Back to Journals » Substance Abuse and Rehabilitation » Volume 13
Cocaine Use Disorder (CUD): Current Clinical Perspectives
Authors Schwartz EKC, Wolkowicz NR , De Aquino JP, MacLean RR, Sofuoglu M
Received 24 February 2022
Accepted for publication 22 August 2022
Published 3 September 2022 Volume 2022:13 Pages 25—46
DOI https://doi.org/10.2147/SAR.S337338
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Justinn Cochran
Elizabeth KC Schwartz,1,2 Noah R Wolkowicz,1,2 Joao P De Aquino,1,2 R Ross MacLean,1,2 Mehmet Sofuoglu1,2
1Department of Psychiatry, Yale University School of Medicine, New Haven, CT, USA; 2Department of Psychiatry, VA Connecticut Healthcare System, West Haven, CT, USA
Correspondence: Elizabeth KC Schwartz, Tel +1-203-932-5711, Fax +1-203-937-3472, Email [email protected]
Abstract: Cocaine use disorder (CUD) is a devastating disorder, impacting both individuals and society. Individuals with CUD face many barriers in accessing treatment for CUD, and most individuals with CUD never receive treatment. In this review, we provide an overview of CUD, including risk factors for CUD, common co-occurring disorders, acute and chronic effects of cocaine use, and currently available pharmacological and behavioral treatments. There are no FDA-approved pharmacological treatments for CUD. Future studies with larger sample sizes and testing treatment combinations are warranted. However, individuals with CUD and co-occurring disorders (eg, a mood or anxiety disorder) may benefit from medication treatments. There are behavioral interventions that have demonstrated efficacy in treating CUD – contingency management (CM) and cognitive-behavioral therapy for substance use disorders (CBT-SUD) in particular – however many barriers remain in delivering these treatments to patients. Following the discussion of current treatments, we highlight some promising emerging treatments, as well as offer a framework that can be used in building a treatment plan for individuals with CUD.
Keywords: cocaine, cocaine use disorder, treatment, pharmacotherapy, behavioral interventions
Introduction
Cocaine use disorder (CUD) – the compulsive use of cocaine despite its medical, psychological, and behavioral consequences – is a severe public health problem, affecting millions of people globally. In the United States (US) alone, approximately 2.2 million people use cocaine regularly (compared to 600,000 methamphetamine users), 1.5 million of whom meet the Diagnostic and Statistical Manual for Mental Disorders (DSM-5) criteria for CUD.1,2
Recent nationwide epidemiological data show that both cocaine use and cocaine-related problems, including CUD, are increasing in adults as well as in adolescents.3–5 In the US, the percentage of overdose deaths involving cocaine is increasing, doubling in one year alone between 2015 and 2016 – in 2017, among the 70,237 drug overdose deaths in the US, about 20% involved cocaine.6 Currently, the rate of cocaine overdose deaths is higher than opioid overdose deaths in Black men and women.7 In 2011, cocaine use contributed to more than 500,000 emergency room visits in the US8 due to overdose, medical disorders, accidents, and violence. In addition to contributing to medical emergencies, cocaine use causes many disabling and costly health problems, including heart attack, stroke, neuropsychiatric complications, and increases the risk of contracting HIV and hepatitis C.9,10 Cocaine use is also associated with frequent encounters with the criminal justice system, including crime, arrests, and imprisonment – and thus altered family structures, which disproportionately affects minorities in part due to racial disparities in crack-cocaine sentencing.11–14 More than one-third of homeless adults report lifetime cocaine use problems.15 The availability of effective and widely accessible treatments for CUD remains an urgent challenge.
The development of novel and efficacious treatments for CUD has been an area of intense research over the past 3 decades. The results of these studies have been the subject of several excellent systematic reviews and meta-analyses.16–19 In this review article, we provide a clinically relevant overview of the current literature on CUD. We first summarize the clinical epidemiology of CUD and then follow with an overview of the neurobehavioral consequences of short- and long-term cocaine use. We then summarize the current pharmacological and behavioral treatment approaches for CUD, and discuss emerging treatment approaches.
Diagnostic Criteria
The DSM-5 defines CUD as clinically significant impairment or distress caused by at least 2 of 11 criteria in the preceding 12 months.20 The 11 criteria can be organized into the following 4 groups: 1) physiologic, including craving, tolerance, and withdrawal, 2) loss of control of cocaine use, 3) cocaine use taking precedence over other activities (including responsibilities at home, work, or school), and 4) other negative consequences from cocaine use. According to the DSM, the presence of two or three symptoms indicates mild CUD, the presence of four or five symptoms indicates moderate CUD, and the presence of six or more symptoms indicates severe CUD.
The DSM-5 also provides definitions for different levels of remission. Early remission is defined as the absence of symptoms, except for cravings, for more than 3 months and less than 12 months, and sustained remission is defined as 12 months without symptoms, except for cravings. CUD, like all substance use disorders, tends to be chronic and relapsing in nature, and, similar to other chronic diseases, many patients require multiple episodes and modalities of treatment, which can together over time contribute to sustained recovery.21
The DSM-5 does not offer a definition of recovery, and there is no standardized definition of the term. While recovery has many meanings, most extend beyond cessation of substance use, emphasizing improved health and broader changes in behavior and sometimes even identity.22
Clinical Epidemiology
Overview
According to the 2019 National Survey on Drug Use and Health, which included people aged 12 and older, 5.5 million people reported past year cocaine use.23 Among cocaine users, about 20% will meet the criteria for CUD at some point in their lifetime.24,25 Among individuals who report cocaine use (including even just once), approximately 15% are estimated to progress to CUD within the following 10 years26 – a rate of progression higher than those found for cannabis (8%) and alcohol (12–13%). Additionally, the speed of progression from first cocaine use to CUD is much faster than the speed of progression from first use of alcohol to alcohol use disorder or from the first use of cannabis to cannabis use disorder, with one in 16 to 20 cocaine users becoming dependent within the first year of cocaine use.26 The National Epidemiological Survey of Alcohol and Related Conditions study, utilizing a large-scale community-based sample, found that the probability estimate of transitioning from first substance use to dependence was 7.1% for people who use cocaine, compared to 2.0% for those who use nicotine, alcohol, or cannabis.24 Although over time ongoing cocaine use continues to carry a high risk of progressing to CUD, given that the peak risk for initiation of cocaine use occurs at around age 20, much of the burden of CUD is carried by a younger population who often then struggle to quit for many years. For example, Simpson et al found that one year after completion of treatment (which in the study ranged from outpatient to residential), 21% of individuals initially diagnosed with CUD continued to use cocaine weekly, and the proportion of weekly cocaine users rose to 25% at 5 years.11 Moreover, 5 years after treatment, 18% reported having been arrested, emphasizing the high rates of the psychosocial impact, including legal stigma, of CUD.
Risk Factors
Many risk factors for CUD have been identified, including genetic, environmental, and other demographic and individual-level factors. Progression from experimentation with cocaine to the development of addiction has a significant genetic component.27 While heritability estimates vary, CUD is thought to be one of the most heritable mental health disorders28 – the risk for developing CUD is estimated to be up to 65% hereditary in women and up to 79% heredity in men.29,30 In comparison, the heritability for both alcohol and opioid use disorders is estimated to be 50% for both men and women.31,32 However, unlike tobacco and alcohol use disorders, specific genes directly linked to CUD remain to be identified. Environmental factors increase the risk of substance use disorders in general; however, CUD, similar to stimulant use disorders, is largely influenced by shared environment.33 Environmental factors can disproportionately affect minority populations. For example, black individuals have reported increased use of cocaine in the more addictive form of crack,34 which, in one study, was shown to be accounted for by increased availability and shared social conditions in some communities.35 Black individuals also experience longer gaps between problematic use and treatment entry.36 Other risk factors for developing CUD include impulsivity,37 childhood ADHD diagnosis,38 childhood adverse experiences,39 fewer years of education,40 lower parental level of education,41 polysubstance abuse,42,43 and presence of co-occurring mental health disorders.24 Using cocaine in more addictive forms such as crack-cocaine or via IV compared to intranasal routes,44 and using cocaine more frequently and in higher amounts, all increase the risk for CUD.25 Frequency of cocaine use seems to increase the risk of CUD more strongly than the amount of use; however, frequency and amount of use synergistically combine to increase the risk of progressing to CUD.25
Co-Occurring Mental Health Disorders
Co-occurrence between CUD and other mental health disorders is frequently observed in both treatment samples and large epidemiological studies. Two studies of treatment-seeking cocaine users found similar rates – 73.5% and 65% – of co-occurring lifetime mental health diagnoses, not including a co-occurring substance use disorder.45,46 Among responders in the US National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), 45% of cocaine users reported a lifetime mood disorder, and 31% reported a lifetime anxiety disorder.47 Compared to those who have never used cocaine, current cocaine use is associated with an almost tripled risk for depression (6% vs 16%) and a more than doubled risk for anxiety disorders (11% vs 5%).48 A meta-analysis estimated an 11 to 28% lifetime prevalence of bipolar disorder among individuals with CUD, compared to 1 to 3% for those in the general population.49
CUD is associated with high rates of both polysubstance use and co-occurring substance use disorders. In a study investigating mono vs polydrug use abuse, 77.8% of individuals who used cocaine reported polysubstance use (n = 21,970) – in terms of using substances simultaneously, the most common combination reported was cocaine and alcohol.50 In a separate study, in a sample of 227 individuals with CUD, the prevalence of lifetime heroin, alcohol, and benzodiazepine dependence were 46%, 29% and 25%, respectively.46 Concurrent use of cocaine and other drugs has multiple health risks. For example, co-use of cocaine and opioids contributes to cocaine overdose deaths,51 and co-use of alcohol and cocaine increases blood cocaine levels by 30% and increases cardiotoxicity by forming cocaethylene, a potentially lethal byproduct.52 About 75–84% of cocaine users also smoke cigarettes (the leading cause of preventable death in the US),53,54 and the quit rate for smoking among cocaine users is much lower than for those who do not use other illicit substances (13% vs 56%).54
In general, co-occurring substance use disorders and other mental health disorders negatively impact the treatment outcome of each other.55,56 This is true for CUD as well. The presence of mental health and substance use co-occurrence has a strong impact on the development and maintenance of CUD – contributing to the severity of CUD and to poorer treatment retention and outcomes.57–59 For example, the presence of co-occurring depression is associated with greater euphoria from cocaine use,60,61 more intense cravings, and more severe withdrawal symptoms during early abstinence from cocaine.62,63 Co-occurring depression also predicts a higher likelihood of relapse.64 Similar to depression, cocaine use is a well-known risk factor for suicidal ideation and suicide attempt,65–67 emphasizing the risks associated with the co-occurrence of CUD and depression.
There are many theories as to why such high rates of co-occurrence exist between substance use disorders and other mental health disorders. Mental health disorders and SUDs may have shared genetic vulnerabilities. According to the self-medication hypothesis, individuals try to relieve symptoms of a psychiatric disorder (such as depression) or side effects of medications (such as sedation).68,69 Features of anxiety and depression (such as negative internal mood states, including stress) may trigger substance use.
Another popular idea to explain high rates of co-occurrence is the shared vulnerability underlying substance use disorders and other mental health disorders (depression, bipolar disorder, and anxiety disorder). Genetic studies that found overlapping shared vulnerability across substance use and other psychiatric disorders support this hypothesis. For example, rather than being only driven by genetic variations in underlying biological processes directly linked to drug actions or drug metabolism, it is likely that addiction is driven by genetic variants that also mediate activity in brain circuits and regions involved in domains such as personality traits, reward, and mental health conditions.70
Clinically, it can often be difficult to distinguish an independent (or primary) mood disorder from a mood disorder secondary to (or caused by) a substance. This distinction often relies on determining the temporal sequences of mood symptoms and substance use, which is aided by following a patient longitudinally. Primary mood disorders are diagnosed when symptoms precede substance use or exist during periods of at least several weeks of abstinence. For individuals with long histories of overlapping mood symptoms and substance use, making the distinction between an independent and secondary mood disorder can be challenging, particularly as mood and substance use disorders tend to recur in nature, and patients with an SUD may have a difficult time achieving long periods of abstinence. For example, for individuals with co-occurring CUD and depression, it may be difficult to determine if depression is primary or caused by cocaine withdrawal. Given the high rates of co-occurring mental health disorders seen in SUDs and the overlapping symptomatology across these disorders, new paradigms may be needed for how we think about these conditions that we often try to diagnostically separate and treat individually. Ultimately, both substance-induced and “primary” mood disorders need clinical attention and treatment, even when making this distinction is challenging or not possible.
