Back to Journals » Infection and Drug Resistance » Volume 14
Coadministration of Ginger Extract and Fluconazole Shows a Synergistic Effect in the Treatment of Drug-Resistant Vulvovaginal Candidiasis
Authors Khan A , Azam M, Allemailem KS , Alrumaihi F , Almatroudi A , Alhumaydhi FA , Ahmad HI, Khan MU, Khan MA
Received 5 February 2021
Accepted for publication 30 March 2021
Published 21 April 2021 Volume 2021:14 Pages 1585—1599
DOI https://doi.org/10.2147/IDR.S305503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Arif Khan,1,* Mohd Azam,2,* Khaled S Allemailem,2 Faris Alrumaihi,2 Ahmad Almatroudi,2 Fahad A Alhumaydhi,2 Hafiz Iqtidar Ahmad,3 Masih Uzzaman Khan,4 Masood Alam Khan1,*
1Department of Basic Health Sciences, College of Applied Medical Sciences, Qassim University, Buraydah, 51452, Saudi Arabia; 2Department of Medical Laboratories, College of Applied Medical Sciences, Qassim University, Buraydah, 51452, Saudi Arabia; 3Department of Tashreeh Wa Munafeul Aza, Faculty of Unani Medicine, Aligarh Muslim University, Aligarh, 202002, India; 4Department of Pharmaceutical Chemistry & Pharmacognosy, Unaizah College of Pharmacy, Qassim University, Buraydah, 51452, Saudi Arabia
*These authors contributed equally to this work
Correspondence: Masood Alam Khan
College of Applied Medical Sciences, Qassim University, Buraydah, 51452, Saudi Arabia
Tel +966-507059437
Email [email protected]
Background: Azoles are the most common antifungal drugs used in the treatment of vulvovaginal candidiasis (VVC). The frequency of azole-resistant Candida isolates has increased dramatically in the last two decades. Here, we assessed the antifungal activity of a combination of fluconazole (FLZ) and methanolic extract of ginger (Meth-Gin) against drug-resistant vulvovaginal candidiasis (VVC) in a murine model.
Methods: The in vitro activity of FLZ or a combination of FLZ and Meth-Gin was determined against Candida albicans by the agar well diffusion, macrodilution, time-kill and the biofilm eradication methods. The therapeutic efficacy of the formulations was assessed by analyzing the fungal load, pro-inflammatory cytokines, percent apoptotic cells and the histological changes in the vaginal tissues of the mice. Moreover, the renal toxicity the drug formulation was evaluated by analyzing the levels of the blood urea nitrogen (BUN) and creatinine.
Results: The results of in vitro study demonstrated that FLZ did not show any activity against C. albicans, whereas a combination of FLZ and Meth-Gin demonstrated greater activity as shown by the data of the zone of growth inhibition, MIC and time-kill assay. FLZ or Meth-Gin treatment could not completely cure VVC, whereas a combination of FLZ and Meth-Gin was greatly effective in the treatment of VVC. The vaginal tissue from mice of the infected control group had the highest fungal load of 155370 ± 20617 CFUs. Treatment with FLZ at a dose of 40 mg/kg reduced the fungal load to 120863 ± 10723 CFUs. Interestingly, the mice treated with a combination of FLZ (40 mg/kg) and Meth-Gin (200 mg/kg) had a fungal load of 256 ± 152 CFUs. Besides, FLZ and Meth-Gin combination effectively reduced the pro-inflammatory cytokines (IL-1β, TNF-α and IL-17) and the percentage of apoptotic cells in the vaginal tissues. Likewise, the histological analysis revealed the epithelial necrosis, shedding and ulceration in the vaginal tissue, whereas treatment with FLZ and Meth-Gin combination reversed the histopathological changes in the vaginal epithelium and lamina propria.
Conclusion: The findings of the current study suggest that the co-administration of Meth-Gin and FLZ may have a potential therapeutic effect in the treatment of azole-resistant candidiasis.
Keywords: fluconazole, candidiasis, ginger extract, inflammation, cytokines
Introduction
The occurrence of Candida infections has lately amplified due to a sharp rise in the numbers of immunocompromised patients.1–3 Candida albicans is the most common fungal pathogen that causes a vaginal infection called “Vulvovaginal candidiasis (VVC)”. Beside C. albicans, C. glabrata induced VVC has increased about 27% from 1990 to 2009.4,5 Azoles are the most frequently used antifungal drugs that target enzyme 14α–demethylase and lower the levels of ergosterol in C. albicans.6 Moreover, azoles also increase the reactive oxygen species (ROS) levels in the fungal biofilm.7 But their extensive use has resulted in the emergence of azole-resistant Candida isolates8,9 Besides attempting to search the new antifungal chemotherapeutics, the rejuvenation of the existing antifungal drugs also becomes important to compensate the depleted armory of the antifungal drugs.10 The use of antibiotic combinations seems to be an important strategy to counter the phenomenon of drug resistance.11–13 Chloroquine (CQ) and fluconazole (FLZ) exhibited a synergistic effect against FLZ-resistant isolates of C. albicans.14 We earlier showed that liposomal-CQ increased the efficacy of FLZ against less susceptible isolate of Cryptococcus neoformans.15
In recent years, the use of plant-derived medicines has been extensively explored in the treatment of infectious diseases.16–18 A combination of phytochemicals with standard antibiotics has been shown to be very beneficial to fight drug-resistant pathogens.19–22 The combination of menthol with nystatin and itraconazole showed a synergistic effect against the clinical isolates of Candida spp.23 Moreover, the essential oil of Mentha suaveolens exhibited activity against the murine vaginal candidiasis.24 Donadu et al demonstrated that Helichrysum microphyllum and Hypericum perforatum oils demonstrated antifungal activity against Candida isolates taken from the patients of candidiasis.25 Moreover, essential oils from Hornstedtia bella and Atalantia sessiflora have shown their activity against Candida spp.26,27 Ginger (Zingiber officinale) is an important spice and a medicinal herb that possesses the antioxidant, anti-inflammatory and hepatoprotective properties.28 An aqueous extract of ginger exhibited an anti-biofilm formation in bacteria, including Escherichia coli, Salmonella typhimurium and Pseudomonas aeruginosa.29 Ginger contains many constituents that are blended with many antimicrobial activities30–35 (Table 1). Ginger components such as 6-gingerols and 6-shogaol have shown their efficacy in inhibiting the biofilm and hyphae formation in C. albicans.30 Moreover, an ethanolic extract of ginger showed the inhibitory effect against the biofilm formation in C. albicans and C. krusei.36 In the present study, we have evaluated the effect of methanolic extract of ginger (Meth-Gin) in combination with FLZ against VVC in a mouse model. The findings of the present study demonstrated that coadministration of Meth-Gin remarkably augmented the activity of FLZ against murine VVC.
 |
Table 1 Principal Constituents of Ginger and Their Antimicrobial Activity |
Materials and Methods
Materials
Fluconazole was purchased from the Santa Cruz Biotechnology (Dallas, TX, USA). Sabouraud dextrose agar and Tryptic soya broth were obtained from Hi Media (Mumbai, India). Estradiol valerate and other chemicals were bought from Sigma-Aldrich (St. Louis, USA). ELISA kits for IL-1β, TNF-α and IL-17 were purchased from Abcam (Cambridge, UK).
