Back to Journals » Infection and Drug Resistance » Volume 14
Co-Infection Pneumonia with Mycobacterium abscessus and Pneumocystis jiroveci in a Patient without HIV Infection Diagnosed by Metagenomic Next-Generation Sequencing
Authors Xie D , Xian Y, You J, Xu W, Fan M, Bi X, Zhang K
Received 1 December 2020
Accepted for publication 13 February 2021
Published 4 March 2021 Volume 2021:14 Pages 879—888
DOI https://doi.org/10.2147/IDR.S292768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Dan Xie,* Ying Xian,* Jingya You, Wen Xu, Min Fan, Xiaogang Bi, Kouxing Zhang
Department of General Intensive Care Unit, Lingnan Hospital, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kouxing Zhang
Department of General Intensive Care Unit, Lingnan Hospital, The Third Affiliated Hospital of Sun Yat-Sen University, 2693 Kaichuang Avenue, Guangzhou, 510530, People’s Republic of China
Email [email protected]
Introduction: Co-infection pneumonia with Mycobacterium abscessus (M. abscessus) and Pneumocystis jirovecii (P. jirovecii) is rarely reported in previously healthy patients without HIV infection. The diagnosis of pneumonia of M. abscessus and P. jirovecii remains challenging due to its nonspecific clinical presentation and the inadequate performance of conventional diagnostic methods.
Case Report: We report the case of a 44-year-old previously healthy male transferred to our hospital in February 2020 with a 4-month history of productive cough and one month of intermittent fever. At local hospital, the metagenomic next-generation sequencing(mNGS) detected P. jirovecii sequences in blood; with the antifungal therapy (Caspofungin, trimethoprim–sulfamethoxazole [TMP-SMX] and methylprednisolone [MP]), the patient still had hypoxemia, cough and fever. Then he was transferred to our hospital, the mNGS of bronchoalveolar lavage fluid (BALF) detected the sequences of M. abscessus and P. jirovecii. CD4+ T-lymphocytopenia in the peripheral blood cells was presented and HIV serology was negative. Caspofungin, TMP-SMX, clindamycin and MP were used to treat P. jirovecii pneumonia (PJP). Moxifloxacin, imipenem cilastatin and linezolid were used to treat M. abscessus infection. Clinical progress was satisfactory following antifungal combined with anti-M. abscessus therapy.
Conclusion: Co-infection pneumonia with M. abscessus and P. jirovecii as reported here is exceptionally rare. mNGS is a powerful tool for pathogen detection. M. abscessus infection could be a risk factor for P. jirovecii infection. This case report supports the value of mNGS in diagnosing of M. abscessus and P. jirovecii, and highlights the inadequacies of conventional diagnostic methods.
Keywords: Pneumocystis jiroveci, Mycobacterium abscessus, mNGS, co-infection, HIV negative
Introduction
Pneumocystis jiroveci (P. jiroveci) is a common opportunistic fungus causing Pneumocystis jiroveci pneumonia (PJP) in HIV infected patients. It is also a major opportunistic infection in HIV-negative immunocompromised patients, such as malignancy, stem cell transplantation (SCT), solid organ transplantation, rheumatologic disease, and respiratory disease.1 Mycobacterium abscessus (M. abscessus) is a rapidly growing mycobacteria (RGM) of non-tuberculosis mycobacterium (NTM), which is an opportunistic pathogen of pulmonary infection in HIV infected patient. In HIV-negative people, M. abscessus infects both patients without underlying risk factors,2,3 and patients with a history of chronic lung diseases, such as cystic fibrosis and bronchiectasis, which led it to being the first RGM to be isolated in these contexts.2,4 In HIV-negative patients, there were a few cases of co-infection of P. jiroveci and Mycobacterium tuberculosis.5–7 But the co-infection of M. abscessus and P. jiroveci had not been reported before by search of Pubmed with the key words “‘Mycobacterium abscessus’ AND ‘Pneumocystis’”.
