Back to Journals » Medical Devices: Evidence and Research » Volume 7
Clinical utility of the Covidien Closure Fast™ Endovenous Radiofrequency Ablation Catheter
Authors Braithwaite S, Braithwaite B
Received 17 January 2014
Accepted for publication 20 March 2014
Published 4 June 2014 Volume 2014:7 Pages 179—185
DOI https://doi.org/10.2147/MDER.S48141
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Simon A Braithwaite,1 Bruce D Braithwaite2
1University College Hospital Medical School, London, UK; 2Department of Cardiovascular Medicine, University of Nottingham, Nottingham, UK
Abstract: The Closure Fast™ Endovenous Radiofrequency Ablation Catheter is the latest version of a minimally invasive system for the treatment of patients with superficial venous disease. The Closure Fast™ catheter heats the vein wall to 120°C, causing denaturation of the collagen of the vein wall and contraction of the vessel such that no blood can flow through it. Nearly one million systems have been sold since the product was launched. Many, if not all, patients can be treated under local anesthesia with the Closure Fast™ catheter. Duplex ultrasound reports occlusion rates for the treated vein of 94%–98% at 1 year and 85%–93% at 3 years. The system produces average postoperative pain scores of less than 2 out of 10 on a visual analog score. In the first postoperative week, 76% of patients do not require analgesia. Some 45% of patients return to normal activity on the first postoperative day. Serious complications appear to be rare following the Closure Fast™ procedure. Transient paresthesia occurs in 0.2% of cases, thrombophlebitis in 1%–10%, and thromboembolic events in up to 1.4%, mainly heat-induced thrombosis. Closure Fast™ adds significant costs to treating superficial venous disease but studies have shown it to be cost-effective when used in an office setting.
Keywords: Closure Fast, catheter, Endovenous Radiofrequency Ablation Catheter
Aims
This paper aims to analyze current published research on the Closure Fast™ Endovenous Radiofrequency Ablation Catheter.
Introduction
The introduction of minimally invasive endovenous radiofrequency ablation (RFA) has revolutionized the treatment of venous incompetence. Endovenous RFA was approved for used by the National Institute for Health and Clinical Excellence (NICE) in the UK in 2001 and the guidance published in September 2003. NICE guidance states that “Current evidence on the safety and efficacy of radiofrequency ablation of varicose veins appears adequate to support the use of this procedure as an alternative to saphenofemoral ligation and stripping, provided that the normal arrangements are in place for consent, audit and clinical governance.”1 There are several perceived advantages of endovenous RFA over traditional surgery, including reduced pain, faster recovery times, and lower complication rates.
The Closure Fast™ (formally known as VNUS® ClosureFAST, Covidien, Dublin, Ireland) Endovenous Radiofrequency Ablation Catheter is the latest version of a minimally invasive system for the treatment of patients with superficial venous disease. Covidien manufactures the catheter and the associated procedure is termed Venefit™. Nearly one million systems have been sold since the product was launched (personal communication with Covidien Sales, January, 2014). Along with laser ablation systems, and foam sclerotherapy and its predecessors (Closure™ and Closure plus™),2 Closure Fast™ has helped to revolutionize the way that patients with varicose veins can be treated.
Methods
A systematic review of online databases, web of knowledge, and PubMed was carried out. The following search terms were used: “ClosureFast,” “Radiofrequency Ablation catheter,” “varicose veins,” “saphenous vein.” A total of 30 papers were deemed acceptable for the criteria required to answer the question posed by this paper.
Mechanism of action
Closure Fast™ is one of a class of systems that uses thermal energy to destroy both the endothelium and the collagen within the walls of veins. The Closure Fast™ catheter heats the vein wall to 120°C. This temperature has been shown to be sufficient to cause proteins in the wall of the vein to denature. Thermal injury results in changes in the collagen of the vein wall and contraction of the vessel such that no blood can flow through it.3 Over time, the vein is gradually reabsorbed by the body’s natural mechanisms so that there is little, if any risk of recanalization.