Preparation of Methanolic Extract of Ginger
The dried ginger powder was defatted by using cyclohexane followed by stirring for 3 hours. The powder was placed in the Soxhlet extraction system (Buchi laboratories, Flawil, Switzerland) for 48 hours, adding the methanol as a solvent in the collecting beaker. The remaining solvent was evaporated completely under the reduced pressure using vacuum controlled rotary evaporator to remove the traces of methanol.
Test Strain
An isolate of C. albicans (KFH 121) was obtained from the Microbiology Laboratory of the King Fahad Hospital, Buraydah, Saudi Arabia. The isolate was maintained on Sabouraud Dextrose Agar (SDA). The identification of C. albicans was confirmed by the germ tube induction test.
Antifungal Susceptibility Testing
The in vitro activity of FLZ or Meth-Gin or a combination of FLZ and Meth-Gin was determined by the agar well diffusion and macrodilution methods.8 C. albicans was seeded on the SDA plates and the holes of 8 mm diameter were made to load 100 µL of FLZ (100 µg) or Meth-Gin (500 µg) or FLZ (100 µg) + Meth-Gin (250 µg) and FLZ (100 µg) + Meth-Gin (500 µg). The plates were incubated at 37 °C for 24 hours and the zone of growth inhibition was measured. The minimum inhibitory concentration (MIC) of FLZ and Meth-Gin were determined by the broth macrodilution antifungal susceptibility method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI), Wayne.37 The concentrations of FLZ were taken in the range of 0.5 to 256 µg/mL, whereas Meth-Gin was taken in the range of 20 µg/mL to 4 mg/mL. A 100 μL of yeast suspension containing 1 × 105 CFUs of C. albicans was inoculated in the test tubes containing 3 mL of Tryptic soya broth (TSB) with various concentrations of FLZ or Meth-Gin. The MIC of FLZ or Met-Gin was considered the lowest drug concentration that showed 50% inhibition in C. albicans growth. In order to assess any synergy between FLZ and Meth-Gin, a combination of above mentioned concentration of FLZ and Meth-Gin were taken and their activity against C. albicans was determined. The concentration of FLZ and Meth-Gin in which there was 50% growth inhibition was considered the MIC value.
To Analyze a Synergy Between FLZ and Meth-Gin
The fractional inhibitory concentration index (FICI) was used to evaluate the interaction of FLZ and Meth-Gin. The concentration ranges of FLZ and Meth-Gin were same as mentioned above to determine the MIC value. The FIC index was expressed using the following equation:
ƩFIC = FIC-FLZ + FIC-Meth-Gin = MICFLZcomb/MICFLZalone + MICMeth-Gin comb/MICMeth-Gin alone
where MICFLZ alone and MICMeth-Gin alone are MIC values for FLZ and Meth-Gin used alone. MICFLZcomb and MICMeth-Gin comb are MICs of FLZ and Meth-Gin when used in combination. The MIC values of FLZ and Meth-Gin alone or in combinations were determined as the lowest concentrations of the drug that showed 50% reduction in C. albicans growth as compared to the growth in the absence of any drug.
The interaction between FLZ and Meth-Gin was interpreted as a synergistic when FIC index was less than 0.5. When FIC index was between 0.5 and 4, the interaction was considered indifferent, and as an antagonist when FIC index was more than 4.
Time-Kill Assay
After overnight culture, C. albicans was diluted with YPD medium and the culture was incubated at 37°C until the log-phase of growth was achieved. C. albicans suspension (5 × 105 CFUs) was transferred to the flasks containing 20 mL of YPD and FLZ (8 μg/mL) or Meth-Gin (2 mg/mL) or a combination of FLZ (8 μg/mL) and Meth-Gin (0.25, 0.5, 1 and 2 mg/mL). The flasks were kept in a shaker bath and samples (0.5 mL) were taken in duplicates at baseline and 1, 3, 6, 12, 24 hours. Samples were centrifuged at 5000 rpm for 15 minutes and reconstituted with sterile PBS to the original volumes to minimize any drug carryover effect. The numbers of CFUs were quantified by plating the serial dilutions onto SDA plates and incubated for 48 hours. The fungal density of each sample was determined by counting the CFUs.
Analysis of C. albicans Death by Confocal Microscopy
Candida albicans (1 × 105 CFUs) was cultured in TSB in the presence or absence of FLZ (16 μg/mL) or Meth-Gin (500 μg/mL) or a combination of FLZ-8 μg/mL + Meth-Gin-500 and FLZ-16 μg/mL + Meth-Gin-500 for 24 hours. After gentle washing with PBS, the cells were stained with propidium iodide (PI) and washed with PBS. The cells were observed and analyzed by confocal microscopy using 20× magnification objective as described earlier.38
Effect of FLZ or Meth-Gin or a Combination of FLZ and Meth-Gin on the Preformed C. albicans Biofilm
The effect of FLZ or Meth-Gin or a combination of FLZ and Meth-Gin on the preformed biofilm by C. albicans was analyzed as described earlier.39 C. albicans was cultured in TSB to obtain the density of approximately 1×106 CFUs/mL. C. albicans (100 µL) was inoculated into 96-well plate and incubated at 37°C for 24 hours. Without disrupting the biofilm, the culture medium was replaced with a fresh TSB containing FLZ (8, 16, 32 and 64 µg/mL) or Meth-Gin (250, 500, 1000 and 2000 µg/mL) or a combination of FLZ (8 μg/mL) and Meth-Gin (500, 1000 and 2000 µg/mL). The plates were incubated for 24 hours at 37°C, gently washed with sterile phosphate-buffered saline (PBS) and dried at room temperature for 20 minutes. The crystal violet solution (0.1%) was added to each well for 20 minutes to stain the biofilm followed by washing with PBS. After drying, 100 µL of 95% ethanol was added into each well in order to solubilize the stain. The optical density (OD) was measured at 595 nm (BIO-TEK EL310 Microplate Auto-Reader, Biotek instruments).
