Back to Journals » Drug Design, Development and Therapy » Volume 14
Co-Delivery Anticancer Drug Nanoparticles for Synergistic Therapy Against Lung Cancer Cells
Received 7 August 2020
Accepted for publication 2 October 2020
Published 23 October 2020 Volume 2020:14 Pages 4503—4510
DOI https://doi.org/10.2147/DDDT.S275123
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Anastasios Lymperopoulos
Yuzhou Shen, Jicheng TanTai
Department of Thoracic Surgery, Shanghai Chest Hospital, Shanghai Jiao Tong University, Shanghai 200030, People’s Republic of China
Correspondence: Jicheng TanTai Email [email protected]
Introduction: This study aims to develop a novel co-delivery gefitinib and quercetin system loaded with PLGA-PEG nanoparticles and evaluate their antitumor activity in vitro and in vivo.
Methods: Gef/Qur NPs were prepared and characterized. The release of drugs, stability, cellular uptake and cytotoxicity were evaluated in vitro. The antitumor effects and systemic toxicity of different formulations were also investigated.
Results: Gef/Qur NPs displayed a smaller particle size and a PDI and zeta potential of 0.11 and − 23.5 mV, respectively. The hydrophobic Gef and Qur content in NPs reached up to 65.2% and 56.4%, respectively, and their high entrapment efficiencies recorded 83.7% and 82.3%, respectively. The in vitro release of Gef/Qur from the NPs was sustained for 12 h. Compared with control groups, Gef/Qur NPs showed higher cellular uptake and cell inhibition rates. In vivo studies identified the lungs as the target tissue and the region of maximum drug release. Through pharmacodynamics analysis, we found that two drugs (Gef and Qur) were incorporated into one nanoparticle carrier, which played a good role in generating synergistic effect.
Discussion: It is concluded that PLGA-PEG is an ideal drug carrier for the co-delivery of Gef/Qur to treat lung cancer.
Keywords: gefitinib, quercetin, PLGA-PEG, nanoparticles, in vitro release, antitumor effect
Introduction
A multi‐step and multi‐factorial disease, lung cancer has a variety of histological subtypes, and is the most fatal cancer worldwide. It is estimated that 2.09 million new cases of lung cancer occurred globally in 2018, ranking first among all cancer types. In some developed countries such as Austria and Germany, lung cancer is one of the most common cancers.1 The incidence rate of newly diagnosed lung cancer in China is over 1/3, inducing a heavy burden on patients, families, society and the country as a whole. Lung cancer is the most common cancer in China and the main cause of cancer-related death. The risk factors associated with it have been well studied under limited conditions by medical staff engaging in lung cancer prevention. However, diagnosis often occurs so late that about two-thirds of patients would have lost the chance of radical surgery. These patients usually die within one to two years. The 5-year survival rate in 2008 was very low. The age standardized (World) mortality rate of lung cancer in China was 28.7/100,000, significantly higher than the world average (19.4/100,000).2,3 At present, systematic administration of chemotherapeutic drugs is still the main treatment for lung cancer, despite the high incidence of side effects and insufficient drug exposure to the lungs.
Gefitinib (Gef), as the first selective inhibitor of EGFR tyrosine kinase domain, is widely used in chemical therapy for many kinds of tumors.4 It acts by inhibiting EGFR tyrosine kinase and belongs to Biopharmaceutical Classification System class II. Its low solubility (sparingly soluble at pH 1) in upper gastric fluid affects the onset of action, bioavailability, and therapeutic activity. The logarithm P value of GFT is 4.15, which indicates that Gef has strong hydrophobicity. The daily oral dose is 250 mg and the bioavailability is 44%.5 The most common adverse drug reactions are hepatobiliary diseases, gastrointestinal diseases, metabolism and nutrition disorders, skin and subcutaneous diseases, etc.6 Therefore, it is necessary to improve the oral bioavailability of Gef and reduce the daily oral dose. Unfortunately, however, after a time period of drug exposure, some patients’ response to Gef will be greatly reduced due to acquired resistance.7–10
Quercetin (Qur), or 3,3ʹ,4ʹ,5,7-pentahydroxyflavone, is categorized as a flavonol, one of the six subclasses of flavonoid compounds. It exhibits a wide range of biological activities.11 The significant antitumor, antiallergy and anti-inflammatory effects of quercetin have been extensively reviewed.12,13 There is evidence that Qur can target different types of cancer cells, including leukemia, breast cancer, esophageal cancer, colon cancer, prostate cancer, nasopharyngeal carcinoma, endometrial cancer and lung cancer.14,15 It can inhibit the proliferation of these malignant cells; however, the exact molecular mechanism of its effect is not clear. In addition, Qur has low water solubility, poor absorption and rapid metabolism (bioavailability of about 1–5%),16 all of which can generate in vivo results that differ from the powerful in vitro efficacy of Qur.