Sex Differences
CUD is associated with critical sex-based differences. Rates of lifetime CUD are higher among men – 3% for men vs 1.8% for women.71 Women, however, progress faster than men from first cocaine use to addiction. This rapid progression is termed a “telescoped course”, and it may be related to women being more likely to use crack cocaine, which is highly addictive.72 The profile of co-occurring mental health disorders among men and women with CUD also differ. Among individuals with CUD, men are more likely to have co-occurring SUDs and ADHD, while women are more likely to have co-occurring anxiety disorders, eating disorders, and post-traumatic stress disorder.72–75 Among individuals who enter treatment for CUD, men and women report equal rates of homelessness; however, women are more likely than men to report past trauma and receive pharmacotherapies for psychiatric conditions, suggestive of a higher level of complexity and a higher disease burden.75,76 Women who use cocaine also typically present to treatment reporting more socioeconomic problems than men.74 They are more likely than men to be unemployed and receive public aid,76 and they report greater interpersonal problems.74 They are also more likely than men to live with children,76 and thus must balance treatment with childcare. Following treatment, women are more likely than men to return to cocaine use in the context of distressing emotional states and interpersonal conflict.77 Collectively, these findings underscore the need for more sex-specific and tailored treatment approaches, as discussed below.
Acute and Chronic Neurobehavioral Effects of Cocaine
Acute Effects
There are several acute effects of cocaine that may drive typical patterns of use. As a psychostimulant, cocaine activates the sympathetic nervous system, causing increased arousal, vigor, activity, wakefulness, and elevated mood, as well as reduced appetite and sleep. Cocaine’s acute effects last for approximately 20–30 minutes; during this time users report feeling an intense euphoria or “high.” Acutely, and like other drugs of abuse, cocaine increases dopamine release in the brain’s mesolimbic reward systems, and this effect is believed to underlie the euphoric and addictive potential of cocaine. At higher doses and with more rapid routes of administration (eg, smoked or intravenous vs intranasal), cocaine tends to induce more robust euphoria, thereby increasing the likelihood of developing addiction.78
In a study of 36,309 adults in the US, 73% of cocaine users reported using an average of 0.8 grams on 0.4 days per week, compared to the remaining 27% who reported using between 2.6 and 19 grams of cocaine daily, at least 3 or more days per week.25 During cocaine binges, cocaine is used in large quantities during a discrete period until resources to do so have run out or the user is unable to continue use. These periods are associated with a higher risk of participating in criminal behavior, contracting sexually transmitted diseases such as HIV, and engaging in other impulsive behaviors with dire consequences for the individual and society.79,80
Neurobehavioral Features of Individuals with Chronic Cocaine Use
Key neurobehavioral changes occur with continued regular use of cocaine. These changes include the emergence of withdrawal following cessation of cocaine use and reduced euphoria from a given dose of cocaine (ie, tolerance), which can drive dose escalation. Symptoms of cocaine withdrawal include fatigue, psychomotor slowing, anxiety, depression, sleep disturbance, increased appetite, and intense craving for cocaine use. Although milder than withdrawal symptoms that accompany alcohol or opioid use, cocaine withdrawal symptoms can drive further cocaine use due to physical and psychological distress. Approximately 82–86% of cocaine users experience cocaine withdrawal – these estimates are based on two recent studies, one of which included individuals using cocaine at least twice a week for 6 months, and one of which included individuals who met criteria for CUD.81,82 Those who experience cocaine withdrawal are more likely to use cocaine in larger amounts, report stronger euphoria, and have more severe medical, psychiatric, and psychosocial problems.81
According to the incentive-sensitization theory, addiction develops due to an increased sensitivity to drug-related cues, even while the drug’s euphoric effects are diminished. This phenomenon is driven by learned associations and neuroplastic brain changes, leading to a drastic increase of “wanting” or “desiring” of the substance (eg, craving) which is often decoupled from a corresponding “liking” of that substance.83 One way to assess such hypersensitivity to drug-related cues is by measuring an automatic cognitive process called attentional bias for that drug.84 Once an increased attentional bias to cocaine develops, one more quickly notices cocaine-related cues and also has difficulty switching attention to more neutral stimuli.85,86 It has been suggested that increased attentional bias for cocaine cues may serve as a cognitive marker for CUD (reviewed in Sofuoglu et al 2014).87 The incentive-sensitization model is particularly relevant to CUD, as its core features are intense cravings (or “wanting” the substance), and high responsiveness to drug cues – as opposed to prominent withdrawal symptoms, which tend to accompany alcohol or opioid use.81
Chronic cocaine use is associated with several chronic neurocognitive deficits. People who chronically use cocaine display deficits in attention (particularly sustained attention), visual and working memory, verbal fluency, sensory-perceptual functions, response inhibition, and impulsivity (reviewed in Potvin).85,88,89 Notably, many of these deficits persist after several months of abstinence,85 indicating that they are not caused by immediate drug effects or acute phases of withdrawal. In a meta-analysis of 15 studies comparing cocaine users to healthy controls, cocaine use caused the greatest deficits in visual and working memory and attention.88 Furthermore, neuro-imaging studies, commonly utilizing functional magnetic resonance imaging (fMRI), conducted on cocaine users show decreases in activation or abnormal blood flow in brain regions that underlie executive and attentional function, such as anterior cingulate,90 lateral prefrontal,90 prefrontal,91 and orbitofrontal cortices.92 It is unclear if these cognitive deficits precede or follow cocaine use. Individuals with pre-existing cognitive deficits are more likely to use substances and develop SUDs.93 Alternatively, chronic use of cocaine and other drugs (eg, alcohol, tobacco) may contribute to cognitive deficits in a dose-dependent manner.94 Regardless of their cause, cognitive deficits observed in individuals with CUD have clinical relevance. For example, among cocaine users, treatment non-completers tend to perform worse in cognitive measures;95 consistent with this, better executive function predicts longer retention in treatment.96 Deficits in executive functioning can lead to decreased “top-down” processes important in recovery. For example, executive functions such as sustained attention and response inhibition are likely needed to help regulate drug use behavior,97,98 and it follows that breakdown in these processes enhances vulnerability to substance use and relapse. These types of cognitive deficits may also serve as a treatment target for both pharmacologic and behavioral interventions, as we outline below (see Treatments Targeting Cognitive Deficits).
Finally, neuroimaging studies have revealed differences in underlying neural networks in individuals with CUD compared to healthy controls who do not use substances. As with other drugs of abuse, chronic exposure to cocaine causes neuroplastic changes that ultimately dysregulate neural circuitry. While different brain regions are affected by different stages of addiction, the mesocorticolimbic99 circuit is the core circuit affected. While acute exposure to cocaine increases synaptic dopamine release in the mesolimbic circuit, chronic repeated exposure is associated with reduced dopamine function,100 underlying the anhedonia that accompanies substance use disorders. Attempting to correct imbalances in brain circuitry is the aim of emerging treatments such as transcranial magnetic stimulation (see Non-Invasive Brain Stimulation Methods, below).
Overview of Current Treatment Approaches
Pharmacotherapies
Over the past few decades, numerous studies have investigated pharmacologic treatments for CUD. However, thus far, no medication has met the Food and Drug Administration’s (FDA) criteria for approval, which consists of treatment efficacy demonstrated in at least two adequately powered (typically n > 200) randomized, placebo-controlled trials (RCT). While no drug class has proven to be effective,17 individual compounds with therapeutic promise have been used off-label. In this section, we discuss some of the most relevant findings per drug class.
Antidepressants
Results from a Cochrane review,101 a systematic review and meta-analysis,17 and an umbrella review102 suggest that, as a class, antidepressants have no consistent effect on any clinically relevant measure of CUD studied thus far (including cocaine use, sustained abstinence, retention, and harm outcomes). Instead, antidepressants have been associated with higher drop-out rates, possibly by causing adverse events – although the quality of the evidence for this finding has been deemed low.17,101
Among the studies that have found therapeutic effects of antidepressants for CUD is a double-blind placebo-controlled trial administering citalopram, a selective serotonin reuptake inhibitor (SSRI) antidepressant. In this study, citalopram was superior to placebo in reducing cocaine use, when combined with a behavioral intervention (either cognitive-behavioral therapy or contingency management).103 Likewise, in a 12-week double-blind RCT, sertraline, another SSRI, delayed time to returning to using cocaine among 86 cocaine-dependent individuals with depressive symptoms who had already been abstinent for two weeks when the trial began.104 Finally, in another trial, bupropion, with a mechanism of action that differs from SSRIs, enhanced the efficacy of contingency management in promoting abstinence from cocaine.105,106
Psychostimulants
By mimicking some actions of cocaine through increased dopaminergic activity but with key differences in pharmacokinetic properties – such as slower onset of effects and a longer half-life, and thus less abuse risk – psychostimulants have been used off-label to promote abstinence from cocaine. While a Cochrane review that included 26 trials (N = 2366) found low strength evidence that psychostimulants may promote abstinence in 14 trials (defined by 3 weeks of non-use in 13 of the studies and 2 weeks of non-use in 1 study), no differences in cocaine use, study retention, or harm outcomes were found.105 In another study, a high-dose (60 mg) but not low dose (30 mg) of sustained-release preparation of dextroamphetamine reduced cocaine use.107 Finally, another RCT administering 60 mg sustained-release dextroamphetamine in heroin- and cocaine-dependent individuals also being treated with methadone and diacetylmorphine found that dextroamphetamine was well tolerated and decreased days of cocaine use.108
Dopamine Agonists
A Cochrane review of 17 studies found no difference between dopamine agonists (bromocriptine, amantadine, and pergolide) in any clinically relevant outcome – including positive urine samples for cocaine metabolites and study retention.109 Another Cochrane review of 24 trials found no difference between dopamine agonists (including bromocriptine, amantadine, and pramipexole) compared to placebo in treatment retention or abstinence from cocaine use among people with CUD.110 The authors also found no evidence that combining a dopamine agonist with a psychosocial intervention improved treatment outcomes.110 A study investigating amantadine to reduce cocaine use and cravings found it was no more effective than placebo, in a group of cocaine-dependent individuals who were receiving methadone for co-occurring opioid use disorder.111 A separate study examining amantadine’s efficacy in reducing cocaine withdrawal symptoms and improving CUD outcomes found that amantadine decreased cocaine use, measured by urine samples and self-reports, in a group of cocaine-dependent people who were experiencing severe withdrawal symptoms at the start of the study.112 In this study, amantadine did not promote abstinence in the individuals who experienced less severe cocaine withdrawal at the start of the study, consistent with the authors' speculation that amantadine reduces cocaine withdrawal symptoms.
Modafinil is another well-known cognitive enhancer with the potential to treat CUD. Modafinil affects multiple neurotransmitter systems, and increases dopamine by blocking dopamine transporters.113 In an inpatient setting, modafinil improved working memory and sustained attention in cocaine-dependent individuals (N= 16), compared to individuals randomized to escitalopram, escitalopram plus modafinil, or placebo.114 In a separate study, modafinil treatment was associated with more days of abstinence from cocaine, when combined with weekly one-hour psychotherapy sessions.115 In another study, modafinil increased the likelihood of negative urine samples and chances of achieving cocaine abstinence for more than 3 weeks after treatment.116 However, in a follow-up study, the authors were unable to replicate this effect.117 A meta-analysis including 11 studies found that overall, modafinil was not superior to placebo in improving measures of CUD, yet the authors noted that in a subgroup analysis of 6 studies conducted in the US, modafinil increased cocaine abstinence rates.118 The authors concluded that, based on modafinil’s tolerability and good safety profile, it deserves further investigation.