Mice
Female Swiss mice were obtained from the College of Applied Medical Sciences, Qassim University, Saudi Arabia. Mice were kept in the hygienic and pathogen-free conditions. The study was authorized by the ethical committee of the College of Applied Medical Sciences, Qassim University. The experiments were conducted following the guidelines of the University of London Animal Welfare Society, Wheathampstead, England.
Infection Model
A dose of 500 µg of estradiol valerate was dissolved in 100 µL of sesame oil and injected subcutaneously into each mouse. On 3 day post estradiol valerate injection, each mouse was infected with 5 × 106 CFUs of C. albicans through the intravaginal route as described in our earlier study.40
Therapeutic Efficacy of FLZ Against VVC
After 48 hours of infection, the mice in various groups were orally treated with FLZ at the doses of 10, 20 and 40 mg/kg for a week. Mice were divided into four groups and each group contained ten mice (n = 10).
1. Infected
2. FLZ-10 mg/kg
3. FLZ-20 mg/kg
4. FLZ-40 mg/kg
Therapeutic Activity of Meth-Gin in the Treatment of VVC
In a different experiment, infected mice from each group were treated with a daily dose of 50, 100 and 200 mg/kg of Meth-Gin through the oral route for a week. On day 8 post infection, three mice from each group were sacrificed and the vaginal tissues were excised to quantify the fungal load.40 Mice were divided into five groups and each group contained ten mice (n = 10).
1. Infected
2. Meth-Gin-50 mg/kg
3. Meth-Gin-100 mg/kg
4. Meth-Gin-200 mg/kg
5. Meth-Gin-400 mg/kg
To Assess the Efficacy and Safety of FLZ or Meth-Gin in the Treatment of VVC
On day 8 post-treatment, three mice from each group were sacrificed to take the vaginal tissue and blood. In order to determine the fungal burden, the vaginal tissues were homogenized in cold-PBS and various dilutions of tissue homogenates were spread on SDA plates. After 48 hours of incubation, the CFUs were counted and multiplied by the dilution factor.40 The levels of the blood urea nitrogen (BUN) and creatinine were determined to investigate the toxic effects of the treatment on kidney.8
Co-Administration of Meth-Gin and FLZ in the Treatment of VVC
Mice were treated with a single daily dose of a combination of Meth-Gin (100 mg/kg) and FLZ (20 and 40 mg/kg) for a week. On day 8 post infection, three mice from each group were sacrificed and their vaginal tissues were excised to determine the fungal load. Mice were divided into six groups and each group contained ten mice (n = 10).
1. Infected
2. Meth-Gin-200 mg/kg
3. FLZ-20 mg/kg
4. FLZ-40 mg/kg
5. Meth-Gin-200 mg/kg + FLZ-20 mg/kg
6. Meth-Gin-200 mg/kg + FLZ-40 mg/kg
To Assess the Efficacy and Safety of a Combination of Meth-Gin and FLZ
On day 8 post-treatment, three mice from each group were sacrificed and their vaginal tissue was taken out. The vaginal tissues were homogenized in cold-PBS and various dilutions of tissue homogenate were spread on SDA plates. The plates were incubated for 48 hours and the numbers of CFUs were counted.40 To understand the effect of treatment on the renal toxicity, the levels of blood urea nitrogen (BUN) and creatinine were analyzed in the blood as described earlier.41
Determination of Inflammatory Cytokines in the Vaginal Tissues
The vaginal tissues were homogenized in ice-cold lysis buffer containing protease inhibitor cocktail. The samples were centrifuged at 5000 rpm for 15 minutes. The supernatant was used to analyze IL-1β, TNF-α and IL-17 by the ELISA as described in our earlier study.42
Analysis of Apoptotic Vaginal Cells by Flow Cytometric Analysis
Cell apoptosis was identified by the flow cytometry using FITC-conjugated annexin V and propidium iodide (PI) staining. The single-cell suspension of vaginal tissue was prepared by using gentleMACsTM Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). The cells were filtered through 70 um mesh cell strainer followed by centrifugation, and then suspended in the binding buffer. The vaginal cells were stained with Annexin V-FITC/PI apoptosis staining kit (Miltenyi Biotec, Germany). The samples were acquired by MACSQuant Analyzer 10 and the data were analyzed using FlowJo software v10.7.
Histopathological Analysis
The vaginal tissues from mice were fixed into 10% neutral-buffered formalin. Paraffin-embedded blocks were prepared and serial sections of 5 μm thickness were cut. They were stained with Hematoxylin and Eosin (H and E) staining as described earlier.41 In brief, the tissue sections were deparaffinised, hydrated and stained with hematoxylin for 45 seconds. The slides were rinsed and stained with eosin for 45 seconds. Again, the slides were rinsed thoroughly and mounted with DPX. The histopathological changes in tissues were observed by examining the slides under a light microscope (Leica, USA).
Statistics
The fungal load data and hematological and cytokine parameters were analyzed by one-way ANOVA followed by a Bonferroni post test using GraphPad Prism software, version 6.0 (La Jolla, CA, USA).
Results
Susceptibility of C. albicans to FLZ and Meth-Gin
The SDA plate containing vehicle did not show any zone of inhibition (Figure 1A), whereas the SDA plate containing Meth-Gin (500 µg) showed 10 mm size of the inhibition zone (Figure 1B). However, the plate containing FLZ (100 µg) did not show any clear zone of inhibition, but showed some fungistatic effect around the well (Figure 1C). Interestingly, a synergy was observed between FLZ and Meth-Gin as the well containing a combination of FLZ (100 µg) with 250 and 500 µg of Meth-Gin showed inhibition zones of 30 and 35 mm, respectively (Figure 1D and E).
In the current study, the MIC of FLZ for C. albicans was found to be 64 µg/mL. Candida albicans with MIC value ≥64 µg/mL of FLZ has been categorized as a resistant strain according to the CLSI guidelines. The MIC for Meth-Gin against the present C. albicans isolate was found to be 3 mg/ ml. The MIC of FLZ and Meth-Gin in combination were considerably reduced and found to be 16 µg/mL and 1 mg/mL, respectively. The mean FIC index for a combination of FLZ and Meth-Gin was calculated to be 0.416 that indicates a synergistic interaction between FLZ and Meth-Gin.