Although literature has reported the efficacy of each of these two drugs, beyond this, there has been no study on the combinatorial therapeutic effects or on the co-encapsulation of these drugs for systemic injection. Therefore, we envisioned that nanomedicine approaches could significantly improve pharmacokinetics and precisely tailor the intracellular interplay of two drugs, thereby potentiating higher synergistic efficacy compared with the oral administration or either drug alone. PLGA has variable properties that give different physicochemical characteristics to the polymer. Surface-modified PLGA NPs with poly(ethylene glycol) (PEG) have also been prepared using the copolymer of PLGA and PEG to have nanoparticles (NPs) formulation with enhanced long-circulating properties.17–19 The present study aims to develop a co-delivery system of Gef and Qur with PLGA-PEG nanoparticles (Gef/Qur NPs) that could be efficiently internalized into lung cancer cells. Gef/Qur NPs were prepared and characterized. The release of drugs, stability, cellular uptake and cytotoxicity were evaluated in vitro. The antitumor effects and systemic toxicity of different formulations were also investigated.
Materials and Methods
Materials
Gef was purchased from DiBo Chemical Co., Ltd (Hubei, China) and Qur from Changyue Co., Ltd (Xian, China). PLGA–PEG diblock copolymer (50:50 PLGA attached to mPEG 5000, 15%. wt) was obtained from Melo Biotech Co., Ltd. (Shanghai, China). PC-9 lung cancer cell line was provided by the Institute of Biochemistry and Cell Biology, Chinese Academy of Sciences (Shanghai, China). All the other reagents used were of analytical grade and were used without further purification.
Animals
The experiment was carried out on mice with a weight of 20 ± 2 g. The animals were kept in cages in a room at a temperature of 25±2°C, with a 12/12 light-dark cycle and free access to food and water. All animal experiments were performed in accordance with institutional guidelines, following a protocol approved by the Ethics Committees of Shanghai Jiao Tong University. The guide of the National Institutes of Health for the care and use of laboratory animals was strictly followed.
Preparation of NPs
Gef/Qur NPs were prepared from PLGA–PEG diblock copolymers by modified emulsification solvent evaporation method.20 Briefly, the polymer (0.5 mg) and an appropriate amount of drug (Gef 5mg; Qur 5mg) were dissolved in ethyl acetate (5mL) to obtain a solution of 10% (w/v) PLGA–PEG, 1.0 mg/mL (w/v) Gef and 1.0 mg/mL (w/v) Qur, which was then added to a solution of 2.5% (w/v) poly(vinyl alcohol) (PVA). The mixture was then shaken vigorously and treated with sonication (200 Hz, 2min). The resulting nanosuspension was then left to stir for 2 h to evaporate the organic solvent (45 ± 2°C). After subsequent ultracentrifugation/washing with distilled water, NPs were obtained, resuspended in 1% sucrose aqueous solution and lyophilized, and then kept at -–20°C for further use.
Characterization
The particle size distribution, zeta potential and polydispersity index (PDI) of the NPs were measured by a dynamic light scattering analyzer.21 The transmission electronic microscopy (TEM) observations were conducted using a JEOL JEM-100CX2 TEM with an acceleration of 180 kV. The samples were pretreated with negative staining using uranyl acetate on a copper grid.