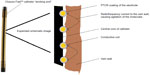
The patent for Closure Fast™ was filed in 2005 and granted in 2009 (US patent 7517349 B2).4 It is described as an electrosurgical instrument and method for treating varicose veins using an elongated catheter. “The distal end carries a coiled electrode with a positive temperature coefficient of resistance (PTCR) surface and an electrode with a pressure sensitive variable resistance to provide a smart surface for controlling radiofrequency current flow at the interface between the electrosurgical surface and the tissue.”4 In plain English, this means a coil of wire around a catheter with a feedback system that ensures that the wire heats tissue to a constant temperature of 120°C, independent of the temperature and conductivity of the tissue, and avoids the formation of charring. Along with its predecessors and radiofrequency competitors, the Closure Fast™ uses bi-polar radiofrequency to cause ohmic heating of tissue. The conductive coil delivers a radiofrequency current to the vein wall, causing agitation of the molecules in the wall, heating, and then denaturation of the proteins (Figure 1). As the tissue heats up, it warms the PTCR coating of the electrode, which in turn reduces the radiofrequency flow and avoids overheating and subsequent charring of the tissue. This problem occurred with the Closure™ and Closure plus™ systems and requires cleaning of the electrode. It is also known to occur with the Celon system.
Description of Venefit technique
Along with other thermal systems, Celon,5 and laser and steam,6 the Closure Fast™ catheter can only be used in relatively straight vessels such as the great saphenous, small saphenous, and anterior saphenous veins.7 It is necessary to ablate these vessels in order to reduce the risk of a failed procedure.8 The patients should be positioned supine for treatment of the great saphenous or anterior thigh vein, and in the prone position for ablation of the small saphenous or inter-saphenous veins.
The Venefit procedure, using Closure Fast™, is similar to other methods of minimally invasive vein therapy. Briefly, the patient’s symptoms need to be assessed and their varicose veins examined using duplex ultrasound. Once it has been established that the truncal vein is incompetent, the procedure involves percutaneous cannulation and insertion of a 7F sheath into the target vein.9 Using ultrasound, the Closure Fast™ catheter is advanced through the sheath and into the lumen of the vein to the most proximal part that needs to be treated. The final placement of the catheter tip must be 2 cm from the saphenofemoral junction. If the vein has a tortuous or kinked section, a 0.18-inch guidewire can be advanced along the vein and the catheter can be introduced in a standard over-the-wire technique.
The “working end” of the Closure Fast™ comes in two lengths – 7 cm and 3 cm. Clinicians mostly use the longer system, with the shorter one being reserved for situations where only a short length of vein needs to be treated. The 3 cm length was introduced in 2010, possibly to address one of the perceived advantages of a competing system (the Olympus Celon RF system).5
The site of cannulation is determined by the anatomy of the patient, but clinicians should aim to introduce the device as distally as possible, in order to treat the maximum amount of incompetent vein. There is little point in introducing the 7 cm system within 10 cm of the junction between the superficial truncal vein and its connection to the deep vein. In shorter sections of this type, the 3 cm device can be used.
Provided the clinician can cannulate the vein and introduce a sheath, most truncal veins can be treated. Problems can occur if the preoperative duplex fails to identify areas of scarring within the vein; this can make it difficult to pass the catheter and results in an abandoned procedure with the attendant frustration and cost. It is therefore essential that the clinician fully examines the truncal vein with ultrasound to exclude any areas of scarring. In a long vein with a single area of scarring or severe tortuosity, the catheter can be introduced above and below the abnormal area via two punctures and two sheaths. These same problems and solutions occur with laser fibers and the Celon catheter. If there is too much scarring, then foam sclerotherapy of the vein should be considered.