The pneumonia caused by M. abscessus and P. jirovecii present similar clinical features including fever and cough, which are nonspecific.2,5 Traditional diagnostic techniques in the microbiology laboratory for P. jirovecii and M. abscessus have some limits: P. jirovecii still cannot be reliably grown in vitro and P. jirovecii organisms in respiratory specimens following immunofluorescence staining suffer from low sensitivity;8 when the direct microscopic examination is positive for acid-fast bacilli, it is necessary to exclude the diagnosis of tuberculosis and other NTM organisms; M. abscessus cultures are not pigmented (neither scoto- nor photochromogen) and are similar to many other RGMs, such as M. chelonae;9 and the PCR of P. jirovecii and M. abscessus is not available in clinical settings in most hospitals in China including our hospital.
Metagenomic next generation sequencing (mNGS) is a powerful tool for detecting pathogens that can be performed directly on clinical specimens.10,11 It had been used for detection of P. jiroveci,8,10 and M. abscessus.12 In this report, we describe a previously healthy HIV-negative male coinfected with M. abscessus and P. jiroveci detected by mNGS.
Case Report
A 44-year-old previously healthy male was transferred to our hospital in February 2020 with a 4-month history of productive cough and one month of intermittent fever. He had no relevant transplantation and no significant medical history (particularly no corticosteroid and no immunosuppressive drug history), and never had any episode of tuberculosis or chronic pulmonary disorders. He was a never-smoker and no alcoholism. He had no family history of chronic lung disease. He had no recent travel history of Wuhan. Four months prior, the patient developed cough with white phlegm, accompanied with chest tightness and shortness of breath when he coughed severely. Sometimes he had blood-streak sputum. He was suspected as upper respiratory tract infection and given symptomatic treatment, but the cough was not relieved. One month later, chest x-ray showed pulmonary infection. With the treatment of clarithromycin, ribavirin and ampicillin for 5 days, cough was relieved. However, one month prior, he developed low-grade fever. 22 days prior, he had a fever with maximum body temperature of 39.4°C. He was admitted to local hospital. Blood tests showed a white blood cell (WBC) count of 8.32×10E9/L and C-reactive protein (CRP) of 128.06mg/L. Chest computed tomography (CT) scan showed bilateral infiltrations. Piperacillin tazobactam, oseltamivir and doxycycline were initiated after admission to treat pulmonary infection. One day later, chest CT showed the aggravated infection of both lungs. He developed to be hypoxia, septic shock. Acute respiratory distress syndrome (ARDS) was diagnosed. Then he was treated with meropenem, voriconazole, doxycycline. Reexamination of chest CT showed the infection was relieved. Five tests of nucleic acid of Corona Virus Disease 2019 (COVID-19) were all negative, which were carried out by regional Centers for Disease Control and Prevention (CDC). Eight days prior, the metagenomic next-generation sequencing (mNGS) of blood detected the sequences of P. jirovecii and Cytomegalovirus (CMV). Caspofungin, trimethoprim–sulfamethoxazole (TMP-SMX) and methylprednisolone (MP) were used for antifungal therapy. One day prior, the oxygen saturation decreased again. Then he was transferred to our hospital and admitted to our department.