The Gef/Qur NPs were dissolved in an assay solution composed of 50% methanol, and incubated at 40 °C for 30 min. The Gef and Qur concentration were determined to be at 254 nm using the HPLC method. The drug loading coefficient (DL%) and encapsulation efficiency (EE%) of the NPs were calculated by the following equations:
DL%=WE/WL×100
where WE was the drug amount encapsulated in NPs and WL was the total weight of NPs and
EE%= WE/WT ×100
where WE was the drug amount encapsulated in NPs and WT was the total amount of drug added.
Stability Study
The proposal of stability study was mainly revised according to the guidelines of the Chinese Pharmacopoeia. The NPs were placed in a stable chamber at room temperature and saturated with sodium chloride solution with a relative humidity of 75%±5%. In the 0, 1st, 2nd, 3rd month of testing, they were assessed to determine whether the particle size, PDI, zeta potential, EE% and DL% had changed.
In vitro Drug Release
The drug release in vitro was assayed using dialysis. The Gef/Qur NPs or free drugs were placed in a dialysis bag (MWCO 12 kDa) and dialyzed in 180 mL phosphate-buffered saline (PBS, pH 7.4) containing 10% alcohol at 37 °C on a shaker (100 rounds per minute). At a predetermined time-point, 1 mL of release medium was removed and replaced with an equal amount of fresh release medium. Experiments were performed in triplicate.
In vitro Cytotoxicity Assay
The cytotoxicity of Gef/Qur NPs was assessed in PC-9 lung cancer cell lines and compared to blank NPs, free drugs and single drug NPs. Briefly, cells were seeded in 96-well plates followed by 24 h of incubation in RPMI-1640 medium with 10% FBS and 1% streptomycin−penicillin. Twenty-four hours later, cells were incubated with a different sample of varying concentrations of blank NPs, free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs (from 0.05 µg/mL to 20 µg/mL) for 24 h and cell viability was assessed with MTT (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay in accordance with the following procedure: 100 µL of complete growth culture medium and 60 µL of MTT solution (5 mg/mL in PBS) were added to each well for 4 h of incubation. The absorbance, measured with a microplate reading instrument, was 540 nm. The results were expressed as percentages relative to the results obtained with a non-toxic control.
Cellular Uptake
The cellular internalization of free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs was visualized by confocal microscopy using DiR as the staining mark. PC-9 cells were grown in RPMI 1640 medium supplemented with 10% (v/v) FBS and 5% antibiotics (100 IU/mL of penicillin G sodium and 100 µg/mL of streptomycin sulfate). PC-9 cells were inoculated in cell culture dishes with 1×105 cells per dish as the initial density. Cells were then incubated with DiR labeled free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs (equivalent to 0.1 µg/mL of DiR) for 2 h at 37°C±0.5°C.
Subsequently, cells were washed several times with PBS, and fixed with 4% paraformaldehyde for 10 minutes before observed under a confocal microscope. For quantitative drug uptake estimates, the density of cells inoculated in 24-well plates was 1×105 cells per plate. When they reached 70–80% confluence, cells were incubated with DiR labeled free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs (equivalent to 0.1 µg/mL of DiR) for 2 h and then washed several times with cold PBS. Subsequently, cells were dissolved by the addition of Triton X-100 (0.1%). Fluorescence intensities were measured by flow cytometry at an excitation wavelength of 750 nm and an emission wavelength of 782 nm.
In vivo Antitumor Activity
The PC-9 model was established as described previously.22 The treatments were started on the day when the tumor volume reached 100–150 mm, and this day was marked as day 0. On the following day, the mice were randomly and evenly divided into 6 groups (8 each): group 1 was given a 5% glucose injection, group 2 and 3 were given free Gef and free Qur, group 4 and 5 were given Gef NPs and Qur NPs, and group 6 was given Gef/Qur NPs, all via the tail vein on day 1, 4, 7, 10 and 13 at a dose of 20 mg/kg. The diameter of the tumor was measured with a digital caliper. The tumor volume (mm3) was calculated using the formula tumor volume=length×width2×0.5. Throughout the study, the mice were weighed regularly to monitor potential toxicities.