Antipsychotics/Dopamine Blockers
A recent Cochrane review of 14 studies (N = 719) investigating the efficacy of antipsychotic pharmacotherapy for the treatment of CUD (via actions on dopaminergic and serotonergic systems) found that antipsychotics reduced treatment drop-out rates but had no significant effect on any other outcomes related to CUD.119 Similarly, a systematic review and meta-analysis including 8 RCTs investigating antipsychotics for the treatment of CUD concluded that antipsychotics may improve treatment retention.17 Clozaril, however, has shown some promise in reducing substance use, including cocaine use, in patients who are receiving this medication for a co-occurring psychotic disorder.120,121
Anticonvulsants and Muscle Relaxants
Stimulation of the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) decreases activation of the dopamine reward circuitry. Thus, anticonvulsants, which increase GABA activity, may decrease cocaine-induced dopamine release and reinforcing effects. A systematic review of 20 RCTs (N = 2068) found no significant difference between anticonvulsants and placebo in cocaine use, craving, rates of anxiety and depression, and treatment retention.122 Studies investigating topiramate, an anticonvulsant that stimulates GABA and inhibits glutamate, have found conflicting results. A meta-analysis of five RCTs (N = 518) examining topiramate for CUD found that, compared to placebo, topiramate increased abstinence (low strength of evidence) but did not affect study retention (moderate strength of evidence).17 A recent RCT, completed after the above meta-analysis was published, found that, compared to placebo, topiramate decreased the amount and frequency of cocaine use for the first 4 weeks of the study, but by week 12 there was no difference between treatment with topiramate and placebo.123
Repurposing of Medications Approved to Treat Other SUDs
With the exception of disulfiram – which has mixed evidence to treat CUD – no significant differences in outcomes have been found for medications approved to treat other SUDs. Disulfiram inhibits the enzyme dopamine-beta-hydroxylase, which converts dopamine to norepinephrine, thus increasing synaptic dopamine levels. Some clinical trials show that disulfiram may decrease cocaine use in those with co-occurring opioid use disorder,124 and also in those with co-occurring alcohol use disorder with and without co-administration of naltrexone.125 In a different study, disulfiram reduced cocaine use in individuals with CUD who were not alcohol dependent or who did not drink alcohol during treatment, compared to those who were using alcohol or who were alcohol dependent.126 A meta-analysis of 12 RCTs found low strength evidence that disulfiram does not increase abstinence and instead decreases retention; however, the authors noted that the evidence was insufficient for drawing conclusions due to the heterogeneity of effects measured.17
Ketamine
Finally, ketamine has shown some promise as a novel treatment for SUDs including CUD. NMDA receptors are believed to be the main glutamate receptor involved in learned behavior, and ketamine, through NMDA receptor antagonism, modulates glutamate signaling. A recent RCT found that one single subanesthetic ketamine infusion, compared to an infusion of midazolam, improved several treatment outcomes of CUD – including lowering the likelihood of using cocaine and decreasing cravings and relapse risk – in a group of 55 cocaine-dependent individuals who also initiated mindfulness-based relapse prevention therapy (MBRPT) as part of the study.127 MBRPT was combined with ketamine because the authors speculated that the two treatments would activate similar brain networks and therefore enhance each other. Impressively, the group who received ketamine and therapy had higher rates of abstinence even at a 6-month follow-up. These promising findings warrant future studies with larger samples.
There are many reasons why, despite numerous studies, no medications have been approved for CUD. These include methodological issues, small sample sizes leading to underpowered studies, high drop-out rates, and heterogeneity of both study design and sample population. Additionally, as observed recently by Brandt et al, a great many pharmacological treatments for CUD have been explored; however, only a few studies have been carried out for each individual medication.18 Thus, they argue that medications that have demonstrated positive signals in early studies should be given further investigation. Exploring combination therapies is another promising area.
Psychosocial Treatments for Cocaine Use Disorder
Psychosocial treatments for CUD are limited in variety but nonetheless are the current standard of practice for this disorder, representing the culmination of nearly four decades of clinical research.128–131 Here, we discuss two approaches that have received the greatest degree of empirical support: Contingency Management (CM)130,132 and Cognitive-Behavioral Therapy for Substance Use Disorders (CBT-SUD).133,134 We begin by providing a brief history of each treatment’s development before describing its structure, application, and empirical support. Lastly, we note treatment limitations and discuss ongoing efforts to refine these psychosocial interventions for CUD.
Contingency Management
CM was first implemented in the US within opioid treatment clinics, in which the possibility of frequent objective drug-use monitoring (eg, via urine toxicology screens) and related, salient natural rewards (eg, take-home methadone doses) allowed researchers to demonstrate the value of positive contingencies in reducing substance use.131,135–137 CM was later applied to CUD during the 1980s, prompted in large part by the lack of effective pharmacotherapies for this condition.129,136,138 These initial efforts were crucial for establishing CM as a formal treatment and in the culmination of the treatment practiced today.
Application of CM draws heavily from operant conditioning principles, focusing on systematically adjusting reinforcers and punishers in a patient’s environment to increase behavior in alignment with treatment goals.139 Abstinence, assessed via urine toxicology screens 2–3 times per week, is frequently indicated as the main treatment goal and reward for negative (drug-free) toxicology screens serve as the primary reinforcer in CM. That is, patients who provide negative screens receive monetary-based vouchers130,140 or opportunities for prizes141,142 redeemable for retail goods or services. Voucher/prize-value is systematically increased following consecutive negative screens to incentivize sustained abstinence; conversely, positive screens or failure to submit urine screens at scheduled times results in resetting of reward values to their initial levels. CM for CUD is typically offered in an outpatient setting over 12-weeks132,143 and has been provided in both individual and group formats.144
Within this common framework, reinforcement is organized around several principles including rapidly and accurately detecting cocaine use, positively reinforcing abstinence close-in-time to detection, loss of positive reinforcement upon drug use, and incorporation of reinforcers to compete with drug use.129 As the evidence base supporting CM has grown, its refinements in its practice have similarly developed, with, for example, better outcomes resulting in more immediate provision of reinforcement following objective verification of abstinence or provision of higher-magnitude reinforcers.145,146 Additionally, individuals who demonstrate greater within-treatment abstinence tend to show better long-term outcomes after treatment ends.147
CM has routinely demonstrated a high level of efficacy in treating CUD, with multiple studies and RCTs showing effectiveness of CM over standard care and in patients who have co-occurring SUDs or mental health disorders.148–154 Studies have also indicated that CM is effective across several different clinical groups, including community155 and veteran populations.150 For example, Bentzley et al conducted a meta-analysis comparing several different treatments for CUD including psychotherapies, various medications (eg, antidepressants, psychostimulants, opioids), and placebo groups; collectively, results suggested only CM was associated with greater likelihood of negative urine toxicology screens for cocaine.148
Despite CM’s extensive empirical support, several ongoing barriers impede its widespread adoption including societal and provider stigma, concerns about the durability of treatment effects, and pragmatic concerns such as implementation costs and personnel availability.156 Additionally, there is some concern with how CM can be implemented alongside other treatment approaches such as 12-step facilitation. Approximately two-thirds of substance use clinics report that the 12-step method is the predominant treatment approach.157,158 Clinic staff that use a 12-step approach may hesitate to implement CM due to philosophical or practical concerns.159 However, CM is a remarkably effective intervention generally and in some meta-analyses has outperformed other active treatments for CUD.148,149,160,161 Furthermore, the emphasis on abstinence is consistent with 12-step treatment perspectives. While reductions from peak treatment abstinence levels can occur, multiple studies show lasting effects of CM up to 1-year post-treatment completion.162–164 Finally, while CM can be an expensive treatment to implement (eg, patients in trials conducted by Higgins et al could earn nearly $1000)140, a large body of research illustrates that CM can be implemented at costs as low as $240/per patient and conducted in cost-effective formats, such as in therapy groups. 155,165
Cognitive-Behavioral Therapy for Substance Use Disorders (CBT-SUD)
CBT-SUD for CUD grew from broader efforts to apply cognitive-behavioral principles to the treatment of addictive behaviors. Drawing from applications of CBT in depression and anxiety, initial applications of CBT in addictive behaviors were validated by Marlatt et al in patients seeking treatment for alcohol use.136,166,167 Following its initial validation, CBT-SUD was then applied to treat CUD, largely through the work of Carroll et al. These efforts culminated in the publication of Carroll’s seminal manual, A Cognitive-Behavioral Approach: Treating Cocaine Addiction.128,133,134 CBT-SUD has since become a widely familiar model amongst practitioners with a strong evidence base.
CBT-SUD focuses on helping patients understand their cocaine use and teaching them new skills to help manage it. A core aspect of this approach is the integration of “functional analysis”, which aids patients in understanding the antecedents and consequences prompting/maintaining cocaine use. From this foundation, patients receive skills training on how to understand, recognize, and intervene during cravings/urges, identify and challenge unhelpful cognitions related to cocaine use, effectively problem-solve, implement assertive communication to refuse drug offers, reduce drug-related cues in their environment, and recognize and intervene on seemingly minor decisions that can increase risk of cocaine use.134 Within this framework, sessions are often structured to ensure time is allocated for psychoeducation, skill/home practice review, and introduction of novel skills. Several “core” skill modules (eg, functional analysis, coping with cravings, drug refusal skills)134 are integral components of this manualized protocol, though later iterations have expanded these topics to include numerous “adjunctive” modules (eg, mood management, listening skills, social and recreational counseling)168. In CBT-SUD, patients are asked to complete regular home-practice between each session that, as indicated above, is then reviewed by providers in subsequent meetings. CBT-SUD is typically conducted in 1-hour weekly, individual sessions occurring over 12 to 16 weeks; however, this treatment has commonly been applied in group formats.169
There is a large body of evidence supporting the efficacy of CBT in treating CUD. Several RCTs have shown CBT-SUD is effective in reducing cocaine use.126,170,171 Notably, some evidence has indicated “sleeper effects” of CBT-SUD for CUD, in which improvements in cocaine use continue after treatment completion.133 Rawson et al, for example, conducted an RCT in which methadone-maintained participants with CUD were randomly assigned to either CBT, CM, CBT+CM, or treatment as usual (TAU).163 Their results indicated reductions in cocaine use after the treatment period (16 weeks) for both CM and CBT, though CM demonstrated slightly larger effects immediately post-treatment; however, at 26- and 52-week follow-ups, CBT participants continued demonstrating improvements, resulting in equivalent improvements between CBT and CM at these timepoints. Thus, CBT-SUD is not only effective but its effects may persist beyond the completion of treatment.
Despite CBT-SUD’s empirical support, several treatment considerations and implementation barriers are important to note. First, with respect to treatment considerations, CBT-SUD’s efficacy is in part dependent on patient skill and generalization.172,173 This is exemplified in work by Decker et al that showed patients who completed less than half of assigned home practice demonstrated greater cocaine use than patients completing more than half of assigned home practice; these effects remained significant even after statistically controlling for baseline cocaine use frequency and session attendance.174 Second, fidelity has remained an ongoing challenge to effectively implementing CBT-SUD,171,175 with research suggesting higher levels of didactic training and supervision are necessary to maintain adequate model-adherence in practice.176 Even among individuals who have been thoroughly trained to use evidence-based treatments, like CBT-SUD, clinicians are not as adherent to treatment protocols even though they believe themselves to be competent.177 Collectively, these issues present significant practical demands from practitioners and administrations to ensure adequate delivery of CBT-SUD for patients with CUD.
These barriers prompted adaptations in the delivery of CBT-SUD, with the most notable example being a computer-assisted program known as “CBT4CBT”.178–181 CBT4CBT utilizes multimedia (eg, video, text, games, cartoons) features to help coach patients through learning CBT-SUD coping skills. The platform provides a standardized method of delivering clinician-adjunctive services to help treat cocaine and other substance use disorders. Evidence for CBT-SUD demonstrates comparable efficacy and effect duration with its traditional modality generally for substance use disorders,178,182 as well as specifically for CUD .183–185 In Carroll et al’s RCT of CBT4CBT, methadone-maintained patients with CUD who completed an 8-week course of the program reported high acceptability of CBT4CBT, alongside greater odds of obtaining ≥3 consecutive weeks of cocaine abstinence and negative urine screens for all drugs; further, these effects were retained at a 6-month follow-up.183 Finally, cost-effectiveness research has suggested stark superiority of CBT4CBT versus traditional implementations of CBT-SUD.186
Summary of Psychosocial Treatments for CUD
In summary, psychosocial treatments represent the current gold-standard for CUD treatment. Within this broader category, CM and CBT-SUD currently have the greatest empirical support in positive treatment outcomes (eg, reduced cocaine use), collectively garnered over approximately 3 decades of research and application. Nonetheless, ongoing barriers remain including implementation barriers such as stigma towards these practices, pragmatic limitations (eg, cost, staff availability), and model adherence. Though ongoing research efforts are refining the extent and application of these modalities (eg, computer-assisted methods like CBT4CBT),179 there remains a need for greater dissemination and implementation of both CM and CBT-SUD for CUD.
Integrated Care and Combined Treatments
Integrated treatment refers to when treatment of both the SUD and the co-occurring mental health disorder are delivered by the same clinician or clinical team. This approach has been considered the ideal treatment for individuals with co-occurring mental health disorders and SUDs at all levels of treatment (inpatient, outpatient, etc.).187–189 Although integrated treatment approaches are supported in the literature, a recent Cochrane review found low-quality evidence of no difference between integrated models of care and standard models of care for multiple outcomes including substance use and global functioning.190 There are several reasons that could explain this finding, including the difficulty in standardizing integrated dual diagnosis treatment.191 Aside from the difficulties in ensuring standardization of integrated treatment, there are other barriers to this type of care in both research and clinical practice. Most treatment centers are still not even designed to offer this type of care. For example, a survey of 180 community addiction programs spanning residential treatment programs, outpatient programs, and intensive outpatient programs found that only 20% offered integrated or dual diagnosis services,192 and a separate study which sampled 256 programs across the US found that only 18% of addiction treatment programs and 9% of mental health programs offered integrated treatment approaches.193
Multimodal care typically includes combining psychosocial interventions (such as CM or CBT) with medications, and this approach often has better outcomes than treatment with a single intervention. For example, in a study investigating the efficacy of desipramine combined with CM for the treatment of opioid and cocaine dependence, while both CM and desipramine were individually effective in increasing opioid and cocaine-negative urines, the combined treatment was more effective in improving these outcomes than either treatment alone.194 Similarly, individuals treated with 40mg fluoxetine and the environmental contingency of decreased clinic visit requirements for fewer cocaine-positive urine samples had fewer cocaine-positive urine samples than those treated with only 20 mg fluoxetine or placebo.195 Another study of 106 opioid-dependent cocaine users found evidence that combining CM with bupropion reduced cocaine use more than bupropion alone, and for a longer period than for CM alone.106 Taken together, these studies underscore the potential synergism between behavioral and pharmacological treatments for CUD.