The Confocal Microscopy Analysis Revealed a Synergistic Effect of FLZ and Meth-Gin Against C. albicans
The antifungal effect of FLZ alone or in combination with Meth-Gin against C. albicans was measured by the confocal microscopy after staining with propidium iodide (PI). As shown in Figure 2, there was no significant difference in the viability of the cells, which were exposed to FLZ as compared to vehicle-treated C. albicans. Whereas, a continuous decrease was observed in the viability of C. albicans treated with Meth-Gin alone or a combination of Meth-Gin and FLZ (Figure 2)
 |
Figure 2 The effect of FLZ or Meth-Gin or a combination of FLZ and Meth-Gin on cell viability of C. albicans by confocal microscopy using propidium iodide staining. |
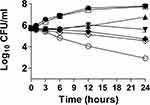
Synergism of FLZ and Meth-Gin Against C. albicans by Time-Kill Studies
The antifungal effect of FLZ alone or in combination of Meth-Gin against C. albicans was analyzed by time kill studies. The extent of C. albicans killing was determined by a decrease in the numbers of CFUs at different time points. The findings of the present study demonstrated that there was a time-dependent effect of FLZ and Meth-Gin combination against C. albicans (Figure 3). FLZ (8 µg/mL) alone exhibited very meager activity against C. albicans at 24 hour time point. However, FLZ (8 µg/mL) in combination with Meth-Gin (1 and 2 mg/mL) reduced ≥3 log10 CFUs/mL of C. albicans (Figure 3). Nevertheless, the combination of FLZ (8 μg/mL) and Meth-Gin (0.5 mg/mL) produced a 2.1-log10 CFUs/mL reduction as compared to the vehicle treatment at 24 hour time point. It suggested that a combination of FLZ and Meth-Gin showed an augmented antifungal activity against C. albicans. Interestingly, Meth-Gin (2 mg/kg) also reduced ≥3 log10 CFUs/mL of C. albicans. FLZ (8 µg/mL) in combination with 1 or 2 mg/mL of Meth-Gin killed 99.99 of C. albicans as compared to FLZ (8 µg/mL) treatment alone (Figure 3).
FLZ and Meth-Gin Combination, Not FLZ Alone, Effectively Eradicated Preformed Biofilm
We tested various concentrations of FLZ (8, 16, 32 and 64 µg/mL) against the preformed C. albicans biofilm. FLZ at the highest concentration (64 µg/mL) eliminated 29% of the preformed biofilm as compared to the vehicle treatment (Figure 4A), albeit it is found to be statistically significant (P<0.001). Simultaneously, we tested the concentration-dependent effect of Meth-Gin in the elimination of biofilm. Meth-Gin treatment eradicated 41% of the biofilm at a dose of 2000 µg/mL (Figure 4B), which was significant as compared to the vehicle treatment (P<0.001). For FLZ treatment could not effectively remove the preformed biofilm. To understand whether the treatment with FLZ and Meth-Gin combination is effective to eliminate the biofilm, the results demonstrated that a combination of FLZ and Meth-Gin efficiently removed the preformed C. albicans biofilm (Figure 4C). FLZ (8 µg/mL) in combination with Meth-Gin at the doses of 500, 1000 and 2000 µg/mL reduced the biofilm to 59.6%, 37.6% and 17.3%, respectively, as compared to vehicle control (P<0.001). Moreover, the anti-biofilm activity of FLZ and Meth-Gin was also found to be significantly greater as compared to FLZ treatment (P<0.001).
Treatment with FLZ Did Not Cure Murine VVC
The therapeutic efficacy of FLZ was assessed by analyzing the fungal load in the vaginal tissues of C. albicans infected mice (Figure 5A). FLZ at the doses of 10 and 20 mg/kg did not significantly reduce the vaginal fungal burden as compared to the fungal burden in the untreated infected mice (Figure 5A). However, FLZ at a dose of 40 mg/kg significantly decreased the fungal burden to 92230 ± 8416 CFUs as compared to 133010 ± 17289 CFUs in the untreated infected mice (P<0.05).
In order to evaluate FLZ-induced renal toxicity, the BUN and creatinine levels were analyzed in the blood of the treated mice (Figure 5B and C). FLZ therapy at a dose of 10 mg/kg elevated the BUN level from 22.67 ± 7 to 34 ± 5.3 mg/dl (P>0.05), whereas FLZ treatment at the doses of 20 and 40 mg/kg significantly raised the BUN level to 49.33 ± 7 and 68.33 ± 14.6 mg/dl, respectively (P<0.05 and P<0.001). On the other hand, there was no significant rise in creatinine level in mice treated with FLZ at the above mentioned doses (Figure 5C).
Meth-Gin Treatment Was Effective in Reducing the Severity of VVC
The activity of Meth-Gin against VVC was analyzed by determining the fungal burden in the vaginal tissue. Meth-Gin reduced the fungal burden in a dose-dependent manner (Figure 6A). Meth-Gin at a dose of 400 mg/kg was the most effective against VVC and reduced the fungal burden to 17031 ± 11259 as compared to 154037 ± 10053 CFUs/gm of the vaginal tissue in the untreated mice (P<0.001). Noteworthy, a treatment with Meth-Gin, at the doses of 100 and 200 mg/kg, reduced the fungal burden to 108040 ± 15294 and 62897 ± 15893 CFUs/gm (P<0.05 and P<0.001, respectively). However, Meth-Gin at a dose of 50 mg/kg was not effective against VVC (Figure 6A). The toxicity of Meth-Gin was assessed by evaluating the BUN and creatinine levels in the blood. The results demonstrated that Meth-Gin at the doses of 50, 100, 200 and 400 mg/kg did not induce any significant elevation in the BUN and creatinine levels (Figure 6B and C).
Coadministration of Meth-Gin and FLZ Showed a Synergistic Effect Against VVC
The activity of FLZ in combination with Meth-Gin was assessed against murine VVC. The results demonstrated that FLZ at the doses of 20 and 40 mg/kg was not effective to cure VVC (Figure 7A), whereas the administration of FLZ in combination with Meth-Gin was found to be very effective to treat VVC (Figure 7A). The coadministration of FLZ (40 mg/kg) and Meth-Gin (200 mg/kg) substantially decreased the fungal load (Figure 7A), which was highly significant lower as compared to FLZ treatment alone (P<0.001). Like to this, FLZ (20 mg/kg) along with Meth-Gin (200 mg/kg) reduced the fungal burden to 8523 ± 3226 CFUs as compared to the fungal burden of 140373 ± 15475 CFUs in the vaginal tissue of mice treated with FLZ (20 mg/kg) (P<0.001).