Statistical Analysis
One-way analysis of variance (ANOVA) was used to compare the mean of different treatment groups. A P-value of less than 0.05 was considered statistically significant, unless otherwise stated.
Results and Discussion
Preparation and Characterization
In order to further effectively solubilize free hydrophobic Gef and Qur molecules, Gef/Qur NPs were prepared by the emulsification solvent evaporation method. Amphiphilic copolymers of PLGA and PEG spontaneously formed core–shell structural NPs, and their particle size and zeta potential were measured by DLS. It was estimated that the drug loading of NPs was very high, and the parameters of NPs are shown in Table 1. Compared with the blank nanoparticles (140 nm), Gef/Qur NPs showed a smaller particle size (126.8 nm), which may be due to the increased cohesive force that formed smaller cores resulted from the interactions between Gef/Qur and PLGA-PEG segments. The PDI and zeta potential of Gef/Qur NPs were 0.11 and −23.5 mV, respectively. The low PDI (<0.2) indicated that NPs have a narrow size distribution. The negative charge as measured by zeta potential (<−20 mV) confirmed the shielding effect of PEG on NPs surface, which provided enough repulsion force among particles and improved physical stability. The hydrophobic Gef and Qur content in NPs reached up to 65.2% and 56.4%, respectively, with entrapment efficiencies being 83.7% for Gef and 82.3% for Qur. In addition, TEM was used to confirm the size and morphology of Gef/Qur NPs (Figure 1A and B). The amphiphilic copolymer assembled into a nearly spherical morphology with a narrow size distribution and clear boundary, confirming their self-assembly behavior.
 |
Table 1 The Characteristics and Stability Data of Gef/Qur NPs. (n=3) |
 |
Figure 1 (A) The nanostructure of Gef/Qur PLGA-PEG NPs; (B) The transmission electron microscope of Gef/Qur PLGA-PEG NPs (Magnification ×100,000). |
Stability
The stability data of Gef/Qur NPs is also summarized in Table 1. In the stability test, NPs maintained a good round shape, and none of Gef/Qur NPs demonstrated significant changes in physicochemical characteristics. In addition, no aggregation or precipitation of NPs was observed during the 3-month storage period. The stability study indicates that a proper formulation (lyophilized liposomes) may increase the storage time of the drug.
In vitro Drug Release
The in vitro release kinetics exhibited by the Gef/Qur NPs and free drugs were evaluated via dialysis. Free Gef and Free Qur were rapidly released and reached a cumulative release of 95% of the total drug within 4–6 h. In comparison with free drugs, a longer time was required for the release of Gef or Qur from Gef/Qur NPs. Figure 2 demonstrates that the encapsulated drugs underwent a sustained release. The release curve in PBS could be divided into two phases: the initial phase of fast release and a later phase of stable release. After the initial phase, the drug was released stably at a lower speed in the later phase through diffusion resulted from the continuous degradation of the polymer. As shown in Figure 2, during the entire study period, a sustained Gef (Qur) release to a total of about 72% (64%) was found in the group of Gef/Qur NPs. It showed that Gef/Qur NPs could be used as a lasting and effective drug delivery system. Meanwhile, the release curve of the stable samples after 3 months showed no significant difference compared with that of the initial samples.
 |
Figure 2 The accumulative release of Gef/Qur PLGA-PEG NPs and other formulations in the medium at pH=7.4 containing 10% alcohol at 37 °C. The results were expressed as mean ± SD (n=3). |
In vitro Cytotoxicity Assay
Figure 3 shows the antiproliferative effect of Gef/Qur NPs on PC-9 lung cancer cells. The cells were treated with different formulations, and the cytotoxicity was measured by MTT 24 hours later. All the preparations showed time- and concentration-dependent cytotoxicity to PC-9 cells. The negative control was blank NPs and the cell survival rate was 100%. This result shows the safety of the unloaded vector. Gef (Qur) NPs and free Gef (Qur) have similar cytotoxicity characteristics as expected. With the increase of drug concentration, more cell death was observed. Gef/Qur NPs were the most potent among all the formulations with respect to cell growth inhibition. These results showed that the co-delivery system had obvious synergistic effects. As summarized in Table 2, the IC 50 was 4.12, 3.89, 2.57, 2.12 and 0.65 µg/mL for free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs, respectively.