Emerging Treatments
Treatments Targeting Cognitive Deficits
As discussed above, there are neurocognitive deficits associated with regular cocaine use. Many of these overlap with changes seen in the mental health disorders that are commonly co-occurring among people with CUD. As this overlap in symptomatology exists, cognitive domains hold promise as treatment targets for both pharmacologic and behavioral interventions.196,197
Behavioral Treatments
Changes in attentional bias, seen in both CUD and anxiety and depressive disorders, are a promising treatment target (reviewed in).87 Attentional bias modification (ABM) is a relatively new approach in which participants learn how to shift attention away from drug cues and towards neutral cues.198 Authors of a recent study investigated ABM’s potential to reduce drug use behaviors in individuals with CUD – they found no differences between the group receiving ABM and the group receiving control therapy, although both groups report decreased use and cravings.199 Another study found that intensity of attentional bias may be a predictor of imminent relapse to cocaine use, as measured and tracked by a hand-held portable device.200 Building on these findings, portable and user-friendly interventions may help people identify the need to adjust the intensity and frequency of treatment or gain a better understanding of their own unique triggers to use cocaine, in real-time.
Pharmacological Approaches
N-acetylcysteine, by balancing glutamate function, may help reduce attentional bias to cocaine-related cues. This was supported by results of a study of 14 individuals who, during n-acetylcysteine treatment, had reduced attentional bias to cocaine cues.201 They also had reduced cocaine choices, as measured by the Drug Choice Procedure in which participants were asked to choose between cocaine and a monetary reinforcer.
The cholinergic system plays a role in many cognitive processes including attention, memory, mood, motivation, reward, and stress response. Findings from multiple lines of evidence indicate that the cholinergic system is another promising potential target for the treatment of CUD.202 Cholinesterase inhibitors, such as galantamine and rivastigmine, increase synaptic acetylcholine levels and have been used to enhance cognitive function in neuropsychiatric disorders such as Alzheimer’s disease. Recently, among abstinent cocaine users (N = 28), galantamine improved sustained attention and working memory functions after only 10 days.203 In another study conducted among cocaine-dependent individuals (N = 41), rivastigmine improved performance on a measure of working memory after 7 days of treatment.204 Another study found a trend for galantamine to decrease cocaine use in methadone-maintained patients with opiate use disorder and CUD (N = 14);205 and in a larger trial (N = 120) galantamine significantly reduced cocaine use in methadone-maintained patients.184 Notably, post-hoc analyses of data from this trial showed that galantamine also reduced opioid use.206 Conversely, a recent randomized placebo-controlled trial of galantamine in patients with only CUD showed no reduction in cocaine use with galantamine treatment at either 8 mg/day (N = 31) or 16 mg/day (N = 30).207 Taken together, and combined with galantamine’s safety and tolerability, results from these studies indicate that galantamine holds promise as a novel pharmacotherapy for CUD.
Although the psychostimulant methylphenidate has shown promise in improving response inhibition in cocaine-dependent individuals,208,209 clinical trials investigating methylphenidate’s ability to improve symptoms of CUD have yielded mixed results.210 Methylphenidate, however, was shown to decrease cocaine use in individuals with co-occurring ADHD and CUD, when compared with controls,211 thereby supporting its use for patients with CUD and co-occurring ADHD.
Sex-Specific Treatments
Studies investigating sex and gender differences in cravings, relapse, stress response, and other features of addiction (see Sex Differences) support the need for tailored treatment approaches to address sex and gender-specific needs. This includes the possibility of providing different medications to men and women. The female sex hormone progesterone, produced during the second half of the menstrual cycle, has been found to decrease cravings and euphoria produced by substances of abuse including cocaine, as well as improve cognitive function.212–214 Progesterone may also reduce stress responses,215 which are more prominent in cocaine-dependent women compared to men.216 A stronger stress response is a known risk factor for substance use and relapse, particularly in women drug users. A narrative review looking at 16 studies, nine of which included patients with CUD, found cumulative evidence supporting progesterone in its ability to decrease cravings and subjective positive effects of cocaine.215 Oxytocin may also play a role in modulating stress response. In a recent study of 112 adults with CUD, women reported greater stress provoked by a social stress test than men, but had decreased cortisol response when the hormone oxytocin was administered intranasally 40 minutes prior to the test.217 A logical next step would be to examine if intranasal oxytocin reduces cocaine use triggered by stress in women.
Non-pharmacological interventions aimed at stress reduction may also hold promise to reduce cocaine use, particularly for women. Authors of a study investigating the relationship of sex and cocaine dependence on cravings found that cocaine-dependent woman had increased corticostriatal-limbic activity – which correlated with craving – in response to stress, compared to cocaine-dependent men, who had activation of these areas in response to drug cues.218 The authors reported that these findings emphasize the importance of therapies targeting stress reduction, such as mindfulness skills,219 for women. Another example is offering services that help with caretaker responsibilities.
Non-Invasive Brain Stimulation Methods
Compared to individuals who do not use substances, individuals with CUD exhibit dysfunctional circuitry (see Neurobehavioral Features of Individuals with Chronic Cocaine Use), which can be modulated by brain stimulation methods. In transcranial magnetic stimulation (TMS), which is non-invasive and generally well tolerated, magnetic pulses are applied to modulate activity in specific cortical regions, thus alleviating certain symptoms by regulating underlying brain networks. TMS has been FDA approved to treat major depressive disorder and obsessive-compulsive disorder, and it is being explored for a variety of other conditions including substance use disorders. Most studies target the dorsolateral prefrontal cortex (DLPFC) with the aim of both increasing prefrontal cortex functioning and strengthening the modulation by the DLPFC of the dopaminergic mesolimbic circuitry (reviewed by Diana et al, 2017).220 In transcranial direct current stimulation (tDCS), a weak electrical current is passed between two electrodes placed on the scalp, which then modulates cortical excitability. tDCS also most commonly targets the DLPFC, due to the reasoning mentioned above. There have been several studies examining these neurostimulation techniques as potential treatments for CUD (recently reviewed by Bolloni et al, 2018, Rachid, 2018, and Lupi et al, 2017).221–223 In summary, several studies found that TMS and tDCS have efficacy in reducing cravings224–228 or reducing risky behaviors229 found in CUD. However, several methodological issues deserve attention. These include small sample sizes, in some cases lack of a sham control group, differences in stimulation protocols used, and an unclear understanding of the mechanisms of the treatments. Several authors emphasize the potential efficacy of these novel approaches in treating CUD and suggest ways to standardize these treatments, as well as point to the strength of combining these techniques with neuroimaging to better understand and target relevant neural circuits.220,223,230
Immunotherapies
Since the early 90s, researchers have attempted to produce an anti-cocaine vaccine, which would block cocaine’s effects (reviewed by Kinsey et al in).231 While cocaine by itself does not provoke an immune response, it can stimulate the production of antibodies when bound to a larger carrier protein. As a result, in the presence of cocaine, these antibodies bind to cocaine, preventing it from reaching the brain and therefore blocking its euphoric and reinforcing effects. One cocaine vaccine – termed TA-CD – that was investigated through Phase III trials was synthesized by linking succinylnorcocaine – a chemical derivative of cocaine – to a carrier protein derived from the cholera B toxin (rCTB).232 In a double-blind, placebo-controlled randomized trial, cocaine-users who developed high antibody titers following vaccination reduced cocaine use; however, only 38% of vaccinated individuals had sufficient antibody levels, and the antibodies remained elevated for only 2 months.233 Notably, the favorable retention rate in this study was attributed to the fact that the participants were in methadone maintenance treatment, which required frequent clinical visits. In a follow-up clinical trial with cocaine users who were not on methadone, no significant treatment differences were found. In fact, those who had developed higher vaccine-induced antibody levels actually had more positive-cocaine urines, indicating increased cocaine use. The authors speculated that the individuals with greater antibody levels and more positive-cocaine urines may have increased cocaine use to overcome a blockade of euphoria caused by the vaccine.234 Other cocaine vaccines are being explored in pre-clinical studies.
Personalized Treatments
Given the heterogeneity of the patient population involved – each individual with CUD has varying illness severity, personal characteristics, backgrounds, and social support – personalized, multi-dimensional treatment approaches are needed. Identifying risk factors for SUDs, including genetic,235 behavioral, and environmental, may help to predict treatment course and thus assist in treatment selection. For example, it has been suggested that those with higher impulsivity may respond better to behavioral interventions targeting this symptom cluster,236 and, as mentioned above, that female substance users may especially benefit from a multidisciplinary team that can provide treatment for interpersonal stress such as mindfulness-based therapies.
Identifying Treatments and Supporting Recovery
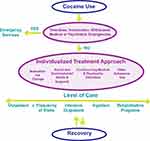
Only 19% of individuals with CUD receive much-needed treatment.237 Referral to evidence-based substance use treatment and other needed services is critical, and any time individuals with CUD interface with a health care team (in the emergency department, in primary care, etc.) is an important opportunity to link individuals to appropriate care. Treating CUD directly is just one aspect of care that should be considered. In fact, each individual can benefit from a comprehensive care plan that targets multiple domains (see Figure 1). After ruling out or managing mental health and medical emergencies – including ones related to intoxication, overdose, or withdrawal – it is important to address several critical domains of the patient’s experience. These include addressing CUD and other co-occurring substance use disorders, mental health and emotional needs, medical and physical needs, and social and environmental needs. Each individual’s motivational status and goals should also be assessed. These domains should be continuously considered to help guide an individual’s treatment plan, including the appropriate level of care (outpatient, inpatient, residential, etc.) which may be affected by the presence of co-occurring medical conditions or other critical needs including homelessness, or lack of adequate social support. The American Society of Addiction Medicine (ASAM) criteria can be used to help make this type of assessment and guide treatment.238
The Substance Abuse and Mental Health Services Administration (SAMHSA) offers a working definition of recovery as “a process of change through which individuals improve their health and wellness, live a self-directed life, and strive to reach their full potential”.239 This highlights recovery as a multi-step, evolving process that encompasses many domains.
In summary, in this review, we have highlighted many challenges that exist in the field of CUD therapeutics, outlined evidence-based treatments, and underscored promising novel therapies. It is our hope that we have also highlighted the many existing opportunities to support individuals with CUD in their recovery process. These opportunities must be seized by professionals from multiple disciplines – from medicine to psychology and from social work to occupational therapy. While it may take time for each individual with CUD to find their own unique combination of treatments that will work best, it is critical to keep individuals engaged in care until their own most effective path toward recovery can be discovered.
Funding
This research was supported by the New England Veterans Administration VISN 1 Mental Illness Research, Education and Clinical Center (MIRECC).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Administration SAaMHS. Key Substance Use and Mental Health Indicators in the United States: Results from the 2017 National Survey on Drug Use and Health. HHS Publication No. SMA 18-5068, NSDUH Series H-53. Rockville, MD: Center for Behavioral Health Statistics and Quality; 2018.
2. SAMHSA. Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health; National Findings. SAMHSA; 2014.
3. Simpson KJ, Moran MT, McCall KL, et al. Increasing heroin, cocaine, and buprenorphine arrests reported to the Maine Diversion Alert Program. Forensic Sci Int. 2019;303:109924. doi:10.1016/j.forsciint.2019.109924
4. Schneider KE, Krawczyk N, Xuan Z, Johnson RM. Past 15-year trends in lifetime cocaine use among US high school students. Drug Alcohol Depend. 2018;183:69–72. doi:10.1016/j.drugalcdep.2017.10.028
5. John WS, Wu LT. Trends and correlates of cocaine use and cocaine use disorder in the United States from 2011 to 2015. Drug Alcohol Depend. 2017;180:376–384. doi:10.1016/j.drugalcdep.2017.08.031
6. Hedegaard H, Bastian BA, Trinidad JP, Spencer M, Warner M. Drugs most frequently involved in drug overdose deaths: United States, 2011–2016. Natl Vital Stat Rep. 2018;67(9):1–14.
7. Shiels MS, Freedman ND, Thomas D, Berrington de gonzalez A. Trends in U.S. drug overdose deaths in non-Hispanic black, Hispanic, and non-Hispanic white persons, 2000–2015. Ann Intern Med. 2018;168(6):453–455. doi:10.7326/M17-1812
8. Crane EH. Highlights of the 2011 Drugs Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. Rockville, MD: Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2013.