Chest CT on admission showed bilateral infiltrations, ground glass opacity, crazy paving pattern (Figure 1A1 and A2). Blood tests performed on admission showed WBC count of 9.38×10E9/L, absolute value of lymphocyte (LYMP) of 0.65×10E9/L, hemoglobin (Hb) level of 126g/L, platelet count of 291×10E9/L, aspartate aminotransferase (AST) of 37 IU/L, alanine aminotransferase (ALT) of 40 IU/L, blood urea nitrogen (BUN) of 5.6mmol/L, and serum creatinine of 68umol/L. Hypersensitive C-reactive protein (hsCRP) was 43.2mg/L, and procalcitonin (PCT) was 0.025ng/mL. The physical examination showed blood pressure of 125/69mmHg, heart rate of 94 beats/minute, respiratory rate of 25 rates/minute, and temperature of 37.7°C, oxygen saturation of 99% with therapy of high-flow nasal cannula (HFNC) with fraction of inspiration (FiO2) 0.55). Moist rales were audible over bilateral lungs, without lower-extremity edema. Physical examination of the heart and abdomen were normal. The arterial blood gas analysis revealed a pH of 7.45, the partial pressure of oxygen (PO2) was 124mmHg, the partial pressure of carbon dioxide (PCO2) was 45mmHg, PaO2/FiO2 was 113mmHg, alveolar-arterial oxygen gradient ((A-a) DO2) was 212mmHg. Intravenous moxifloxacin (400mg/day), ganciclovir (250mg, every 12 hours), caspofungin (50mg/day), clindamycin (600mg, every 8 hours) and methylprednisolone (40mg/day), and oral trimethoprim–sulfamethoxazole (TMP-SMX) (3 tablets (240/1200mg) every 6 hours, with each tablet containing 80mg trimethoprim and 400mg sulfamethoxazole) were prescribed. Human immunoglobulin for intravenous injection (IVIG) was given with the dosage of 10g for 5 days. Thymalfasin was used with the dosage of 1.6mg for 12 days. Hepatitis B and C viruses, HIV, and Syphilis were all tested negative by serology. The sputum analysis of acid-fast bacilli smear and mycobacterial culture was negative. Mycobacterium tuberculosis complex was negative based on blood test with the GeneXpert MTB/RIF assay. The immunofluorescence staining of sputum smear did not find Pneumocystis organisms. Serum 1,3-beta-D-glucan (BDG) level was normal (<3.836pg/mL). In addition, bronchoalveolar lavage fluid (BALF) culture revealed no growth of bacteria or fungi. The DNA copies of CMV was 9E2 copies/mL. The lactate dehydrogenases level was 429 U/L. Antinuclear antibody (ANA), anti-double stranded DNA antibody(dsDNA), anti-neutrophil cytoplasmic antibody (ANCA) series and extractable nuclear antigen (ENA) series were all negative. Flow cytometry of peripheral blood cells showed CD3+T cells/lymphocyte was 86% (normal range 50%-84%), CD3+CD4+T cells/lymphocyte was 17%, CD3+CD8+T cells/lymphocyte was 55%, CD3+CD4+T cells/CD3+CD8+T cells was 0.31, B cells/lymphocyte was 12%, NK cells (CD3-CD16+ or CD3-CD56+)/lymphocyte was 2%. The thyroid stimulating hormone (TSH) level was normal. Peripheral blood and bronchoalveolar lavage fluid (BALF) samples collected on day 3 were sent for mNGS test, which was performed by Vision Medicals CO. Ltd (Guangzhou, China) using Illumina NextSeq 550 (USA). On day 4, the mNGS detected M. abscessus with 1315 high-confidence sequence reads, CMV with 649 high-confidence sequence reads and P. jirovecii with 44 high-confidence sequence reads in BALF sample. mNGS detected CMV with 74 high-confidence sequence reads and P. jirovecii with 368 high-confidence sequence reads in blood (Table 1). The M. abscessus sequence reads mapped to a M. abscessus reference genome (NC_010397.1), the P. jirovecii sequence reads assembled to a P. jirovecii reference genome (NW_017264779.1), and the sequence reads for CMV allowed assembly of a CMV reference genome (NC_006273.2) (Figure 2).