The renal toxicity of FLZ or a combination of FLZ and Meth-Gin treatment was assessed by analyzing the BUN and creatinine levels (Figure 7B and C). Treatment with FLZ at a dose of 40 mg/kg elevated the BUN level from 24 ± 8.5 to 80 ± 12.5 mg/dl (P<0.001). Mice treated with a combination of FLZ (40 mg/kg) and Meth-Gin (200 mg/kg) had the BUN level of 48.67 ± 10 mg/dl, which was significantly lower as compared to the BUN level in mice treated with FLZ (40 mg/kg) (P<0.05). Like to the BUN level, the creatinine level was significantly elevated to 1.33 ± 0.12 mg/dl in the mice of FLZ treated group (40 mg/kg) as compared to 0.633 mg/dl in mice from the untreated group (P<0.001). Interestingly, the treatment with a combination FLZ (40 mg/kg) and Meth-Gin reduced creatinine level to 0.9 ± 0.11 mg/dl (P<0.05).
Treatment with a Combination of Meth-Gin and FLZ Reduced the Secretion of Pro-Inflammatory Cytokines in the Vaginal Tissues
The levels of pro-inflammatory cytokines, including IL-1β, TNF-α and IL-17 were determined in the vaginal tissue homogenates. Mice in the untreated group exhibited a substantial increase in the levels of IL-1β, TNF-α and IL-17 as compared to their levels in normal mice (Figure 8A, 8B and C). IL-1β in the vaginal tissue from the normal control mice was found to be 71 ± 18 pg/mL that was increased to 362 ± 113 pg/mL in the infected mice (P<0.01). Treatment with a combination of FLZ (40 mg/kg) and Meth-Gin (200 mg/kg) significantly reduced IL-1β to 115 ± 31 pg/mL (<0.05).
TNF-α was also found to be substantially raised in the vaginal tissue of VVC-inflicted mice (Figure 8B). TNF-α in the untreated mice was found to be 748 ± 68 pg/mL that was significantly higher as compared to 161 ± 38 pg/mL in the vaginal tissue from the normal control mice (P<0.001). Treatment with a combination of FLZ (40 mg/kg) and Meth-Gin (200 mg/kg) reduced TNF-α level to 249 ± 72 pg/mL (P<0.001). Moreover, a combination of FLZ (20 mg/kg) and Meth-Gin also reduced TNF-α to 388 ± 121 pg/mL (P<0.01). However, FLZ at the doses of 20 and 40 mg/kg did not significantly decrease TNF-α in the vaginal tissue (Figure 8B). Interestingly, the treatment with Meth-Gin decreased TNF-α level to 434 ± 134 pg/mL, which was significantly lower as compared to TNF-α level in the untreated mice (P<0.05).
Like to IL-1β and TNF-α, IL-17 was increased to 275 ± 62 pg/mL as compared to 31 ± 17 pg/mL in the normal mice (Figure 8C, P<0.001). Treatment with FLZ (20 and 40 mg/kg) did not significantly decrease IL-17 level in the infected mice (Figure 8C, P>0.05). However, a combination of FLZ-40 and Meth-Gin decreased IL-17 level from 275 ± 62 to 84 ± 27 pg/mL (P<0.01). Furthermore, FLZ-40 and Meth-Gin combination was more effective in reducing IL-17 level as compared to FLZ-40 treatment alone (P<0.05).
A Combination of FLZ and Meth-Gin Protected the Vaginal Cells Against VVC-Induced Apoptosis
Candida albicans infection induced apoptosis in the vaginal cells. A total of 40.6% cells showed apoptosis in the vaginal tissue from the untreated mice as compared to 2.35% in the infected mice (Figure 9A, B and G) (P<0.001). Mice treated with FLZ had 35.9% apoptotic cells (P>0.05) in the vaginal tissues, whereas Meth-Gin treatment reduced the percentage of apoptotic cells to 27.7% (Figure 9C, D and G) (P<0.01). A combination of FLZ (20 and 40 mg/kg) and Meth-Gin (200 mg/kg) was found to be very effective and reduced the apoptotic cells to 15.77% and 13.13%, respectively (Figure 9E, F and G), which were significantly reduced as compared to the untreated or FLZ-treated mice (P<0.001).
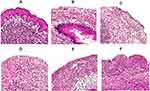
Treatment with FLZ and Meth-Gin Combination Ameliorated Histopathological Changes in the Vaginal Tissues
In order to assess the therapeutic effect of FLZ or a combination of FLZ and Meth-Gin in the treatment of VVC, we analyzed the histological alterations in the vaginal tissues from the mice in the untreated or FLZ or Meth-Gin or FLZ + Meth-Gin treated mice. The vaginal tissue from the control normal mice revealed non-keratinized stratified squamous epithelium and normal lamina propria (Figure 10A). Whereas, the vaginal tissues from the infected mice showed epithelial necrosis, shedding and an extensive ulceration (Figure 10B). Moreover, the presence of C. albicans hyphae can be seen in the vaginal tissue of the untreated mice (red arrow). FLZ or Meth-Gin treatment showed moderate recovery with numerous scattered inflammatory cells and leukocytes (Figure 10C and D). Treatment with a combination of FLZ (20 and 40 mg/kg) and Meth-Gin (200 mg/kg) showed regenerative alterations, including the mild epithelial necrosis with few scattered inflammatory cells in the lamina propria (Figure 10E and F).
Discussion
Azoles are front-line antifungal drugs that are widely used in the treatment of Candidiasis. The massive use of FLZ has resulted in the emergence of drug-resistant Candida isolates. The target of FLZ is lanosterol 14-α demethylase, an enzyme that is coded by ERG11 gene. An over-expression of multi-drug resistant genes, including MDR1, CDR1 or CDR2 or ERG11 contributes to FLZ-resistance in C. albicans isolates.6 Several toxic manifestations, including hypokalemia, respiratory failure and liver inflammation are associated with the use of FLZ treatment.43 A rapid emergence of resistance against FLZ has rendered these antifungals ineffective. Thus, it is very important to formulate a strategy to keep these antifungal drugs effective and alive. In the current work, we investigated the therapeutic effect of Meth-Gin to increase the antifungal activity of FLZ against drug-resistant VVC.
