexistence of pemphigus herpetiformis and systemic lupus erythematosus. J Eur Acad Dermatol Venereol. 2003;17:316–319. doi:10.1046/j.1468-3083.2003.00738.x
19. Walling HW, Sontheimer RD. Cutaneous lupus erythematosus: issues in diagnosis and treatment. Am J Clin Dermatol. 2009;10:365–381. doi:10.2165/11310780-000000000-00000
20. Kuhn A, Sticherling M, Bonsmann G. Clinical manifestations of cutaneous lupus erythematosus. J Dtsch Dermatol Ges. 2007;5:1124–1137. doi:10.1111/j.1610-0387.2007.06554.x
21. Arps DP, Patel RM. Cutaneous hypertrophic lupus erythematosus: a challenging histopathologic diagnosis in the absence of clinical information. Arch Pathol Lab Med. 2013;137:1205–1210. doi:10.5858/arpa.2013-0241-CR
22. Daldon PE, Macedo de Souza E, Cintra ML. Hypertrophic lupus erythematosus: a clinicopathological study of 14 cases. J Cutan Pathol. 2003;30:443–448. doi:10.1034/j.1600-0560.2003.00082.x
23. Anhalt GJ, Kim SC, Stanley JR, et al. Ratrie H 3rd, Mutasim D, Ariss-Abdo L, et al. Paraneoplastic pemphigus. An autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323:1729–1735. doi:10.1056/NEJM199012203232503
24. Maruta CW, Miyamoto D, Aoki V, Carvalho RGR, Cunha BM, Santi CG. Paraneoplastic pemphigus: a clinical, laboratorial, and therapeutic overview. An Bras Dermatol. 2019;94:388–398. doi:10.1590/abd1806-4841.20199165
25. Yong AA, Tey HL. Paraneoplastic pemphigus. Australas J Dermatol. 2013;54:241–250. doi:10.1111/j.1440-0960.2012.00921.x
26. Kimyai-Asadi A, Jih MH. Paraneoplastic pemphigus. Int J Dermatol. 2001;40:367–372. doi:10.1046/j.1365-4362.2001.01169.x
27. Mimouni D, Anhalt GJ, Lazarova Z, et al. Paraneoplastic pemphigus in children and adolescents. Br J Dermatol. 2002;147:725–732. doi:10.1046/j.1365-2133.2002.04992.x
28. Kim JH, Kim SC. Paraneoplastic pemphigus: paraneoplastic autoimmune disease of the skin and mucosa. Front Immunol. 2019;10:1259. doi:10.3389/fimmu.2019.01259
29. Choi Y, Nam KH, Lee JB, et al. Retrospective analysis of 12 Korean patients with paraneoplastic pemphigus. J Dermatol. 2012;39:973–981. doi:10.1111/j.1346-8138.2012.01655.x
30. Joly P, Richard C, Gilbert D, et al. Sensitivity and specificity of clinical, histologic, and immunologic features in the diagnosis of paraneoplastic pemphigus. J Am Acad Dermatol. 2000;43:619–626. doi:10.1067/mjd.2000.107488
31. Liu AY, Valenzuela R, Helm TN, Camisa C, Melton AL, Bergfeld WF. Indirect immunofluorescence on rat bladder transitional epithelium: a test with high specificity for paraneoplastic pemphigus. J Am Acad Dermatol. 1993;28:696–699. doi:10.1016/0190-9622(93)70095-B
32. Lehman JS, Camilleri MJ. Diagnostic utility of direct immunofluorescence findings around hair follicles and sweat glands in immunobullous disease. J Cutan Pathol. 2013;40:230–235. doi:10.1111/cup.12037
33. Barnadas MA, Curell R, Alomar A, Gelpí C. Paraneoplastic pemphigus with negative direct immunofluorescence in epidermis or mucosa but positive findings in adnexal structures. J Cutan Pathol. 2009;36:34–38. doi:10.1111/j.1600-0560.2008.00993.x
34. Chanprapaph K, Tankunakorn J, Suchonwanit P, Rutnin S. Dermatologic manifestations, histologic features and disease progression among cutaneous lupus erythematosus subtypes: a prospective observational study in Asians. Dermatol Ther. 2021;11:131–147. doi:10.1007/s13555-020-00471-y
35. Żychowska M, Żychowska M. Dermoscopy of discoid lupus erythematosus - A systematic review of the literature. Int J Dermatol. 2021;60:818–828. doi:10.1111/ijd.15365
36. Anaya JM. The diagnosis and clinical significance of polyautoimmunity. Autoimmun Rev. 2014;13:423–426. doi:10.1016/j.autrev.2014.01.049
37. Lin TL, Wu CY, Juan CK, Chang YT, Chen YJ. Long-term risk of autoimmune diseases other than systemic lupus erythematosus in cutaneous lupus erythematosus-alone patients: a 10-year nationwide cohort study. Dermatology. 2022;238:92–100. doi:10.1159/000515524
38. Kridin K, Laufer-Britva R, Kridin M, Comaneshter D, Batat E, Cohen AD. The relationship between pemphigus and systemic lupus erythematosus: a cross-sectional study, systematic review, and meta-analysis. Immunol Res. 2019;67:116–122. doi:10.1007/s12026-019-9065-4
39. Zhang YP, Wu J, Han YF, Shi ZR, Wang L. Pathogenesis of cutaneous lupus erythema associated with and without systemic lupus erythema. Autoimmun Rev. 2017;16:735–742. doi:10.1016/j.autrev.2017.05.009
40. Wenzel J, Uerlich M, Wörrenkämper E, Freutel S, Bieber T, Tüting T. Scarring skin lesions of discoid lupus erythematosus are characterized by high numbers of skin-homing cytotoxic lymphocytes associated with strong expression of the type I interferon-induced protein MxA. Br J Dermatol. 2005;153:1011–1015. doi:10.1111/j.1365-2133.2005.06784.x
41. Mak A, Kow NY. The pathology of T cells in systemic lupus erythematosus. J Immunol Res. 2014;2014:419029. doi:10.1155/2014/419029
42. Grassi M, Capello F, Bertolino L, Seia Z, Pippione M. Identification of granzyme B-expressing CD-8-positive T cells in lymphocytic inflammatory infiltrate in cutaneous lupus erythematosus and in dermatomyositis. Clin Exp Dermatol. 2009;34:910–914. doi:10.1111/j.1365-2230.2009.03297.x
43. Nguyen VT, Ndoye A, Bassler KD, et al. Classification, clinical manifestations, and immunopathological mechanisms of the epithelial variant of paraneoplastic autoimmune multiorgan syndrome: a reappraisal of paraneoplastic pemphigus. Arch Dermatol. 2001;137:193–206.