In order to reduce the risk of collateral damage to nerves and other tissue, it is essential that the Venefit procedure is performed in combination with tumescent anesthesia. This involves the injection of crystalloid solution, usually combined with a locally acting anesthetic, along the length of the vein to be treated. As compression is used, veins which have sections of more than 1.5 cm diameter can be treated using Closure Fast™. Once the catheter is in place with the patient’s foot elevated, using ultrasound, the tumescent crystalloid fluid is injected into the perivenous sheath so that the whole vein is surrounded by a “halo” of fluid which compresses the vein wall onto the catheter and insulates structures that surround the vein (Figure 2). Tumescent anesthesia is also used with laser and steam10,11 systems and means that many, if not all, patients can be treated under local anesthesia. This is often cited as being better for patients, allows procedures to be done in an “outpatient” or “office” environment, and reduces the cost of the procedure.12
Once tumescent anesthesia has been correctly infused into the patient’s leg, the catheter is then attached to a generator (Figure 3), which, when switched on, delivers the radiofrequency energy. Each section of vein (7 cm or 3 cm) is heated for 20 seconds before the energy is automatically switched off. The catheter is then manually withdrawn to the next marker on the catheter (7 cm or 3 cm, respectively) and energy is then applied to the next section. In this way, a typical great saphenous vein can be treated in under 3 minutes (49 cm takes 7 × 20 seconds =140 seconds) without the operator having to withdraw the catheter at a steady rate as is required for laser ablation or the Celon system. It is therefore very easy to use. Ultrasound examination of the vein wall normally demonstrates that it is thickened and more echogenic than before the procedure with a smaller total vein diameter.13 This normally indicates successful treatment. Double treatment of each 7 cm section has been suggested to improve outcomes but one small study of 67 treatments showed no benefit at 1 year, although the reduction in diameter was greater.14
  | Figure 3 Image of the Closure Fast™ catheter (ClosureFAST, Covidien, Dublin, Ireland) connected to the Venefit™ Generator. |
Once completed, the Closure Fast™ system and sheath are removed. The system can be used multiple times on the same patient during the same operating session and is then disposed of as it is designed for single patient use. There are reports of reprocessed devices being used in order to save institutions US$178 per procedure without any apparent detrimental outcome.15 There are no reports of cross-infections as a result.
Once the Venefit™ procedure has been completed, any residual varices can be left alone, removed by phlebectomies, or treated by foam sclerotherapy.13
Findings from literature search
There have been several studies reporting the efficacy of the Closure Fast™ system. Duplex ultrasound reports occlusion rates of 100% at 1 week,16 97%–99.7% at 3 months,13,17 94%–98% at 1 year,13,16,18,19 and 85%–93% at 3 years.11,13
When compared with other techniques such as laser, steam, traditional surgery, and foam sclerotherapy, it appears that Closure Fast™ is equally efficacious but may have a lower pain and side effect profile.6
Pain
One of the advantages of Closure Fast™ is reduced postoperative pain when compared with other endothermal methods and traditional surgery. In studies done under local anesthesia, Closure Fast™ produced average pain scores of between 3 and 4 out of 10 on a visual analog scale during the procedure (from the injection of tumescence). The average postoperative pain scores were less than 2 in the first week, with up to 76% of patients not requiring analgesia and those that did taking only paracetamol and simple non-steroidal anti-inflammatory drugs.18,20,21 The 46 patients in the RECOVERY study had pain scores of less than 1 at 48 hours reducing still further over the first week.22
Return to normal activity
In a study of 104 patients, 45% returned to normal activity on the first postoperative day, with 100% at 1 week.20 Other studies have reported the number of days unable to work after the procedure to be 1 day, with a range of 0–28 days.17,18,21
Complications
Serious complications appear to be rare following the Closure Fast™ procedure. No articles were identified in a literature search that specifically assessed complications following the use of Closure Fast™. The complications described below have been obtained from the various studies describing clinical outcomes but include a recent article analyzing over 2,000 treatments, 317 of whom had Closure Fast™.23
There have been no published reports of intra-operative complications as have been seen with laser treatment.24 The senior author has, however, seen photographs of the coiled element unwound after the operator using the needle for tumescent anesthesia damaged it.
Another intra-operative injury reported by personal communications included direct thermal damage to the skin when the 7 cm “working” end was partly in the patient and partly in the sheath used to access the target vein. Clinicians need to ensure that the sheath is removed when they see the “hatched” markings on the catheter, indicating that the “working end” is close to the vein puncture site. Skin burns occurred in 0.7% of patients in two studies reporting the complication.23
Both of these intra-operative complications were due to operator error rather than device failures.
Paresthesia, probably related to either thermal injury or the effects of inflammation on cutaneous nerves occurs in 0.2%–13.7% of patients.11,13,16,18,22 Most symptoms are transitory and tend to settle within a few weeks of treatment.