In recent years, many approaches for the combination therapy have been proposed to treat infectious diseases.11–15 This is very important, particularly, to counter the phenomenon of the multi-drug resistance. Various phytochemicals have been suggested to show a synergistic effect with multiple antibiotics.20 Many plant-derived substances have shown their effectiveness to fight Candida infections, particularly those less susceptible to current antifungal drugs.45 The use of alcoholic extract of Flos rosae chinensis has been shown to increase the activity of FLZ against the drug-resistant C. albicans.46 The extract of Punica granatum and its purified compound punicalagin showed a synergistic effect with FLZ.47 Giordani et al showed that essential oil of Thymus vulgaris potentiated the activity of amphotericin B against C. albicans.48 Furthermore, Allium sativum has been reported to increase the antifungal activity of ketoconazole against Trichophyton spp.49 In another study, Pelargonium graveolens components, geraniol and citronellol, were reported to exert the additive effects with amphotericin B and ketoconazole against Aspergillus spp.50
Ginger showed anti-inflammatory and antioxidant effects when used in combination with anti-tubercular therapy in patients.51 Ginger and its bioactive component 6-shogaol have been effective to alleviate the lung inflammation in an asthma model.52 In this study, we investigated the synergistic effect of methanolic extract of Ginger (Meth-Gin) and FLZ against C. albicans both in in vitro and in a mouse model of VVC. The results of the present study demonstrated that FLZ alone did not exhibit any visible activity against C. albicans. Moreover, FLZ was ineffective to eradicate the preformed biofilm and to cure murine VVC. However, Meth-Gin showed some efficacy against C. albicans, but it did not completely cure VVC. Earlier reports demonstrated that the components of Ginger, 6-shogaol and 6-gingerol, had an anti-biofilm activity against C. auris and C. albicans.31 The biofilm formation is considered one of the most important virulence strategy of C. albicans in the pathogenesis of VVC.53 Moreover, it reduces the susceptibility of the pathogens to antibiotics and contributes to broaden the phenomenon of antibiotic-resistance. The results of the current study demonstrated that FLZ alone was not effective to eradicate the preformed biofilm. However, FLZ in combination with Meth-Gin almost completely eradicated the preformed biofilm. It suggests that Meth-Gin can increase the susceptibility of C. albicans biofilm to FLZ.
None of the past studies showed the activity of Ginger extract alone or in combination with antifungal drug against VVC. The findings of the present study demonstrated that FLZ treatment not only was ineffective to cure VVC but also induced renal toxicity in mice. Earlier, Ginger administration has been shown to alleviate Schistosoma mansoni infection-induced deleterious effects by modulating inflammatory cytokines and liver inflammation enzymes.54 Moreover, Ginger has been shown to possess protective effects against cadmium- and lead-induced nephrotoxicity.55,56 The administration of Meth-Gin not only increased the activity of FLZ against VVC but also reduced FLZ-induced renal toxicity in the treated mice. Proinflammatory cytokines, including IL-1β, TNF-α and IL-17 play an important role in protecting against various pathogens. However, the excessive secretion of inflammatory cytokines exaggerates the pathogenesis of VVC by increasing the severity of inflammation. It reduces the clearance of the pathogen from the host.57 Earlier, the role of pro-inflammatory cytokines have been documented in a mouse model of VVC.58 Ginger possesses anti-inflammatory properties and inhibits the production of TNF-α, IL-6 and other inflammatory markers.59 Here, we investigated the effect of Meth-Gin and FLZ combination on the secretion of IL-1β, TNF-α and IL-17 in the vaginal tissues. The levels of IL-1β, TNF-α and IL-17 were found to be elevated in the vaginal tissue of the infected mice. Treatment with Meth-Gin or a combination of FLZ and Meth-Gin substantially decreased the levels of IL-1β, TNF-α and IL-17. Contrarily, FLZ at the doses of 20 and 40 mg/kg did not effectively reduce the levels of pro-inflammatory cytokines. In a recent report, Roselletti et al showed the apoptosis of vaginal epithelial cells in the clinical samples from the women affected by vaginal infection.60 The findings of the present study showed that vaginal tissue from the untreated mice had a remarkably increased percentage of apoptotic cells, whereas mice treated with a combination of Meth-Gin and FLZ showed significantly lower numbers of apoptotic cells.
The polymorphonuclear neutrophils (PMNs) are important cells of the innate immune system as the first line of defense against the fungal pathogens, including C. albicans. A symptomatic VVC is related to the recruitment of PMNs that contributes to the symptoms of inflammation. The results of the present study demonstrated that the vaginal tissues from the untreated infected mice showed higher numbers of leukocytes. Whereas, the mice treated with a combination of FLZ and meth-Gin showed reduced infiltration of leukocytes in the vaginal tissue. The data of pro-inflammatory cytokines and the fungal load were supported by the histological findings. The vaginal tissue from the untreated infected mice showed the vaginal wall degeneration, epithelial necrosis and ulceration. Whereas, the treatment with a combination of FLZ and Meth-Gin reversed the VVC-induced pathological alterations in the vaginal tissues.
Keeping into consideration the findings of the present study, it may be concluded that coadministration of Meth-Gin and FLZ may be highly effective to treat drug-resistant VVC. Moreover, the administration of Ginger extract may alleviate VVC-induced inflammation and apoptosis. Thus, the use of Ginger extract may prove to be an effective herbal formulation to increase the activity and reduce the toxicity of FLZ against candidiasis.
Acknowledgments
We would like to thank the Deanship of Scientific Research, Qassim University, Buraydah, Saudi Arabia for the support.
Funding
This study is not supported by any funding agency.
Disclosure
There are no conflicts of interest to declare.
References
1. Martins N, Ferreira IC, Barros L, Silva S, Henriques M. Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia. 2014;177(5–6):223–240. doi:10.1007/s11046-014-9749-1