9. Nnadi CU, Mimiko OA, McCurtis HL, Cadet JL. Neuropsychiatric effects of cocaine use disorders. J Natl Med Assoc. 2005;97(11):1504–1515.
10. Khalsa JH, Elkashef A. Interventions for HIV and hepatitis C virus infections in recreational drug users. Clin Infect Dis. 2010;50(11):1505–1511. doi:10.1086/652447
11. Simpson DD, Joe GW, Broome KM. A national 5-year follow-up of treatment outcomes for cocaine dependence. Arch Gen Psychiatry. 2002;59(6):538–544. doi:10.1001/archpsyc.59.6.538
12. Williams CT, Latkin CA. Neighborhood socioeconomic status, personal network attributes, and use of heroin and cocaine. Am J Prev Med. 2007;32(6 Suppl):S203–S210. doi:10.1016/j.amepre.2007.02.006
13. Palamar JJ, Davies S, Ompad DC, Cleland CM, Weitzman M. Powder cocaine and crack use in the United States: an examination of risk for arrest and socioeconomic disparities in use. Drug Alcohol Depend. 2015;149:108–116. doi:10.1016/j.drugalcdep.2015.01.029
14. Blumstein A. The notorious 100: 1 crack: powder disparity–the data tell us that it is time to restore the balance. Federal Sentencing Reporter. 2003;16(1):87–92. doi:10.1525/fsr.2003.16.1.87
15. Childress S, Reitzel LR, Maria DS, Kendzor DE, Moisiuc A, Businelle MS. Mental illness and substance use problems in relation to homelessness onset. Am J Health Behav. 2015;39(4):549–555. doi:10.5993/AJHB.39.4.11
16. Kampman KM. The treatment of cocaine use disorder. Sci Adv. 2019;5(10):eaax1532. doi:10.1126/sciadv.aax1532
17. Chan B, Kondo K, Freeman M, Ayers C, Montgomery J, Kansagara D. Pharmacotherapy for cocaine use disorder-a systematic review and meta-analysis. J Gen Intern Med. 2019;34(12):2858–2873. doi:10.1007/s11606-019-05074-8
18. Brandt L, Chao T, Comer SD, Levin FR. Pharmacotherapeutic strategies for treating cocaine use disorder-what do we have to offer? Addiction. 2021;116(4):694–710. doi:10.1111/add.15242
19. Buchholz J, Saxon AJ. Medications to treat cocaine use disorders: current options. Curr Opin Psychiatry. 2019;32(4):275–281. doi:10.1097/YCO.0000000000000518
20. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders.
21. Dennis M, Scott CK. Managing addiction as a chronic condition. Addict Sci Clin Pract. 2007;4(1):45–55. doi:10.1151/ascp074145
22. Nelson J, Bundoc-Baronia R, Comiskey G, McGovern TF. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Mental Health Services Administration (US); Office of the Surgeon General (US); 2016.
23. Quality CfBHSa. Results from the 2019 National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration; Department of Health and Human Services; 2020.
24. Lopez-Quintero C, Cobos JP, Hasin DS. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 2011;115(1–2):120–130. doi:10.1016/j.drugalcdep.2010.11.004
25. Liu Y, Cheong J, Vaddiparti K, Cottler LB. The association between quantity, frequency and duration of cocaine use during the heaviest use period and DSM-5 cocaine use disorder. Drug Alcohol Depend. 2020;213:108114. doi:10.1016/j.drugalcdep.2020.108114
26. Wagner FA, Anthony JC. From first drug use to drug dependence; developmental periods of risk for dependence upon marijuana, cocaine, and alcohol. Neuropsychopharmacology. 2002;26(4):479–488. doi:10.1016/S0893-133X(01)00367-0
27. Pierce RC, Fant B, Swinford-Jackson SE, Heller EA, Berrettini WH, Wimmer ME. Environmental, genetic and epigenetic contributions to cocaine addiction. Neuropsychopharmacology. 2018;43(7):1471–1480. doi:10.1038/s41386-018-0008-x
28. Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6(7):521–532. doi:10.1038/nrg1635
29. Kendler KS, Karkowski LM, Neale MC, Prescott CA. Illicit psychoactive substance use, heavy use, abuse, and dependence in a US population-based sample of male twins. Arch Gen Psychiatry. 2000;57(3):261–269. doi:10.1001/archpsyc.57.3.261
30. Kendler KS, Prescott CA. Cocaine use, abuse and dependence in a population-based sample of female twins. Br J Psychiatry. 1998;173:345–350. doi:10.1192/bjp.173.4.345
31. Deak JD, Miller AP, Gizer IR. Genetics of alcohol use disorder: a review. Curr Opin Psychol. 2019;27:56–61. doi:10.1016/j.copsyc.2018.07.012
32. Tsuang MT, Lyons MJ, Harley RM, et al. Genetic and environmental influences on transitions in drug use. Behav Genet. 1999;29(6):473–479. doi:10.1023/A:1021635223370
33. Ystrom E, Reichborn-Kjennerud T, Neale MC, Kendler KS. Genetic and environmental risk factors for illicit substance use and use disorders: joint analysis of self and co-twin ratings. Behav Genet. 2014;44(1):1–13. doi:10.1007/s10519-013-9626-6
34. Chen K, Kandel D. Relationship between extent of cocaine use and dependence among adolescents and adults in the United States. Drug Alcohol Depend. 2002;68(1):65–85. doi:10.1016/S0376-8716(02)00086-8
35. Lillie-Blanton M, Anthony JC, Schuster CR. Probing the meaning of racial/ethnic group comparisons in crack cocaine smoking. JAMA. 1993;269(8):993–997. doi:10.1001/jama.1993.03500080041029
36. Lewis B, Hoffman L, Garcia CC, Nixon SJ. Race and socioeconomic status in substance use progression and treatment entry. J Ethn Subst Abuse. 2018;17(2):150–166. doi:10.1080/15332640.2017.1336959
37. Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21(4):193–198. doi:10.1016/S0740-5472(01)00202-1
38. Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev. 2011;31(3):328–341. doi:10.1016/j.cpr.2011.01.006
39. Tang S, Jones CM, Wisdom A, Lin HC, Bacon S, Houry D. Adverse childhood experiences and stimulant use disorders among adults in the United States. Psychiatry Res. 2021;299:113870. doi:10.1016/j.psychres.2021.113870
40. Dokkedal-Silva V, Fernandes GL, Morelhao PK, et al. Sleep, psychiatric and socioeconomic factors associated with substance use in a large population sample: a cross-sectional study. Pharmacol Biochem Behav. 2021;210:173274. doi:10.1016/j.pbb.2021.173274
41. Palamar JJ, Ompad DC. Demographic and socioeconomic correlates of powder cocaine and crack use among high school seniors in the United States. Am J Drug Alcohol Abuse. 2014;40(1):37–43. doi:10.3109/00952990.2013.838961
42. Liu Y, Elliott AL, Serdarevic M, Leeman RF, Cottler LB. A latent class analysis of the past-30-day substance use patterns among lifetime cocaine users: findings from a community sample in North Central Florida. Addict Behav Rep. 2019;9:100170. doi:10.1016/j.abrep.2019.100170
43. Florez-Salamanca L, Secades-Villa R, Hasin DS, et al. Probability and predictors of transition from abuse to dependence on alcohol, cannabis, and cocaine: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Drug Alcohol Abuse. 2013;39(3):168–179. doi:10.3109/00952990.2013.772618
44. Gossop M, Griffiths P, Powis B, Strang J. Cocaine: patterns of use, route of administration, and severity of dependence. Br J Psychiatry. 1994;164(5):660–664. doi:10.1192/bjp.164.5.660
45. Rounsaville BJ, Anton SF, Carroll K, Budde D, Prusoff BA, Gawin F. Psychiatric diagnoses of treatment-seeking cocaine abusers. Arch Gen Psychiatry. 1991;48(1):43–51. doi:10.1001/archpsyc.1991.01810250045005
46. Vergara-Moragues E, Gonzalez-Saiz F, Lozano OM, et al. Psychiatric comorbidity in cocaine users treated in therapeutic community: substance-induced versus independent disorders. Psychiatry Res. 2012;200(2–3):734–741. doi:10.1016/j.psychres.2012.07.043
47. Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67(2):247–257. doi:10.4088/jcp.v67n0211
48. Kandel DB, Huang FY, Davies M. Comorbidity between patterns of substance use dependence and psychiatric syndromes. Drug Alcohol Depend. 2001;64(2):233–241. doi:10.1016/S0376-8716(01)00126-0
49. Hunt GE, Malhi GS, Cleary M, Lai HM, Sitharthan T. Comorbidity of bipolar and substance use disorders in national surveys of general populations, 1990–2015: systematic review and meta-analysis. J Affect Disord. 2016;206:321–330. doi:10.1016/j.jad.2016.06.051
50. Kedia S, Sell MA, Relyea G. Mono- versus polydrug abuse patterns among publicly funded clients. Subst Abuse Treat Prev Policy. 2007;2:33. doi:10.1186/1747-597X-2-33
51. Kariisa M, Scholl L, Wilson N, Seth P, Hoots B. Drug overdose deaths involving cocaine and psychostimulants with abuse potential - United States, 2003–2017. MMWR Morb Mortal Wkly Rep. 2019;68(17):388–395. doi:10.15585/mmwr.mm6817a3
52. Pennings EJ, Leccese AP, Wolff FA. Effects of concurrent use of alcohol and cocaine. Addiction. 2002;97(7):773–783. doi:10.1046/j.1360-0443.2002.00158.x
53. Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse. 1993;5(2):117–130. doi:10.1016/0899-3289(93)90056-H
54. Richter KP, Ahluwalia HK, Mosier MC, Nazir N, Ahluwalia JS. A population-based study of cigarette smoking among illicit drug users in the United States. Addiction. 2002;97(7):861–869. doi:10.1046/j.1360-0443.2002.00162.x
55. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) study. JAMA. 1990;264(19):2511–2518. doi:10.1001/jama.1990.03450190043026
56. Rounsaville BJ. Treatment of cocaine dependence and depression. Biol Psychiatry. 2004;56(10):803–809. doi:10.1016/j.biopsych.2004.05.009
57. Poling J, Kosten TR, Sofuoglu M. Treatment outcome predictors for cocaine dependence. Am J Drug Alcohol Abuse. 2007;33(2):191–206. doi:10.1080/00952990701199416
58. Carroll KM, Power ME, Bryant K, Rounsaville BJ. One-year follow-up status of treatment-seeking cocaine abusers. Psychopathology and dependence severity as predictors of outcome. J Nerv Ment Dis. 1993;181(2):71–79. doi:10.1097/00005053-199302000-00001
59. Merikangas KR, Kalaydjian A. Magnitude and impact of comorbidity of mental disorders from epidemiologic surveys. Curr Opin Psychiatry. 2007;20(4):353–358. doi:10.1097/YCO.0b013e3281c61dc5
60. Newton TF, Kalechstein AD, Tervo KE, Ling W. Irritability following abstinence from cocaine predicts euphoric effects of cocaine administration. Addict Behav. 2003;28(4):817–821. doi:10.1016/S0306-4603(01)00273-8
61. Uslaner J, Kalechstein A, Richter T, Ling W, Newton T. Association of depressive symptoms during abstinence with the subjective high produced by cocaine. Am J Psychiatry. 1999;156(9):1444–1446. doi:10.1176/ajp.156.9.1444
62. Elman I, Karlsgodt KH, Gastfriend DR, Chabris CF, Breiter HC. Cocaine-primed craving and its relationship to depressive symptomatology in individuals with cocaine dependence. J Psychopharmacol. 2002;16(2):163–167. doi:10.1177/026988110201600207
63. Helmus TC, Downey KK, Wang LM, Rhodes GL, Schuster CR. The relationship between self-reported cocaine withdrawal symptoms and history of depression. Addict Behav. 2001;26(3):461–467. doi:10.1016/S0306-4603(00)00105-2
64. McKay JR, Pettinati HM, Morrison R, Feeley M, Mulvaney FD, Gallop R. Relation of depression diagnoses to 2-year outcomes in cocaine-dependent patients in a randomized continuing care study. Psychol Addict Behav. 2002;16(3):225–235. doi:10.1037/0893-164X.16.3.225
65. Abdalla RR, Miguel AC, Brietzke E, Caetano R, Laranjeira R, Madruga CS. Suicidal behavior among substance users: data from the Second Brazilian National Alcohol and Drug Survey (II BNADS). Braz J Psychiatry. 2019;41(5):437–440. doi:10.1590/1516-4446-2018-0054
66. Bohnert KM, Ilgen MA, Louzon S, McCarthy JF, Katz IR. Substance use disorders and the risk of suicide mortality among men and women in the US Veterans Health Administration. Addiction. 2017;112(7):1193–1201. doi:10.1111/add.13774
67. Pavarin RM, Fioritti A. Mortality trends among cocaine users treated between 1989 and 2013 in Northern Italy: results of a longitudinal study. J Psychoactive Drugs. 2018;50(1):72–80. doi:10.1080/02791072.2017.1365976
68. Khantzian EJ. The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry. 1985;142(11):1259–1264.
69. Khantzian EJ. Self-regulation and self-medication factors in alcoholism and the addictions. Similarities and differences. Recent Dev Alcohol. 1990;8:255–271.
70. Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry. 2003;160(4):687–695. doi:10.1176/appi.ajp.160.4.687
71. Grant BF, Saha TD, Ruan WJ, et al. Epidemiology of DSM-5 drug use disorder: results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatry. 2016;73(1):39–47. doi:10.1001/jamapsychiatry.2015.2132
72. McCance-Katz EF, Carroll KM, Rounsaville BJ. Gender differences in treatment-seeking cocaine abusers–implications for treatment and prognosis. Am J Addict. 1999;8(4):300–311. doi:10.1080/105504999305703
73. Pedraz M, Araos P, Garcia-Marchena N, et al. Sex differences in psychiatric comorbidity and plasma biomarkers for cocaine addiction in abstinent cocaine-addicted subjects in outpatient settings. Front Psychiatry. 2015;6:17. doi:10.3389/fpsyt.2015.00017
74. Najavits LM, Lester KM. Gender differences in cocaine dependence. Drug Alcohol Depend. 2008;97(1–2):190–194. doi:10.1016/j.drugalcdep.2008.04.012
75. Requena-Ocana N, Flores-Lopez M, Martin AS, et al. Influence of gender and education on cocaine users in an outpatient cohort in Spain. Sci Rep. 2021;11(1):20928. doi:10.1038/s41598-021-00472-7
76. Wong CJ, Badger GJ, Sigmon SC, Higgins ST. Examining possible gender differences among cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2002;10(3):316–323. doi:10.1037/1064-1297.10.3.316
77. Terry-McElrath YM, O’Malley PM, Johnston LD. Reasons for drug use among American youth by consumption level, gender, and race/ethnicity: 1976–2005. J Drug Issues. 2009;39(3):677–714. doi:10.1177/002204260903900310
78. Boutrel B, Koob GF. What keeps us awake: the neuropharmacology of stimulants and wakefulness-promoting medications. Sleep. 2004;27(6):1181–1194. doi:10.1093/sleep/27.6.1181
79. Harzke AJ, Williams ML, Bowen AM. Binge use of crack cocaine and sexual risk behaviors among African-American, HIV-positive users. AIDS Behav. 2009;13(6):1106–1118. doi:10.1007/s10461-008-9450-9
80. Roy E, Arruda N, Jutras-Aswad D, et al. Examining the link between cocaine binging and individual, social and behavioral factors among street-based cocaine users. Addict Behav. 2017;68:66–72. doi:10.1016/j.addbeh.2017.01.012
81. Sofuoglu M, Dudish-Poulsen S, Brown SB, Hatsukami DK. Association of cocaine withdrawal symptoms with more severe dependence and enhanced subjective response to cocaine. Drug Alcohol Depend. 2003;69(3):273–282. doi:10.1016/S0376-8716(02)00328-9
82. Schuckit MA, Daeppen JB, Danko GP, et al. Clinical implications for four drugs of the DSM-IV distinction between substance dependence with and without a physiological component. Am J Psychiatry. 1999;156(1):41–49. doi:10.1176/ajp.156.1.41
83. Berridge KC, Robinson TE. Liking, wanting, and the incentive-sensitization theory of addiction. Am Psychol. 2016;71(8):670–679. doi:10.1037/amp0000059
84. Cox WM, Fadardi JS, Intriligator JM, Klinger E. Attentional bias modification for addictive behaviors: clinical implications. CNS Spectr. 2014;19(3):215–224. doi:10.1017/S1092852914000091
85. Potvin S, Stavro K, Rizkallah E, Pelletier J. Cocaine and cognition: a systematic quantitative review. J Addict Med. 2014;8(5):368–376. doi:10.1097/ADM.0000000000000066
86. Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2011;37(2):117–122. doi:10.3109/00952990.2010.543204
87. Leeman RF, Robinson CD, Waters AJ, Sofuoglu M. A critical review of the literature on attentional bias in cocaine use disorder and suggestions for future research. Exp Clin Psychopharmacol. 2014;22(6):469–483. doi:10.1037/a0037806
88. Jovanovski D, Erb S, Zakzanis KK. Neurocognitive deficits in cocaine users: a quantitative review of the evidence. J Clin Exp Neuropsychol. 2005;27(2):189–204. doi:10.1080/13803390490515694
89. Goldstein RZ, Leskovjan AC, Hoff AL, et al. Severity of neuropsychological impairment in cocaine and alcohol addiction: association with metabolism in the prefrontal cortex. Neuropsychologia. 2004;42(11):1447–1458. doi:10.1016/j.neuropsychologia.2004.04.002
90. Bolla K, Ernst M, Kiehl K, et al. Prefrontal cortical dysfunction in abstinent cocaine abusers. J Neuropsychiatry Clin Neurosci. 2004;16(4):456–464. doi:10.1176/jnp.16.4.456
91. Volkow ND, Mullani N, Gould KL, Adler S, Krajewski K. Cerebral blood flow in chronic cocaine users: a study with positron emission tomography. Br J Psychiatry. 1988;152:641–648. doi:10.1192/bjp.152.5.641
92. Volkow ND, Fowler JS, Wolf AP, et al. Changes in brain glucose metabolism in cocaine dependence and withdrawal. Am J Psychiatry. 1991;148(5):621–626.
93. Wagner M, Schulze-Rauschenbach S, Petrovsky N, et al. Neurocognitive impairments in non-deprived smokers–results from a population-based multi-center study on smoking-related behavior. Addict Biol. 2013;18(4):752–761. doi:10.1111/j.1369-1600.2011.00429.x
94. Bolla KI, Rothman R, Cadet JL. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11(3):361–369. doi:10.1176/jnp.11.3.361
95. Aharonovich E, Nunes E, Hasin D. Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug Alcohol Depend. 2003;71(2):207–211. doi:10.1016/S0376-8716(03)00092-9
96. Verdejo-Garcia A, Betanzos-Espinosa P, Lozano OM, et al. Self-regulation and treatment retention in cocaine dependent individuals: a longitudinal study. Drug Alcohol Depend. 2012;122(1–2):142–148. doi:10.1016/j.drugalcdep.2011.09.025
97. Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33(5):631–646. doi:10.1016/j.neubiorev.2008.08.016
98. Posner MI, Rothbart MK. Research on attention networks as a model for the integration of psychological science. Annu Rev Psychol. 2007;58:1–23. doi:10.1146/annurev.psych.58.110405.085516
99. Volkow ND, Morales M. The brain on drugs: from reward to addiction. Cell. 2015;162(4):712–725. doi:10.1016/j.cell.2015.07.046
100. Volkow ND, Wang GJ, Fowler JS. Imaging studies of cocaine in the human brain and studies of the cocaine addict. Ann N Y Acad Sci. 1997;820:
101. Pani PP, Trogu E, Vecchi S, Amato L, Antidepressants for cocaine dependence and problematic cocaine use. Cochrane Database Syst Rev. 2011;(12):CD002950. doi:10.1002/14651858.CD002950.pub3
102. Ronsley C, Nolan S, Knight R, et al. Treatment of stimulant use disorder: a systematic review of reviews. PLoS One. 2020;15(6):e0234809. doi:10.1371/journal.pone.0234809
103. Moeller FG, Schmitz JM, Steinberg JL, et al. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33(3):367–378. doi:10.1080/00952990701313686
104. Oliveto A, Poling J, Mancino MJ, et al. Sertraline delays relapse in recently abstinent cocaine-dependent patients with depressive symptoms. Addiction. 2012;107(1):131–141. doi:10.1111/j.1360-0443.2011.03552.x
105. Castells X, Cunill R, Perez-Mana C, Vidal X, Capella D. Psychostimulant drugs for cocaine dependence. Cochrane Database Syst Rev. 2016;9:CD007380. doi:10.1002/14651858.CD007380.pub4
106. Poling J, Oliveto A, Petry N, et al. Six-month trial of bupropion with contingency management for cocaine dependence in a methadone-maintained population. Arch Gen Psychiatry. 2006;63(2):219–228. doi:10.1001/archpsyc.63.2.219
107. Grabowski J, Rhoades H, Stotts A, et al. Agonist-like or antagonist-like treatment for cocaine dependence with methadone for heroin dependence: two double-blind randomized clinical trials. Neuropsychopharmacology. 2004;29(5):969–981. doi:10.1038/sj.npp.1300392
108. Nuijten M, Blanken P, van de Wetering B, Nuijen B, van den Brink W, Hendriks VM. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10034):2226–2234. doi:10.1016/S0140-6736(16)00205-1
109. Soares BG, Lima MS, Reisser AA, Farrell M, Dopamine agonists for cocaine dependence. Cochrane Database Syst Rev. 2003;(2):CD003352. doi:10.1002/14651858.CD003352
110. Minozzi S, Amato L, Pani PP, et al. Dopamine agonists for the treatment of cocaine dependence. Cochrane Database Syst Rev. 2015;(5):CD003352. doi:10.1002/14651858.CD003352.pub4
111. Handelsman L, Limpitlaw L, Williams D, Schmeidler J, Paris P, Stimmel B. Amantadine does not reduce cocaine use or craving in cocaine-dependent methadone maintenance patients. Drug Alcohol Depend. 1995;39(3):173–180. doi:10.1016/0376-8716(95)01154-9
112. Kampman KM, Volpicelli JR, Alterman AI, Cornish J, O’Brien CP. Amantadine in the treatment of cocaine-dependent patients with severe withdrawal symptoms. Am J Psychiatry. 2000;157(12):2052–2054. doi:10.1176/appi.ajp.157.12.2052
113. Volkow ND, Fowler JS, Logan J, et al. Effects of modafinil on dopamine and dopamine transporters in the male human brain: clinical implications. JAMA. 2009;301(11):1148–1154. doi:10.1001/jama.2009.351
114. Kalechstein AD, Mahoney JJ, Yoon JH, Bennett R, De la Garza R. Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology. 2013;64:472–478. doi:10.1016/j.neuropharm.2012.06.064
115. Anderson AL, Reid MS, Li SH, et al. Modafinil for the treatment of cocaine dependence. Drug Alcohol Depend. 2009;104(1–2):133–139. doi:10.1016/j.drugalcdep.2009.04.015
116. Dackis CA, Kampman KM, Lynch KG, Pettinati HM, O’Brien CP. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology. 2005;30(1):205–211. doi:10.1038/sj.npp.1300600
117. Dackis CA, Kampman KM, Lynch KG, et al. A double-blind, placebo-controlled trial of modafinil for cocaine dependence. J Subst Abuse Treat. 2012;43(3):303–312. doi:10.1016/j.jsat.2011.12.014
118. Sangroula D, Motiwala F, Wagle B, Shah VC, Hagi K, Lippmann S. Modafinil treatment of cocaine dependence: a systematic review and meta-analysis. Subst Use Misuse. 2017;52(10):1292–1306. doi:10.1080/10826084.2016.1276597
119. Indave BI, Minozzi S, Pani PP, Amato L. Antipsychotic medications for cocaine dependence. Cochrane Database Syst Rev. 2016;3:CD006306. doi:10.1002/14651858.CD006306.pub3
120. Drake RE, Xie H, McHugo GJ, Green AI. The effects of clozapine on alcohol and drug use disorders among patients with schizophrenia. Schizophr Bull. 2000;26(2):441–449. doi:10.1093/oxfordjournals.schbul.a033464
121. Zimmet SV, Strous RD, Burgess ES, Kohnstamm S, Green AI. Effects of clozapine on substance use in patients with schizophrenia and schizoaffective disorder: a retrospective survey. J Clin Psychopharmacol. 2000;20(1):94–98. doi:10.1097/00004714-200002000-00016
122. Minozzi S, Cinquini M, Amato L, et al. Anticonvulsants for cocaine dependence. Cochrane Database Syst Rev. 2015;2015(4):CD006754.