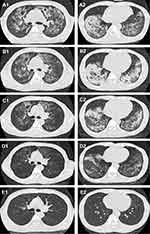 |
Figure 1 Chest CT performed on admission (A1, A2), on day 9 (B1, B2), on day 17 (C1, C2), on day 26 (D1, D2), and five months after discharge (E1, E2). |
 |
Table 1 The Pathogen and Sequence Reads Detected by mNGS in BALF and Blood Samples |
Meanwhile, the oxygen saturation decreased to about 90%, and the partial pressure of oxygen dropped to 54 mmHg. Moxifloxacin (400 mg/day) was switched to imipenem cilastatin (1.0g every 8 hours) for the treatment of M. abscessus infection. Linezolid (600mg every 12 hours) was synergistically used on day 6 to anti-M. abscessus. On day 9, The patient discontinued HFNC, and be treated with nasal catheter for oxygen inhalation, and chest CT was performed and revealed that worsening infiltration in lower lungs and absorbing of other lungs (Figure 1B1 and B2). With the treatment, the patient still had intermittent low-grade fever, with the highest temperature of 38.2 °C, and the heat peak was gradually decreased, the body temperature returned to normal on day 10. The symptom of cough and sputum was also significantly relieved, the hsCRP decreased to 4.8mg/L. The PCT was 0.022ng/mL. On day 12, the patient was transferred to General Medical Wards. Moxifloxacin (400 mg/day), imipenem cilastatin (1.0g every 8 hours) and linezolid (600mg every 12 hours) were used to treat M. abscessus infection. Caspofungin (50mg/day), TMP-SMX (240/1200mg, every 6 hours), clindamycin (600mg, every 8 hours) and MP (20mg/day) were used to treat PJP. The copies of CMV were below 500 copies/mL tested on Day 11, so the ganciclovir was discontinued. On day 17, MP was switched to oral taken with 16mg/day for 7 days, then it was reduced to 12mg/day for 3 days. Chest CT performed on day 17 revealed absorbing infiltrations (Figure 1C1 and C2). On day 18, the count of WBC dropped to 3×10E9/L, recombinant human granulocyte stimulating factor injection was given. On day 20, caspofungin was discontinued, and clindamycin was changed from intravenous to oral administration with the dosage of 450mg/day (150mg every 8hours), moxifloxacin was also switched to oral taken (400mg/day). On day 23, linezolid was discontinued for the plantlet dropping to 89×10E9/L. On day 26, chest CT showed pulmonary infection was significantly absorbed (Figure 1D1 and D2). The level of LYMP elevated to 1.1×10E9/L, with the WBC of 3.97×10E9/L. The levels of AST, ALT, BUN and creatinine was normal. On day 27, he was discharged with 7-day oral medicines (SMZ 240/1200mg every 6hours, clindamycin 150mg every 8hours, MP 8mg/day, clarithromycin 500mg every 12hours, levofloxacin 0.5g/day). The important laboratory results on admission and after treatment are summarized in Table 2. The details of antibiotic and main process of disease are summarized in Figure 3. Five months after discharge, he returned to our hospital for reexamination of chest CT which showed the inflammation in both lungs was basically completely absorbed (Figure 1E1 and E2). During this period, the patient had no symptoms such as cough and fever. Unfortunately, the patient refused blood tests including flow cytometry test.
 |
Table 2 The Important Laboratory Results on Admission and After Treatment |
 |
Figure 3 The details of antibiotic and main process of disease. |
To further confirming P. jirovecii, the BALF and blood samples detected by mNGS were sent for PCR. The commercial PneumoGenius® real-time PCR (PathoNostics, Maastricht, The Netherlands) performed by Shanghai GeneDx Biotech Co., Ltd, according to the manufacturers’ instructions using an LC480 Ⅱ real-time system, detected Pneumocystis mitochondrial large subunit of ribosomal RNA (mtLSU) gene and wild-type sequence at the dihydropteroate synthase (DHPS) locus both in BALF and blood samples. The cycle threshold (Ct) value for mtLSU of BALF and blood were 32.5 and 36.31, respectively (Figure 4).
Discussion
To the best of our knowledge, this is the first report of pulmonary co-infection with M. abscessus and P. jirovecii responsible for respiratory distress in an HIV-negative previously healthy man without any known underlying illness.
P. jirovecii is a ubiquitous organism that commonly causes pneumonia in an AIDS-defining condition, it also caused pneumonia in HIV-negative immunocompromised patients. Predisposing factors of PJP in HIV-negative patients include corticosteroids, cirrhosis, CD4+T-lymphocytopenia, chemotherapy plus corticosteroids, alcoholism and malnutrition, visceral leishmaniasis, and so on.1,6,13–15 In our case, the patient was previously healthy (he had no significant medical history (especially no chemotherapy or corticosteroids), no chronic pulmonary disorders, and no alcoholism). Also, the serology of Hepatitis B and C viruses, HIV, and Syphilis were negative, the autoantibodies (ANA, anti-dsDNA, ANCA, ENA) were all negative. There was no underling disease in this patient. However, we observed CD4+ T-lymphocytopenia in this patient (flow cytometry of peripheral blood showed CD3+CD4+T cells/lymphocyte was 17%). Therefore CD4+ T-lymphocytopenia may be the predisposing factor of PJP in this patient.