 |
Table 2 IC50 Values of All Formulations in PC-9 Cells Following 24-Hour Treatments, Respectively (n=6) |
Cellular Uptake
Free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs were labeled by DiR staining marks. As shown in Figure 4, the nucleus showed red fluorescence, indicating that it was stained by DiR. After PC-9 cells were treated with Gef/Qur NPs, there was a strong red fluorescence in the perinuclear region, indicating that there were enough Gef/Qur NPs entering the cytoplasm. In contrast, red fluorescence was rarely seen in PC-9 cells treated with free drugs. In addition, PC-9 cells pretreated by Gef NPs/Qur NPs also showed slight red fluorescence, which may be attributed to their weak ability to cross cells. The cellular uptake ratio of the NPs, detected by flow cytometry, was 21.2±2.9%, 23.5±2.7%, 43.4±5.2%, 45.6±4.9% and 73.4±6.4% for free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs treated PC-9 cells, respectively. These results demonstrate that the Co-delivery Gef/Qur strategy can facilitate highly efficient uptake of PLGA-PEG NPs by PC-9 cells.
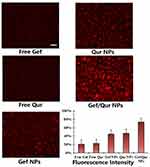 |
Figure 4 Cellular uptake of DiR-loaded NPs, free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs for 2 h by PC-9 cells. Quantitative results of cellular uptake of different groups. The bar was 10 μm. |
In vivo Antitumor Activity
As shown in Figure 5A, the free Gef and free Qur groups showed some ability to inhibit tumors, but did not deliver outstanding performance. Meanwhile, both Gef NPs and Qur NPs significantly inhibited the growth of the PC-9 tumors in mice. However, Gef/Qur NPs started to more effectively inhibit tumor growth than single drug NPs on day 13. The tumor volumes of the Gef/Qur NPs group were smaller than those in others. At the end of the experiment, the tumor volume was 7.2-fold, 6.3-fold, 5.6-fold, 4.3-fold, 3.6-fold and 2.2-fold as much as that at the beginning of the experiment for the group of blank NPs, free Gef, free Qur, Gef NPs, Qur NPs and Gef/Qur NPs. Changes in body weights of tumor-bearing mice were presented in Figure 5B. The body weights of mice recorded throughout the study were fairly constant, indicating that the preparation was not significantly toxic.
Conclusions
This study aims to develop a novel co-delivery gefitinib and quercetin system loaded with PLGA-PEG nanoparticles and evaluate their antitumor activity in vitro and in vivo. Gef/Qur NPs displayed a smaller particle size and the PDI and zeta potential of Gef/Qur NPs were 0.11 and −23.5 mV, respectively. The hydrophobic Gef or Qur content in NPs reached up to 65.2% and 56.4%, respectively, with high entrapment efficiencies of 83.7% for Gef and 82.3% for Qur. The in vitro release of Gef/Qur from the NPs was sustained for 12 h. Compared with control groups, Gef/Qur NPs showed higher cellular uptake and cell inhibition rates. In vivo studies identified the lungs as the target tissue and the region of maximum drug release. Through pharmacodynamics analysis, we found that two drugs (Gef and Qur) were incorporated into one nanoparticle carrier, which played a good role in generating synergistic effect. At last, PLGA-PEG was an ideal drug carrier for the co-delivery of Gef/Qur to treat lung cancer.
Disclosure
The authors declare that they have no conflict of interest.
References
1. Ferlay J, Ervik M, Lam F, et al. Global cancer observatory: cancer today. Lyon, France: International Agency for Research on Cancer; 2018 [
2. Chang S, Dai M, Ren JS, Chen YH, Guo LW. Estimates and prediction on incidence, mortality and prevalence of lung cancer in China in 2008 [article in Chinese]. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33(4):391–394.
3. Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. doi:10.1002/ijc.25516
4. Rawluk J, Waller CF. Gefitinib. Recent Results Cancer Res. 2018;211:235–246.
5. Budha NR, Frymoyer A, Smelick GS, et al. Drug absorption interactions between oral targeted anticancer agents and PPIs: is pH dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther. 2012;92(2):203–213. doi:10.1038/clpt.2012.73
6. EGFR Tyrosine Kinase Inhibitor. Product monograph – IRESSA®. Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor; 2016. Available from: https://www.drugs.com/cdi/gefitinib.html.
7. Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer. 2018;17(1):38.
8. Liu Z, Gao W. Overcoming acquired resistance of gefitinib in lung cancer cells without T790M by AZD9291 or Twist1 knockdown in vitro and in vivo. Arch Toxicol. 2019;93(6):1555–1571. doi:10.1007/s00204-019-02453-2
9. Gao J, Li HR, Jin C, Jiang JH, Ding JY. Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer. Clin Transl Oncol. 2019;21(10):1287–1301. doi:10.1007/s12094-019-02075-1
10. Wan Y, Yuan Y, Pan Y, Zhang Y. Antitumor activity of high-dose pulsatile gefitinib in non-small-cell lung cancer with acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. Exp Ther Med. 2017;13(6):3067–3074. doi:10.3892/etm.2017.4356
11. Kelly GS. Quercetin monograph. Altern Med Rev. 2011;16(2):172–194.
12. Guazelli CF, Fattori V, Colombo BB, et al. Quercetin-loaded microcapsules ameliorate experimental colitis in mice by anti-inflammatory and antioxidant mechanisms. J Nat Prod. 2013;76(2):200–208. doi:10.1021/np300670w
13. Liu H, Xue JX, Li X, Ao R, Lu Y. Quercetin liposomes protect against radiation-induced pulmonary injury in a murine model. Oncol Lett. 2013;6(2):453–459. doi:10.3892/ol.2013.1365
14. Linsalata M, Orlando A, Messa C, Refolo MG, Russo F. Quercetin inhibits human DLD-1 colon cancer cell growth and polyamine biosynthesis. Anticancer Res. 2010;30(9):3501–3507.
15. Chuang-Xin L, Wen-Yu W, Yao C, Xiao-Yan L, Yun Z. Quercetin enhances the effects of 5-fluorouracil-mediated growth inhibition and apoptosis of esophageal cancer cells by inhibiting NF- κ B. Oncol Lett. 2012;4(4):775–778. doi:10.3892/ol.2012.829
16. Reinboth M, Wolffram S, Abraham G, Ungemach FR, Cermak R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br J Nutr. 2010;104(2):198–203. doi:10.1017/S000711451000053X
17. Martins LG, Khalil NM, Mainardes RM. PLGA nanoparticles and polysorbate-80-coated PLGA nanoparticles increase the in vitro antioxidant activity of melatonin. Curr Drug Deliv. 2018;15(4):554–563. doi:10.2174/1567201814666170719112535
18. Joyce P, Prestidge CA. Synergistic effect of PLGA nanoparticles and submicron triglyceride droplets in enhancing the intestinal solubilisation of a lipophilic weak base. Eur J Pharm Sci. 2018;118:40–48. doi:10.1016/j.ejps.2018.03.018
19. Sadat Tabatabaei Mirakabad F, Nejati-Koshki K, Akbarzadeh A, et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pac J Cancer Prev. 2014;15(2):517–535. doi:10.7314/APJCP.2014.15.2.517
20. Paswan SK, Saini TR, Paswan SK, et al. Purification of drug loaded PLGA nanoparticles prepared by emulsification solvent evaporation using stirred cell ultrafiltration technique. Pharm Res. 2017;34(12):2779–2786. doi:10.1007/s11095-017-2257-5
21. Fornaguera C, Solans C, Fornaguera C, et al. Characterization of polymeric nanoparticle dispersions for biomedical applications: size, surface charge and stability. Pharm Nanotechnol. 2018;6(3):147–164. doi:10.2174/2211738506666180706121515
22. Han W, Shi L, Ren L, et al. A nanomedicine approach enables co-delivery of cyclosporin A and gefitinib to potentiate the therapeutic efficacy in drug-resistant lung cancer. Signal Transduct Target Ther. 2018;3(1):16. doi:10.1038/s41392-018-0019-4
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