123. Baldacara L, Cogo-Moreira H, Parreira BL, et al. Efficacy of topiramate in the treatment of crack cocaine dependence: a double-blind, randomized, placebo-controlled trial. J Clin Psychiatry. 2016;77(3):398–406. doi:10.4088/JCP.14m09377
124. Petrakis IL, Carroll KM, Nich C, et al. Disulfiram treatment for cocaine dependence in methadone-maintained opioid addicts. Addiction. 2000;95(2):219–228. doi:10.1046/j.1360-0443.2000.9522198.x
125. Pettinati HM, Kampman KM, Lynch KG, et al. A double blind, placebo-controlled trial that combines disulfiram and naltrexone for treating co-occurring cocaine and alcohol dependence. Addict Behav. 2008;33(5):651–667. doi:10.1016/j.addbeh.2007.11.011
126. Carroll KM, Fenton LR, Ball SA, et al. Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry. 2004;61(3):264–272. doi:10.1001/archpsyc.61.3.264
127. Dakwar E, Nunes EV, Hart CL, et al. A single ketamine infusion combined with mindfulness-based behavioral modification to treat cocaine dependence: a randomized clinical trial. Am J Psychiatry. 2019;176(11):923–930. doi:10.1176/appi.ajp.2019.18101123
128. Carroll KM. Therapy Manuals for Drug Addiction, Manual 1: A Cognitive-Behavioral Approach: Treating Cocaine Addiction. National Institute on Drug Abuse; 1998:55–65.
129. Higgins ST, Delaney DD, Budney AJ, et al. A behavioral approach to achieving initial cocaine abstinence. Am J Psychiatry. 1991;148(9):1218–1224.
130. Higgins ST, Kurti AN, Davis DR. Voucher-based contingency management is efficacious but underutilized in treating addictions. Perspect Behav Sci. 2019;42(3):501–524. doi:10.1007/s40614-019-00216-z
131. Stitzer M, Bigelow G. Contingency management in a methadone maintenance program: availability of reinforcers. Int J Addictions. 1978;13(5):737–746. doi:10.3109/10826087809039299
132. Petry NM, Alessi SM, Ledgerwood DM. Contingency management delivered by community therapists in outpatient settings. Drug Alcohol Depend. 2012;122(1–2):86–92. doi:10.1016/j.drugalcdep.2011.09.015
133. Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51(12):989–997. doi:10.1001/archpsyc.1994.03950120061010
134. Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ. Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction. 1998;93(5):713–727. doi:10.1046/j.1360-0443.1998.9357137.x
135. Stitzer ML, Bickel WK, Bigelow GE, Liebson IA. Effect of methadone dose contingencies on urinalysis test results of polydrug-abusing methadone-maintenance patients. Drug Alcohol Depend. 1986;18(4):341–348. doi:10.1016/0376-8716(86)90097-9
136. Carroll KM. Lost in translation? Moving contingency management and cognitive behavioral therapy into clinical practice. Ann N Y Acad Sci. 2014;1327(1):94. doi:10.1111/nyas.12501
137. Stitzer ML, Grabowski J, Henningfield JE. Behavioral intervention techniques in drug abuse treatment: summary of discussion. NIDA research monograph. 1984.
138. Budney AJ, Higgins ST. Therapy Manual for Drug Addiction Manual 2: A Community Reinforcement Plus Vouchers Approach: Treating Cocaine Addiction. Rockville, MD: National Institute on Drug Abuse; 1998.
139. Heil SH, Davis DR, Arger CA, Higgins ST. Contingency management and the community reinforcement approach. In: Miller SC, Fiellin DA, Rosenthal RN, Saitz R, editors. The ASAM Principles of Addiction Medicine.
140. Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry. 1994;51(7):568–576. doi:10.1001/archpsyc.1994.03950070060011
141. Petry NM. Contingency Management for Substance Abuse Treatment: A Guide to Implementing This Evidence-Based Practice. Routledge; 2013.
142. Petry NM, Martin B, Cooney JL, Kranzler HR. Give them prizes and they will come: contingency management for treatment of alcohol dependence. J Consult Clin Psychol. 2000;68(2):250. doi:10.1037/0022-006X.68.2.250
143. Petry NM, Barry D, Alessi SM, Rounsaville BJ, Carroll KM. A randomized trial adapting contingency management targets based on initial abstinence status of cocaine-dependent patients. J Consult Clin Psychol. 2012;80(2):276. doi:10.1037/a0026883
144. Petry NM, Weinstock J, Alessi SM. A randomized trial of contingency management delivered in the context of group counseling. J Consult Clin Psychol. 2011;79(5):686. doi:10.1037/a0024813
145. Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta‐analysis of voucher‐based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi:10.1111/j.1360-0443.2006.01311.x
146. Higgins ST, Heil SH, Dantona R, Donham R, Matthews M, Badger GJ. Effects of varying the monetary value of voucher‐based incentives on abstinence achieved during and following treatment among cocaine‐dependent outpatients. Addiction. 2007;102(2):271–281. doi:10.1111/j.1360-0443.2006.01664.x
147. Higgins ST, Wong CJ, Badger GJ, Ogden DEH, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol. 2000;68(1):64. doi:10.1037/0022-006X.68.1.64
148. Bentzley BS, Han SS, Neuner S, Humphreys K, Kampman KM, Halpern CH. Comparison of treatments for cocaine use disorder among adults: a systematic review and meta-analysis. JAMA Network Open. 2021;4(5):e218049–e218049. doi:10.1001/jamanetworkopen.2021.8049
149. De Crescenzo F, Ciabattini M, D’Alò GL, et al. Comparative efficacy and acceptability of psychosocial interventions for individuals with cocaine and amphetamine addiction: a systematic review and network meta-analysis. PLoS Med. 2018;15(12):e1002715. doi:10.1371/journal.pmed.1002715
150. DePhilippis D, Petry NM, Bonn-Miller MO, Rosenbach SB, McKay JR. The national implementation of Contingency Management (CM) in the department of veterans affairs: attendance at CM sessions and substance use outcomes. Drug Alcohol Depend. 2018;185:367–373. doi:10.1016/j.drugalcdep.2017.12.020
151. Farronato NS, Dürsteler-MacFarland KM, Wiesbeck GA, Petitjean SA. A systematic review comparing cognitive-behavioral therapy and contingency management for cocaine dependence. J Addict Dis. 2013;32(3):274–287. doi:10.1080/10550887.2013.824328
152. Petry NM, Alessi SM, Hanson T, Sierra S. Randomized trial of contingent prizes versus vouchers in cocaine-using methadone patients. J Consult Clin Psychol. 2007;75(6):983. doi:10.1037/0022-006X.75.6.983
153. Rash CJ, Alessi SM, Petry NM. Cocaine abusers with and without alcohol dependence respond equally well to contingency management treatments. Exp Clin Psychopharmacol. 2008;16(4):275. doi:10.1037/a0012787
154. Weiss LM, Petry NM. Interaction effects of age and contingency management treatments in cocaine-dependent outpatients. Exp Clin Psychopharmacol. 2011;19(2):173. doi:10.1037/a0023031
155. Petry NM, Peirce JM, Stitzer ML, et al. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch Gen Psychiatry. 2005;62(10):1148–1156. doi:10.1001/archpsyc.62.10.1148
156. Petry NM. Contingency management treatments: controversies and challenges. 2010.
157. Roman P, Blum T. National Treatment Center Study: Summary Report. Institute for Behavioral Research, University of Georgia; 1997.
158. Roman PM, Johnson J. National Treatment Center Study Summary Report: Private Treatment Centers. Athens, GA: Institute for Behavioral Research, University of Georgia; 2004.
159. Rash CJ, Petry NM, Kirby KC, Martino S, Roll J, Stitzer ML. Identifying provider beliefs related to contingency management adoption using the contingency management beliefs questionnaire. Drug Alcohol Depend. 2012;121(3):205–212. doi:10.1016/j.drugalcdep.2011.08.027
160. Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta-analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165(2):179–187. doi:10.1176/appi.ajp.2007.06111851
161. Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi:10.1111/j.1360-0443.2006.01581.x
162. Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL. Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol. 2000;68(1):64–72.
163. Rawson RA, Huber A, McCann M, et al. A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry. 2002;59(9):817–824. doi:10.1001/archpsyc.59.9.817
164. Rawson RA, McCann MJ, Flammino F, et al. A comparison of contingency management and cognitive‐behavioral approaches for stimulant‐dependent individuals. Addiction. 2006;101(2):267–274. doi:10.1111/j.1360-0443.2006.01312.x
165. Petry NM, Tedford J, Austin M, Nich C, Carroll KM, Rounsaville BJ. Prize reinforcement contingency management for treating cocaine users: how low can we go, and with whom? Addiction. 2004;99(3):349–360. doi:10.1111/j.1360-0443.2003.00642.x
166. Chaney EF, O’Leary MR, Marlatt GA. Skill training with alcoholics. J Consult Clin Psychol. 1978;46(5):1092. doi:10.1037/0022-006X.46.5.1092
167. Marlatt GA, Donovan DM. Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. Guilford Press; 2005.
168. DeMarce J, Gnys M, Raffa S, Karlin B. Cognitive Behavioral Therapy for Substance Use Disorders Among Veterans: Therapist Manual. Washington, DC: US Department of Veterans Affairs; 2014.
169. McHugh RK, Kim J, Perrins SP, Weiss RD. Group therapy for substance use disorders. In: Textbook of Addiction Treatment. Springer; 2021:433–446.
170. Petitjean SA, Dürsteler-MacFarland KM, Krokar MC, et al. A randomized, controlled trial of combined cognitive-behavioral therapy plus prize-based contingency management for cocaine dependence. Drug Alcohol Depend. 2014;145:94–100. doi:10.1016/j.drugalcdep.2014.09.785
171. Carroll K, Nich C, Rounsaville B. Use of observer and therapist ratings to monitor delivery of coping skills treatment for cocaine abusers: utility of therapist session checklists. Psychother Res. 1998;8:307–320. doi:10.1093/ptr/8.3.307
172. Gonzalez VM, Schmitz JM, DeLaune KA. The role of homework in cognitive-behavioral therapy for cocaine dependence. J Consult Clin Psychol. 2006;74(3):633. doi:10.1037/0022-006X.74.3.633
173. Carroll KM, Nich C, Ball SA. Practice makes progress? Homework assignments and outcome in treatment of cocaine dependence. J Consult Clin Psychol. 2005;73(4):749. doi:10.1037/0022-006X.73.4.749
174. Decker SE, Kiluk BD, Frankforter T, Babuscio T, Nich C, Carroll KM. Just showing up is not enough: homework adherence and outcome in cognitive–behavioral therapy for cocaine dependence. J Consult Clin Psychol. 2016;84(10):907. doi:10.1037/ccp0000126
175. Carroll KM, Nich C, Sifry RL, et al. A general system for evaluating therapist adherence and competence in psychotherapy research in the addictions. Drug Alcohol Depend. 2000;57(3):225–238. doi:10.1016/S0376-8716(99)00049-6
176. Sholomskas DE, Syracuse-Siewert G, Rounsaville BJ, Ball SA, Nuro KF, Carroll KM. We don’t train in vain: a dissemination trial of three strategies of training clinicians in cognitive-behavioral therapy. J Consult Clin Psychol. 2005;73(1):106. doi:10.1037/0022-006X.73.1.106
177. Santa Ana EJ, Martino S, Ball SA, Nich C, Frankforter TL, Carroll KM. What is usual about “treatment-as-usual”? Data from two multisite effectiveness trials. J Subst Abuse Treat. 2008;35(4):369–379. doi:10.1016/j.jsat.2008.01.003
178. Carroll KM, Ball SA, Martino S, et al. Computer-assisted delivery of cognitive-behavioral therapy for addiction: a randomized trial of CBT4CBT. Am J Psychiatry. 2008;165(7):881–888. doi:10.1176/appi.ajp.2008.07111835
179. Carroll KM. CBT4CBT, LLC: developers of computer based training for cognitive behavioral therapy; 2019. Available from: https://cbt4cbt.com/.