M. abscessus is an emerging pathogen of increasing medical and microbiological concern. This bacterium is responsible for lung disease, most often in predisposed patients such as those with cystic fibrosis, chronic obstructive pulmonary disease and bronchiectasis, but this bacterium affects healthy patients in one-third of cases.16 Chronic infection is the most frequent presentation. Symptoms are variable and nonspecific, and a majority of patients suffer from a recurrent or chronic cough.2,16 In our case, prior to developed fever, the patient had three-month productive cough and hemoptysis, x-ray revealed pulmonary infection, with 5-day clarithromycin treatment, the cough relieved. Then he developed fever, hypoxia, and respiratory distress, P. jiroveci sequences were detected in blood by mNGS. But just given the antifungal therapy, the patient still had hypoxemia, cough and fever. Until mNGS detected M. abscessus sequences in BALF, M. abscessus pulmonary infection was considered. Following the combination of anti-M. abscessus therapy, the clinical symptom was significantly improved. So, it was confirmed that the patient was infected with M. abscessus firstly.
M. abscessus and M. tuberculosis shared many characteristics. M. abscessus is an intracellular bacterium and replicates in macrophages. Little is known about the immune response to M. abscessus. The control of M. abscessus infection relies, as for M. tuberculosis, on interferon gamma (IFN-γ) and tumor necrosis factor (TNF)-α production by T lymphocytes. Activation of the toll-likereceptor2 (TLR2) signaling cascade leads to the activation of nuclear factor kappa B, resulting in a pro-inflammatory cytokine response.17,18 M. abscessus MAB2560 enhanced dendritic cell (DC) maturation via TLR4 and activated mitogen-activated protein kinases’ (MAPKs’) production of IL-12.19 The secretion of IL-12 will then activate and polarize naive CD4+ T cells towards Th1 cells to produce IFN-γ. Macrophages and natural killer (NK) cells can both release IL-12 and IFN-γ to guide T cells to the Th1 type phenotype.20 Persistent untreated M. abscessus infection may lead to the depletion of CD4+ T cells and NK cells, which presented as decreasing number of CD4+ T cells and NK cells in our case. Wang H et al reported a case of disseminated cutaneous infection with M. abscessus with low level of CD4+ T cells in the peripheral blood cells and with the treatment of rifampin, isoniazid, ofloxacin, clarithromycin and thymosin for 6 months, the skin lesions were greatly improved and the level of CD4+ T cells in the peripheral blood cells became normal.21 Also, some cases reported the HIV-negative patients of co-infection P. jiroveci and M. tuberculosis had transiently CD4+ T-lymphocytopenia and completely resolved after anti-TB treatment.6,22 The complex interaction between innate immune cells, such as alveolar macrophage (AM)s and DCs with CD4+T-cells is important for an effective host adaptive response, essential for Pneumocystis clearance.23 Therefore, the CD4+ T-lymphocytopenia leaves the host vulnerable to opportunistic fungal infection such as Pneumocystis. So, our patient had developed pulmonary infection caused by M. abscessus firstly which then induced a transient immunosuppression leading to PJP.
Traditional diagnostic techniques in the microbiology laboratory include specimen smear staining, growth and isolation of microorganisms in culture, detection of pathogen-specific antibodies (serology) or antigens and molecular identification of microbial nucleic acids (DNA or RNA), most commonly via PCR. In our case, the acid-fast bacilli staining and culture for M. abscessus was negative; the immunofluorescence staining for P. jirovecii was negative. The PCR of NTM and P. jirovecii was not available in our hospital.