2. Singh S, Fatima Z, Hameed S. Predisposing factors endorsing Candida infections. Infez Med. 2015;23(3):211–223.
3. Spampinato C, Leonardi D. Candida infections, causes, targets, and resistance mechanisms: traditional and alternative antifungal agents. Biomed Res Int. 2013;2013:204237. doi:10.1155/2013/204237
4. Martins HP, Da silva MC, Paiva LC, Svidzinski TI, Consolaro ME. Efficacy of fluconazole and nystatin in the treatment of vaginal Candida species. Acta Derm Venereol. 2012;92(1):78–82. doi:10.2340/00015555-1194
5. Nakamura-Vasconcelos SS, Fiorini A, Zanni PD, et al. Emergence of Candida glabrata in vulvovaginal candidiasis should be attributed to selective pressure or virulence ability? Arch Gynecol Obstet. 2017;296(3):519–526. doi:10.1007/s00404-017-4465-y
6. Bhattacharya S, Sae-Tia S, Fries BC. Candidiasis and Mechanisms of Antifungal Resistance. Antibiotics (Basel). 2020;9:312. doi:10.3390/antibiotics9060312
7. Delattin N, Cammue BP, Thevissen K. Reactive oxygen species-inducing antifungal agents and their activity against fungal biofilms. Future Med Chem. 2014;6(1):77–90. doi:10.4155/fmc.13.189
8. Khan SH, Younus H, Allemailem KS, et al. Potential of methylglyoxal-conjugated chitosan nanoparticles in treatment of fluconazole-resistant Candida albicans infection in a murine model. Int J Nanomedicine. 2020;15:3681–3693. doi:10.2147/IJN.S249625
9. Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS, Rogers PD. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front Microbiol. 2017;7:2173. doi:10.3389/fmicb.2016.02173
10. Arastehfar A, Gabaldón T, Garcia-Rubio R, et al. Drug-resistant fungi: an emerging challenge threatening our limited antifungal armamentarium. Antibiotics (Basel). 2020;9(12):877. doi:10.3390/antibiotics9120877
11. O’Brien B, Chaturvedi S, Chaturvedi V. In vitro evaluation of antifungal drug combinations against multidrug-resistant candida auris isolates from New York outbreak. Antimicrob Agents Chemother. 2020;64(4):e02195–19. doi:10.1128/AAC.02195-19
12. Lu M, Yu C, Cui X, Shi J, Yuan L, Sun S. Gentamicin synergises with azoles against drug-resistant Candida albicans. Int J Antimicrob Agents. 2018;51(1):107–114. doi:10.1016/j.ijantimicag.2017.09.012
13. Denardi LB, Keller JT, Oliveira V, Mario DAN, Santurio JM, Alves SH. Activity of combined antifungal agents against multidrug-resistant candida glabrata strains. Mycopathologia. 2017;182(9–10):819–828. doi:10.1007/s11046-017-0141-9
14. Li Y, Wan Z, Liu W, Li R. Synergistic activity of chloroquine with fluconazole against fluconazole-resistant isolates of Candida species. Antimicrob Agents Chemother. 2015;59(2):1365–1369. doi:10.1128/AAC.04417-14
15. Khan MA, Jabeen R, Mohammad O. Prophylactic role of liposomized chloroquine against murine cryptococcosis less susceptible to fluconazole. Pharm Res. 2004;21(12):2207–2212. doi:10.1007/s11095-004-7672-8
16. Khan MA, Aljarbou AN, Khan A, Younus H. Liposomal thymoquinone effectively combats fluconazole-resistant Candida albicans in a murine model. Int J Biol Macromol. 2015;76:203–208. doi:10.1016/j.ijbiomac.2015.02.015
17. Langeveld WT, Veldhuizen EJ, Burt SA. Synergy between essential oil components and antibiotics: a review. Crit Rev Microbiol. 2014;40:76–94. doi:10.3109/1040841X.2013.763219
18. Duan X, Wang K, Wu J, et al. Comparative efficacy of Chinese herbal injections combined with azithromycin for mycoplasma pneumonia in children: a Bayesian network meta-analysis of randomized controlled trials. J Clin Pharm Ther. 2019;44:675–684. doi:10.1111/jcpt.12855
19. Cai Y, Zhang Q, Fu Y, et al. Effectiveness of Chinese herbal medicine combined with antibiotics for extensively drug-resistant enterobacteria and nonfermentative bacteria infection: real-life experience in a retrospective cohort. Biomed Res Int. 2017;2017:2897045. doi:10.1155/2017/2897045
20. Ayaz M, Ullah F, Sadiq A, et al. Synergistic interactions of phytochemicals with antimicrobial agents: potential strategy to counteract drug resistance. Chem Biol Interact. 2019;308:294–303. doi:10.1016/j.cbi.2019.05.050
21. Salih KA. Synergistic effects of plant extracts and antifungal drugs on C. albicans. J Dev Drugs. 2016;5:3. doi:10.4172/2329-6631.1000165
22. Rolta R, Sharma A, Sourirajan A, Mallikarjunan PK, Dev K. Combination between antibacterial and antifungal antibiotics with phytocompounds of Artemisia annua L: a strategy to control drug resistance pathogens. J Ethnopharmacol. 2021;266:113420. doi:10.1016/j.jep.2020.113420
23. Sharifzadeh A, Khosravi AR, Shokri H, Tari PS. Synergistic anticandidal activity of menthol in combination with itraconazole and nystatin against clinical Candida glabrata and Candida krusei isolates. Microb Pathog. 2017;107:390–396. doi:10.1016/j.micpath.2017.04.021
24. Pietrella D, Angiolella L, Vavala E, et al. Beneficial effect of Mentha suaveolens essential oil in the treatment of vaginal candidiasis assessed by real-time monitoring of infection. BMC Complement Altern Med. 2011;11:18. doi:10.1186/1472-6882-11-18
25. Donadu MG, Usai D, Marchetti M, et al. Antifungal activity of oils macerates of North Sardinia plants against Candida species isolated from clinical patients with candidiasis. Nat Prod Res. 2020;34(22):3280–3284. doi:10.1080/14786419.2018.1557175
26. Donadu MG, Trong LN, Viet HD, et al. Phytochemical compositions and biological activities of essential oils from the leaves, rhizomes and whole plant of Hornstedtia bella Škorničk. Antibiotics (Basel). 2020;9(6):334. doi:10.3390/antibiotics9060334
27. Le NT, Donadu MG, Ho DV, et al. Biological activities of essential oil extracted from leaves of Atalantia sessiflora Guillauminin Vietnam. J Infect Dev Ctries. 2020;14(9):1054–1064. doi:10.3855/jidc.12469
28. Rahmani AH, Shabrmi FMA, Aly SM. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. Int J Physiol Pathophysiol Pharmacol. 2014;6:125–136.
29. Kim HS, Park HD. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS One. 2013;8(9):76106. doi:10.1371/journal.pone.0076106
30. Mao QQ, Xu XY, Cao SY, et al. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods. 2019;8:185. doi:10.3390/foods8060185
31. Lee JH, Kim YG, Choi P, Ham J, Park JG, Lee J. Antibiofilm and antivirulence activities of 6-gingerol and 6-shogaol against Candida albicans due to hyphal inhibition. Front Cell Infect Microbiol. 2018;8:299. doi:10.3389/fcimb.2018.00299
32. Park M, Bae J, Lee DS. Antibacterial activity of [10]-gingerol and [12]-gingerol isolated from ginger rhizome against periodontal bacteria. Phytother Res. 2008;22:1446–1449. doi:10.1002/ptr.2473
33. Kumar L, Chhibber S, Harjai K. Zingerone inhibit biofilm formation and improve antibiofilm efficacy of ciprofloxacin against Pseudomonas aeruginosa PAO1. Fitoterapia. 2013;90:73–78. doi:10.1016/j.fitote.2013.06.017
34. Rampogu S, Baek A, Gajula RG, et al. Ginger (Zingiber officinale) phytochemicals-gingerenone-A and shogaol inhibit SaHPPK: molecular docking, molecular dynamics simulations and in vitro approaches. Ann Clin Microbiol Antimicrob. 2018;17:16. doi:10.1186/s12941-018-0266-9
35. Moon Y, Lee H, Lee S. Inhibitory effects of three monoterpenes from ginger essential oil on growth and aflatoxin production of Aspergillus flavus and their gene regulation in aflatoxin biosynthesis. Appl Biol Chem. 2018;61:243–250. doi:10.1007/s13765-018-0352-x
36. Aghazadeh M, Zahedi Bialvaei A, Aghazadeh M, et al. Survey of the antibiofilm and antimicrobial effects of zingiber officinale (in Vitro Study). Jundishapur J Microbiol. 2016;9:e30167. doi:10.5812/jjm.30167
37. Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard-third edition; CLSI document M27-A3. Wayne, PA, USA:Clinical and Laboratory Standards Institute; 2008a. CLSI.