180. Kiluk BD, DeVito EE, Buck MB, Hunkele K, Nich C, Carroll KM. Effect of computerized cognitive behavioral therapy on acquisition of coping skills among cocaine-dependent individuals enrolled in methadone maintenance. J Subst Abuse Treat. 2017;82:87–92. doi:10.1016/j.jsat.2017.09.011
181. Kiluk BD, Nich C, Buck MB, et al. Randomized clinical trial of computerized and clinician-delivered CBT in comparison with standard outpatient treatment for substance use disorders: primary within-treatment and follow-up outcomes. Am J Psychiatry. 2018;175(9):853–863. doi:10.1176/appi.ajp.2018.17090978
182. Carroll KM, Ball SA, Martino S, Nich C, Babuscio TA, Rounsaville BJ. Enduring effects of a computer-assisted training program for cognitive behavioral therapy: a 6-month follow-up of CBT4CBT. Drug Alcohol Depend. 2009;100(1–2):178–181. doi:10.1016/j.drugalcdep.2008.09.015
183. Carroll KM, Kiluk BD, Nich C, et al. Computer-assisted delivery of cognitive-behavioral therapy: efficacy and durability of CBT4CBT among cocaine-dependent individuals maintained on methadone. Am J Psychiatry. 2014;171(4):436–444. doi:10.1176/appi.ajp.2013.13070987
184. Carroll KM, Nich C, DeVito EE, Shi JM, Sofuoglu M. Galantamine and computerized cognitive behavioral therapy for cocaine dependence: a randomized clinical trial. J Clin Psychiatry. 2018;79(1):17m11669. doi:10.4088/JCP.17m11669
185. DeVito EE, Kiluk BD, Nich C, Mouratidis M, Carroll KM. Drug stroop: mechanisms of response to computerized cognitive behavioral therapy for cocaine dependence in a randomized clinical trial. Drug Alcohol Depend. 2018;183:162–168. doi:10.1016/j.drugalcdep.2017.10.022
186. Olmstead TA, Ostrow CD, Carroll KM. Cost-effectiveness of computer-assisted training in cognitive-behavioral therapy as an adjunct to standard care for addiction. Drug Alcohol Depend. 2010;110(3):200–207. doi:10.1016/j.drugalcdep.2010.02.022
187. Horsfall J, Cleary M, Hunt GE, Walter G. Psychosocial treatments for people with co-occurring severe mental illnesses and substance use disorders (dual diagnosis): a review of empirical evidence. Harv Rev Psychiatry. 2009;17(1):24–34. doi:10.1080/10673220902724599
188. Drake RE, Essock SM, Shaner A, et al. Implementing dual diagnosis services for clients with severe mental illness. Psychiatr Serv. 2001;52(4):469–476. doi:10.1176/appi.ps.52.4.469
189. Mangrum LF, Spence RT, Lopez M. Integrated versus parallel treatment of co-occurring psychiatric and substance use disorders. J Subst Abuse Treat. 2006;30(1):79–84. doi:10.1016/j.jsat.2005.10.004
190. Hunt GE, Siegfried N, Morley K, Brooke-Sumner C, Cleary M. Psychosocial interventions for people with both severe mental illness and substance misuse. Cochrane Database Syst Rev. 2019;12:CD001088. doi:10.1002/14651858.CD001088.pub4
191. Mueser KT, Torrey WC, Lynde D, Singer P, Drake RE. Implementing evidence-based practices for people with severe mental illness. Behav Modif. 2003;27(3):387–411. doi:10.1177/0145445503027003007
192. Lambert-Harris C, Saunders EC, McGovern MP, Xie H. Organizational capacity to address co-occurring substance use and psychiatric disorders: assessing variation by level of care. J Addict Med. 2013;7(1):25–32. doi:10.1097/ADM.0b013e318276e7a4
193. McGovern MP, Lambert-Harris C, Gotham HJ, Claus RE, Xie H. Dual diagnosis capability in mental health and addiction treatment services: an assessment of programs across multiple state systems. Adm Policy Ment Health. 2014;41(2):205–214. doi:10.1007/s10488-012-0449-1
194. Kosten T, Oliveto A, Feingold A, et al. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70(3):315–325. doi:10.1016/S0376-8716(03)00032-2
195. Schmitz JM, Rhoades HM, Elk R, Creson D, Hussein I, Grabowski J. Medication take-home doses and contingency management. Exp Clin Psychopharmacol. 1998;6(2):162–168. doi:10.1037/1064-1297.6.2.162
196. Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive function as a transdiagnostic treatment target in stimulant use disorders. J Dual Diagn. 2016;12(1):90–106. doi:10.1080/15504263.2016.1146383
197. Schumann G, Binder EB, Holte A, et al. Stratified medicine for mental disorders. Eur Neuropsychopharmacol. 2014;24(1):5–50. doi:10.1016/j.euroneuro.2013.09.010
198. Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR. Cognitive bias modification and cognitive control training in addiction and related psychopathology: mechanisms, clinical perspectives, and ways forward. Clin Psychol Sci. 2013;1:912–212. doi:10.1177/2167702612466547
199. Mayer AR, Wilcox CE, Dodd AB, et al. The efficacy of attention bias modification therapy in cocaine use disorders. Am J Drug Alcohol Abuse. 2016;42(4):459–468. doi:10.3109/00952990.2016.1151523
200. Marhe R, Waters AJ, van de Wetering BJ, Franken IH. Implicit and explicit drug-related cognitions during detoxification treatment are associated with drug relapse: an ecological momentary assessment study. J Consult Clin Psychol. 2013;81(1):1–12. doi:10.1037/a0030754
201. Levi Bolin B, Alcorn JL, Lile JA, et al. N-Acetylcysteine reduces cocaine-cue attentional bias and differentially alters cocaine self-administration based on dosing order. Drug Alcohol Depend. 2017;178:452–460. doi:10.1016/j.drugalcdep.2017.05.039
202. Sofuoglu M, Mooney M. Cholinergic functioning in stimulant addiction: implications for medications development. CNS Drugs. 2009;23(11):939–952. doi:10.2165/11310920-000000000-00000
203. Sofuoglu M, Waters AJ, Poling J, Carroll KM. Galantamine improves sustained attention in chronic cocaine users. Exp Clin Psychopharmacol. 2011;19(1):11–19. doi:10.1037/a0022213
204. Mahoney JJ, Kalechstein AD, Verrico CD, Arnoudse NM, Shapiro BA, De La Garza R. Preliminary findings of the effects of rivastigmine, an acetylcholinesterase inhibitor, on working memory in cocaine-dependent volunteers. Prog Neuropsychopharmacol Biol Psychiatry. 2014;50:137–142. doi:10.1016/j.pnpbp.2013.11.001
205. Sofuoglu M, Carroll KM. Effects of galantamine on cocaine use in chronic cocaine users. Am J Addict. 2011;20(3):302–303. doi:10.1111/j.1521-0391.2011.00130.x
206. Carroll KM, DeVito EE, Yip SW, Nich C, Sofuoglu M. Double-blind placebo-controlled trial of galantamine for methadone-maintained individuals with cocaine use disorder: secondary analysis of effects on illicit opioid use. Am J Addict. 2019;28(4):238–245. doi:10.1111/ajad.12904
207. DeVito EE, Carroll KM, Babuscio T, Nich C, Sofuoglu M. Randomized placebo-controlled trial of galantamine in individuals with cocaine use disorder. J Subst Abuse Treat. 2019;107:29–37. doi:10.1016/j.jsat.2019.08.009
208. Goldstein RZ, Woicik PA, Maloney T, et al. Oral methylphenidate normalizes cingulate activity in cocaine addiction during a salient cognitive task. Proc Natl Acad Sci U S A. 2010;107(38):16667–16672. doi:10.1073/pnas.1011455107
209. Li CS, Morgan PT, Matuskey D, et al. Biological markers of the effects of intravenous methylphenidate on improving inhibitory control in cocaine-dependent patients. Proc Natl Acad Sci U S A. 2010;107(32):14455–14459. doi:10.1073/pnas.1002467107
210. Dursteler KM, Berger EM, Strasser J, et al. Clinical potential of methylphenidate in the treatment of cocaine addiction: a review of the current evidence. Subst Abuse Rehabil. 2015;6:61–74. doi:10.2147/SAR.S50807
211. Levin FR, Evans SM, Brooks DJ, Garawi F. Treatment of cocaine dependent treatment seekers with adult ADHD: double-blind comparison of methylphenidate and placebo. Drug Alcohol Depend. 2007;87(1):20–29. doi:10.1016/j.drugalcdep.2006.07.004
212. Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159(4):397–406. doi:10.1007/s00213-001-0944-7
213. Sofuoglu M, Mouratidis M, Mooney M. Progesterone improves cognitive performance and attenuates smoking urges in abstinent smokers. Psychoneuroendocrinology. 2011;36(1):123–132. doi:10.1016/j.psyneuen.2010.07.005
214. Sofuoglu M, Dudish-Poulsen S, Nelson D, Pentel PR, Hatsukami DK. Sex and menstrual cycle differences in the subjective effects from smoked cocaine in humans. Exp Clin Psychopharmacol. 1999;7(3):274–283. doi:10.1037/1064-1297.7.3.274
215. Peltier MR, Sofuoglu M. Role of exogenous progesterone in the treatment of men and women with substance use disorders: a narrative review. CNS Drugs. 2018;32(5):421–435. doi:10.1007/s40263-018-0525-5
216. Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180(1):169–176. doi:10.1007/s00213-004-2129-7
217. Sherman BJ, Baker NL, Brady KT, Joseph JE, Nunn LM, McRae-Clark A. The effect of oxytocin, gender, and ovarian hormones on stress reactivity in individuals with cocaine use disorder. Psychopharmacology. 2020;237(7):2031–2042. doi:10.1007/s00213-020-05516-w
218. Potenza MN, Hong KI, Lacadie CM, Fulbright RK, Tuit KL, Sinha R. Neural correlates of stress-induced and cue-induced drug craving: influences of sex and cocaine dependence. Am J Psychiatry. 2012;169(4):406–414. doi:10.1176/appi.ajp.2011.11020289
219. Brewer JA, Sinha R, Chen JA, et al. Mindfulness training and stress reactivity in substance abuse: results from a randomized, controlled stage I pilot study. Substance Abuse. 2009;30(4):306–317. doi:10.1080/08897070903250241
220. Diana M, Raij T, Melis M, Nummenmaa A, Leggio L, Bonci A. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci. 2017;18(11):685–693. doi:10.1038/nrn.2017.113
221. Bolloni C, Badas P, Corona G, Diana M. Transcranial magnetic stimulation for the treatment of cocaine addiction: evidence to date. Subst Abuse Rehabil. 2018;9:11–21. doi:10.2147/SAR.S161206
222. Rachid F. Neurostimulation techniques in the treatment of cocaine dependence: a review of the literature. Addict Behav. 2018;76:145–155. doi:10.1016/j.addbeh.2017.08.004
223. Lupi M, Martinotti G, Santacroce R, et al. Transcranial direct current stimulation in substance use disorders: a systematic review of scientific literature. J ECT. 2017;33(3):203–209. doi:10.1097/YCT.0000000000000401
224. Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur Neuropsychopharmacol. 2016;26(1):37–44. doi:10.1016/j.euroneuro.2015.11.011
225. Rapinesi C, Kotzalidis GD, Ferracuti S, et al. Add-on high frequency deep transcranial magnetic stimulation (dTMS) to bilateral prefrontal cortex in depressive episodes of patients with major depressive disorder, bipolar disorder I, and major depressive with alcohol use disorders. Neurosci Lett. 2018;671:128–132. doi:10.1016/j.neulet.2018.02.029
226. Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict. 2008;17(4):345–346. doi:10.1080/10550490802139283
227. Camprodon JA, Martinez-Raga J, Alonso-Alonso M, Shih MC, Pascual-Leone A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 2007;86(1):91–94. doi:10.1016/j.drugalcdep.2006.06.002
228. Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM. A randomized placebo-controlled trial of targeted prefrontal cortex modulation with bilateral tDCS in patients with crack-cocaine dependence. Int J Neuropsychopharmacol. 2015;18(12):pyv066. doi:10.1093/ijnp/pyv066
229. Gorini A, Lucchiari C, Russell-Edu W, Pravettoni G. Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci. 2014;8:661. doi:10.3389/fnhum.2014.00661
230. Shen Y, Ward HB. Transcranial magnetic stimulation and neuroimaging for cocaine use disorder: review and future directions. Am J Drug Alcohol Abuse. 2021;47(2):144–153. doi:10.1080/00952990.2020.1841784
231. Kinsey BM, Kosten TR, Orson FM. Anti-cocaine vaccine development. Expert Rev Vaccines. 2010;9(9):1109–1114. doi:10.1586/erv.10.102
232. Shen XY, Orson FM, Kosten TR. Vaccines against drug abuse. Clin Pharmacol Ther. 2012;91(1):60–70. doi:10.1038/clpt.2011.281
233. Martell BA, Orson FM, Poling J, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiatry. 2009;66(10):1116–1123. doi:10.1001/archgenpsychiatry.2009.128
234. Kosten TR, Domingo CB, Shorter D, et al. Vaccine for cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Drug Alcohol Depend. 2014;140:42–47. doi:10.1016/j.drugalcdep.2014.04.003
235. Jones JD, Comer SD. A review of pharmacogenetic studies of substance-related disorders. Drug Alcohol Depend. 2015;152:1–14. doi:10.1016/j.drugalcdep.2015.03.003
236. Schmitz JM, Mooney ME, Green CE, et al. Baseline neurocognitive profiles differentiate abstainers and non-abstainers in a cocaine clinical trial. J Addict Dis. 2009;28(3):250–257. doi:10.1080/10550880903028502
237. (SAHMSA) SAaMHSA. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. SAaMHSA; 2019.
238. Mee-Lee D, Shulman G, Fishman M. The ASAM criteria. In: Treatment Criteria for Addictive, Substance-Related, and Co-Occurring Conditions.
239. (SAMHSA) SAaMHSA. SAMHSA working definition of recovery: 10 guiding principles of recovery. In: Services DoHaH. Rockville: SAaMHSA; 2012.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.