Recently, mNGS has rapidly emerged as a promising single, culture-independent pathogen detection tool that can be performed directly on clinical specimens. mNGS targets all DNA or RNA present in a sample, allowing detection of the entire microbiome as well as the human host genome or transcriptome in patient samples.24 Fortunately, in our case, mNGS directly detected the sequences of P. jirovecii in blood, and the sequences of M. abscessus and P. jirovecii in BALF samples, allowing confirmation of the diagnosis of PJP and the M. abscessus pneumonia, and allowing timely adjustment of treatment regimens. The clinical progress was satisfactory following antifungal therapy combined with anti-M. abscessus therapy. Later, the PneumoGenius® real-time PCR detected the Pneumocystis mtLSU gene both in BALF and blood samples, which was consistent with the results of mNGS. However, PCR can only detect target DNA or RNA in the sample, while mNGS can detect all DNA or RNA present in a sample.
Unfortunately, the patient refused blood test and mNGS of BALF at the follow-up visit. The lymphocytes count and CD4+ T-cells ratio and the sequence reads of M. abscessus and P. jiroveci of this patient after cured is not known, which is the major limitation of this study.
Conclusion
Co-infection pneumonia with M. abscessus and P. jirovecii as reported here is exceptionally rare. M. abscessus infection could be a risk factor for P. jirovecii infection.
mNGS is a powerful tool for pathogen detection. This case report supports the value of mNGS in diagnosing of M. abscessus and P. jirovecii, and highlights the inadequacies of conventional diagnostic methods.
Statement of Ethics
This research complies with the guidelines for human studies and is in accordance with the Declaration of Helsinki. This work was approved by the medical ethics committee of the Third Affiliated Hospital of Sun Yat-sen University, China (No. [2020]-02-246-01). Consent was obtained from the patient for participation in this study and the publication of associated data including radiological images. The authors confirmed that personal identity information of the patient data was unidentifiable from this report.
Acknowledgments
We thank Vision Medicals Co. Ltd (Guangzhou, China) and Shanghai GeneDx Biotech Co., Ltd (Shanghai, China) for providing the technical assistance.
We thank Ziying Wang (Hangzhou Matridx Biotechnology Co., Ltd.) for providing the technical assistance.
Funding
This work was supported by the Major Science and Technology Project of Guangdong Province, China (No. 2013B020224002).
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Young N, McBride S, Morpeth S, Bryce A, Siddiqui A, Bhally H. Pneumocystis pneumonia in HIV-negative adults: missed opportunities for prevention. N Z Med J. 2020;133(1520):27–34.
2. Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175(4):367–416. doi:10.1164/rccm.200604-571ST
3. Varghese B, Shajan SE, Al MO, Al-Hajoj SA. First case report of chronic pulmonary lung disease caused by Mycobacterium abscessus in two immunocompetent patients in Saudi Arabia. Ann Saudi Med. 2012;32(3):312.
4. Floto RA, Olivier KN, Saiman L, et al. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax. 2016;71(1):88–90.
5. Ben-Mustapha I, Belkhouja K, Kheder S, et al. Coinfection with Mycobacterium tuberculosis and Pneumocystis Jirovecii in immunocompetent young woman. Arch Inst Pasteur Tunis. 2013;90(1–4):55–60.
6. Mongardon N, Bruneel F, Henry-Lagarrigue M, Legriel S, Azarian R, Bedos JP. Pneumonia involving Mycobacterium tuberculosis and Pneumocystis jiroveci in HIV-seronegative patients. Eur J Intern Med. 2008;19(7):e70.
7. Filoche P, Adoun M, Caron F, Godet C, Robert R, Meurice JC. Co-infection with Mycobacterium tuberculosis and Pneumocystis jiroveci in a patient without HIV infection. Rev Mal Respir. 2006;23(1 Pt 1):83–87.
8. Chen J, He T, Li X, Wang X, Peng L, Ma L. Metagenomic Next-Generation Sequencing in Diagnosis of a Case of Pneumocystis jirovecii Pneumonia in a Kidney Transplant Recipient and Literature Review. Infect Drug Resist. 2020;13:2829–2836.
9. Mougari F, Guglielmetti L, Raskine L, Sermet-Gaudelus I, Veziris N, Cambau E. Infections caused by Mycobacterium abscessus: epidemiology, diagnostic tools and treatment. Exp Rev Anti Infect Ther. 2016;14(12):1139–1154.