38. Khan A, Alhumaydhi FA, Alwashmi ASS, et al. Diallyl sulfide-mediated modulation of the fatty acid synthase (FASN) leads to cancer cell death in bap-induced lung carcinogenesis in Swiss mice. J Inflamm Res. 2020;13:1075–1087. doi:10.2147/JIR.S284279
39. Balasamy RJ, Ravinayagam V, Alomari M, et al., Cisplatin delivery, anticancer and antibacterial properties of Fe/SBA-16/ZIF-8 nanocomposite. RSC Adv. 2019;9:42395–42408. doi:10.1039/C9RA07461A
40. Ahmad N, Alam MK, Shehbaz A, et al. Antimicrobial activity of clove oil and its potential in the treatment of vaginal candidiasis. J Drug Target. 2005;13:
41. Laskar AA, Khan MA, Rahmani AH, Fatima S, Younus H. Thymoquinone, an active constituent of Nigella sativa seeds, binds with bilirubin and protects mice from hyperbilirubinemia and cyclophosphamide-induced hepatotoxicity. Biochimie. 2016;127:205–213. doi:10.1016/j.biochi.2016.05.020
42. Khan MA, Khan A, Khan SH, et al. Coadministration of liposomal methylglyoxal increases the activity of amphotericin B against Candida albicans in leukopenic mice. J Drug Target. 2021;29:78–87. doi:10.1080/1061186X.2020.1803333
43. Fisher BT, Zaoutis T, Dvorak CC, et al. Effect of caspofungin vs fluconazole prophylaxis on invasive fungal disease among children and young adults with acute myeloid leukemia: a Randomized Clinical Trial. JAMA. 2019;322:1673–1681. doi:10.1001/jama.2019.15702
44. Guevara-Lora I, Bras G, Karkowska-Kuleta J, et al. Plant-derived substances in the fight against infections caused by candida species. Int J Mol Sci. 2020;21:6131. doi:10.3390/ijms21176131
45. Zhang L, Lin H, Liu W, et al. Antifungal activity of the ethanol extract from flos rosae chinensis with activity against fluconazole-resistant clinical candida. Evid Based Complement Alternat Med. 2017;4780746.
46. Endo EH, Cortez DA, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. Potent antifungal activity of extracts and pure compound isolated from pomegranate peels and synergism with fluconazole against Candida albicans. Res Microbiol. 2010;161:534–540. doi:10.1016/j.resmic.2010.05.002
47. Giordani R, Regli P, Kaloustian J, Mikaïl C, Abou L, Portugal H. Antifungal effect of various essential oils against Candida albicans. Potentiation of antifungal action of amphotericin B by essential oil from Thymus vulgaris. Phytother Res. 2004;18:990–995. doi:10.1002/ptr.1594
48. Pyun MS, Shin S. Antifungal effects of the volatile oils from Allium plants against Trichophyton species and synergism of the oils with ketoconazole. Phytomedicine. 2006;13:394–400. doi:10.1016/j.phymed.2005.03.011
49. Shin S. Anti-Aspergillus activities of plant essential oils and their combination effects with ketoconazole or amphotericin B. Arch Pharm Res. 2003;26:389–393. doi:10.1007/BF02976696
50. Kulkarni RA, Deshpande AR. Anti-inflammatory and antioxidant effect of ginger in tuberculosis. J Complement Integr Med. 2016;13:201–206. doi:10.1515/jcim-2015-0032
51. Yocum GT, Hwang JJ, Mikami M, Danielsson J, Kuforiji AS, Emala CW. Ginger and its bioactive component 6-shogaol mitigate lung inflammation in a murine asthma model. Am J Physiol Lung Cell Mol Physiol. 2020;318:L296–L303. doi:10.1152/ajplung.00249.2019
52. Muzny CA, Schwebke JR. Biofilms: an underappreciated mechanism of treatment failure and recurrence in vaginal infections: table 1. Clin Infect Dis. 2015;61(4):601–606. doi:10.1093/cid/civ353
53. Aly HF, Mantawy MM. Efficiency of ginger (Zingbar officinale) against Schistosoma mansoni infection during host-parasite association. Parasitol Int. 2013;62:380–389. doi:10.1016/j.parint.2013.04.002
54. Reddy YA, Chalamaiah M, Ramesh B, Balaji G, Indira P. Ameliorating activity of ginger (Zingiber officinale) extract against lead induced renal toxicity in male rats. J Food Sci Technol. 2014;51:908–914. doi:10.1007/s13197-011-0568-9
55. Gabr SA, Alghadir AH, Ghoniem GA. Biological activities of ginger against cadmium-induced renal toxicity. Saudi J Biol Sci. 2019;26:382–389. doi:10.1016/j.sjbs.2017.08.008
56. Romani L, Fallarino F, De Luca A, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi:10.1038/nature06471
57. Qu S, Chen L, Tian H, et al. Effect of perillaldehyde on prophylaxis and treatment of vaginal candidiasis in a murine model. Front Microbiol. 2019;10:1466. doi:10.3389/fmicb.2019.01466
58. Ezzat SM, Ezzat MI, Okba MM, Menze ET, Abdel-Naim AB. The hidden mechanism beyond ginger (Zingiber officinale Rosc.) potent in vivo and in vitro anti-inflammatory activity. J Ethnopharmacol. 2018;214:113–123. doi:10.1016/j.jep.2017.12.019
59. Roselletti E, Sabbatini S, Perito S, Mencacci A, Vecchiarelli A, Monari C. Apoptosis of vaginal epithelial cells in clinical samples from women with diagnosed bacterial vaginosis. Sci Rep. 2020;10:1978. doi:10.1038/s41598-020-58862-2
60. Yano J, Peters BM, Noverr MC, Fidel PL
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.