44. Cummins DL, Mimouni D, Tzu J, Owens N, Anhalt GJ, Meyerle JH. Lichenoid paraneoplastic pemphigus in the absence of detectable antibodies. J Am Acad Dermatol. 2007;56:153–159. doi:10.1016/j.jaad.2006.06.007
45. Lim JM, Kim JH, Hashimoto T, Kim SC. Lichenoid paraneoplastic pemphigus associated with follicular lymphoma without detectable autoantibodies. Clin Exp Dermatol. 2018;43:613–615. doi:10.1111/ced.13563
46. Leger S, Picard D, Ingen-Housz-Oro S, et al. Prognostic factors of paraneoplastic pemphigus. Arch Dermatol. 2012;148:1165–1172. doi:10.1001/archdermatol.2012.1830
47. Wieczorek M, Czernik A. Paraneoplastic pemphigus: a short review. Clin Cosmet Investig Dermatol. 2016;9:291–295. doi:10.2147/CCID.S100802
48. Fang Y, Zhao L, Yan F, et al. A critical role of surgery in the treatment for paraneoplastic pemphigus caused by localized Castleman’s disease. Med Oncol. 2010;27:907. doi:10.1007/s12032-009-9304-y
49. Frew JW, Murrell DF. Current management strategies in paraneoplastic pemphigus (paraneoplastic autoimmune multiorgan syndrome). Dermatol Clin. 2011;29:607–612. doi:10.1016/j.det.2011.06.016
50. Nanda M, Nanda A, Al-Sabah H, Dvorak R, Alsaleh QA. Paraneoplastic pemphigus in association with B-cell lymphocytic leukemia and hepatitis C: favorable response to intravenous immunoglobulins and prednisolone. Int J Dermatol. 2007;46:767–769. doi:10.1111/j.1365-4632.2007.03225.x
51. Izaki S, Yoshizawa Y, Kitamura K, et al. Paraneoplastic pemphigus: potential therapeutic effect of plasmapheresis. Br J Dermatol. 1996;134:987–989. doi:10.1111/j.1365-2133.1996.tb06349.x
52. Vezzoli P, Berti E, Marzano AV. Rationale and efficacy for the use of rituximab in paraneoplastic pemphigus. Expert Rev Clin Immunol. 2008;4:351–363. doi:10.1586/1744666X.4.3.351
53. Lee A, Sandhu S, Imlay-Gillespie L, Mulligan S, Shumack S. Successful use of Bruton’s kinase inhibitor, ibrutinib, to control paraneoplastic pemphigus in a patient with paraneoplastic autoimmune multiorgan syndrome and chronic lymphocytic leukaemia. Australas J Dermatol. 2017;58:e240–e242. doi:10.1111/ajd.12615
54. Ito Y, Makita S, Maeshima AM, et al. Paraneoplastic pemphigus associated with B-cell chronic lymphocytic leukemia treated with ibrutinib and rituximab. Intern Med. 2018;57:2395–2398. doi:10.2169/internalmedicine.0578-17
55. Hohwy T, Bang K, Steiniche T, Peterslund NA, d’Amore F. Alemtuzumab-induced remission of both severe paraneoplastic pemphigus and leukaemic bone marrow infiltration in a case of treatment-resistant B-cell chronic lymphocytic leukaemia. Eur J Haematol. 2004;73:206–209. doi:10.1111/j.1600-0609.2004.00280.x
56. Bech R, Baumgartner-Nielsen J, Peterslund NA, Steiniche T, Deleuran M, d’Amore F. Alemtuzumab is effective against severe chronic lymphocytic leukaemia-associated paraneoplastic pemphigus. Br J Dermatol. 2013;169:469–472. doi:10.1111/bjd.12324
57. Garza-Mayers AC, McClurkin M, Smith GP. Review of treatment for discoid lupus erythematosus. Dermatol Ther. 2016;29:274–283. doi:10.1111/dth.12358
58. Zucca E, Arcaini L, Buske C, et al.; ESMO Guidelines Committee. Electronic address: [email protected]. Marginal zone lymphomas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:17–29. doi:10.1016/j.annonc.2019.10.010
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.