10. Wang J, Han Y, Feng J. Metagenomic next-generation sequencing for mixed pulmonary infection diagnosis. BMC Pulm Med. 2019;19(1):252. doi:10.1186/s12890-019-1022-4
11. Huang J, Jiang E, Yang D, et al. <p>Metagenomic Next-Generation Sequencing versus Traditional Pathogen Detection in the Diagnosis of Peripheral Pulmonary Infectious Lesions. Infect Drug Resist. 2020;13:567–576. doi:10.2147/IDR.S235182
12. Leo S, Gaïa N, Ruppé E, et al. Detection of Bacterial Pathogens from Broncho-Alveolar Lavage by Next-Generation Sequencing. Int J Mol Sci. 2017;18(9):2011. doi:10.3390/ijms18092011
13. Koffi N, Ngom A, Aka-Danguy E. [Association of pneumocystosis and pulmonary tuberculosis in an HIV-negative patient]. Rev Mal Respir. 1997;14(5):399–400.
14. Toledo Jr. AC, de Castro MRD. Pneumocystis carinii pneumonia, pulmonary tuberculosis and visceral leishmaniasis in an adult HIV negative patient. Braz J Infect Dis. 2001;5(3):154–157. doi:10.1590/S1413-86702001000300008
15. Onorati P, Carfagna P, Palange P, Venditti M, Serra P. CD4+ T-lymphocytopenia and Pneumocystis carinii pneumonia in a patient with miliary tuberculosis. Eur J Intern Med. 2001;12(2):134–136.
16. Griffith DE, Girard WM, Wallace RJ. Clinical Features of Pulmonary Disease Caused by Rapidly Growing Mycobacteria: an Analysis of 154 Patients. Am Rev Respir Dis. 1993;147(5):1271–1278. doi:10.1164/ajrccm/147.5.1271
17. Aulicino A, Dinan AM, Miranda-CasoLuengo AA, et al. High-throughput transcriptomics reveals common and strain-specific responses of human macrophages to infection with Mycobacterium abscessus smooth and rough variants. BMC Genomics. 2015;16(1):1046. doi:10.1186/s12864-015-2246-1
18. Davidson LB, Nessar R, Kempaiah P, Perkins DJ, Byrd TF, Fessler MB. Mycobacterium abscessus Glycopeptidolipid Prevents Respiratory Epithelial TLR2 Signaling as Measured by HβD2 Gene Expression and IL-8 Release. PLoS One. 2011;6(12):e29148. doi:10.1371/journal.pone.0029148
19. Lee SJ, Shin SJ, Lee SJ, et al. Mycobacterium abscessus MAB2560 induces maturation of dendritic cells via Toll-like receptor 4 and drives Th1 immune response. BMB Reports. 2014;47(9):512–517. doi:10.5483/BMBRep.2014.47.9.001
20. Tomioka H, Tatano Y, Maw WW, Sano C, Kanehiro Y, Shimizu T. Characteristics of suppressor macrophages induced by mycobacterial and protozoal infections in relation to alternatively activated M2 macrophages. Clin Dev Immunol. 2012;2012:635451.
21. Wang H, Jin P, Wu Q. Disseminated cutaneous infection with Mycobacterium abscessus in a patient with a low CD4+ T cell count. Eur J Dermatol. 2008;18(3):337–340. doi:10.1684/ejd.2008.0400
22. Onorati P, Carfagna P, Palange P, Venditti M, Serra P. CD4+ T-lymphocytopenia and Pneumocystis carinii pneumonia in a patient with miliary tuberculosis. Eur J Intern Med. 2001;12(2):134–136. doi:10.1016/S0953-6205(01)00113-3
23. Kelly MN, Shellito JE. Current understanding of Pneumocystis immunology. Future Microbiology. 2001;12(2):43–65. doi:10.2217/fmb.09.116
24. Chiu CY, Miller SA. Clinical metagenomics. Nat Rev Genet. 2019;20(6):341–355.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