Skin pigmentation is reported to occur in 0.2%–5.5% of treatments.11,13,16,18,22 This is likely to occur in patients where the treated vein runs close to the skin, as occurs in slim patients. It can also occur when the clinician treats a tributary of the truncal vein rather than treating the vein in its perivenous sheath. It has been suggested that veins close to the skin might be better treated with phlebectomies to avoid such a side effect.
Thrombophlebitis after Closure Fast™ treatment is reported to occur in 1%–10% of patients.8,12,15–18
Iatrogenic arteriovenous fistula formation has been reported to occur following endovenous laser ablation and there is at least one report of it occurring following the Closure Fast™ procedure. In order to avoid this and possibly many of the complications associated with the technique, it is essential that adequate tumescent fluid is injected around the target vein, particularly where it is close to the deep venous system (the saphenofemoral junction).25
Thromboembolic events occurred in up to 1.4% of patients.11,13,16,18,19 There are reports of a specific complication related to endothermal ablation techniques – endovascular heat-induced thrombosis (EHIT). This can occur in up to 8% of treatments and is characterized by protrusion of the thrombus from the truncal vein into the deep vein, causing a partial occlusion. It appears to be more likely following treatment of the small saphenous veins, in large veins, and in patients with an underlying thombophilia.26,27 EHIT also appears to occur if the distance between the deep vein and the most proximal point of treatment is less than 2.5 cm.28 The problem normally presents as a finding on posttreatment duplex scans and can resolve without any anticoagulation treatment. A four-level classification system and management strategies have been suggested for EHIT.28,29 Patients with complete occlusion of the deep vein by EHIT should be treated with anticoagulation while partial occlusion may be left to the clinician’s discretion, although debate continues on the matter. The advice is that all patients should have duplex follow-up after the first week, although many units may not do so.
Cost
The “cost of sales” of the Closure Fast™ system adds a significant expense to treating a patient with venous disease when compared with the cheap disposable stripping devices used for conventional surgery. This additional cost has, in the past, prohibited more widespread use, but there is some evidence that when used for local anesthetic procedures, with minimal additional staff, Closure Fast™ can be a cost-effective alternative.
In Germany, the impact of the introduction of Closure Fast™ to the statutory health insurance catalog was assessed.30 Using a multi-cohort Markov model, which looks at the number of procedures a patient needs to remain free of the symptoms of varicose veins over 5 years, the introduction of Closure Fast™ would save €19.1 million when compared with open surgery. If Closure Fast™ was not used, of the estimated 1.6 million procedures done over 5 years, 38% would have required an inpatient stay. With the introduction of Closure Fast™, this was calculated to reduce to 32%. The indirect cost savings of an early return to work were not calculated, but if they were, then the benefit for the country as a whole would have been greater.
A clinical effectiveness and economic evaluation performed in the UK was not as favorable as the German model, but marginally favored less invasive techniques such as Closure Fast™, when compared with surgery.31 The UK researchers stated that there is considerable uncertainty in the cost differences between treatments. Their analysis suggests that the additional costs of Closure Fast™ would have to be no more than £24 per case when compared with stripping, to be considered cost-effective at a quality of added life year (QALY) of £20,000. In real terms, this means that the cost of personnel and location required to perform the procedure needs to be reduced from the stripping under general anesthesia cost of £1,100 to less than £700 given the cost of the Closure Fast™ system; the introducer kit and generator in the UK is in the order of £400. For most hospitals, that means the procedure needs to be done under local anesthesia in an outpatient setting.
Research from the US confirms that systems like Closure Fast™ are cost-effective when used in the office setting. When performed in an operating theatre, Closure Fast™ would cost the hospital $1,123 (revenue $3,761, cost of procedure $4,884) while an office-based procedure would lead to a “profit” of $845 (revenue $1,919, cost $1,074).12
Conclusion
There have been a variety of articles, meta-analyses, and systematic reviews on the various methods to treat patients with superficial venous disease.6,32 At scientific meetings across the world, there continues to be heated debates on which method is best, often fuelled by personal preferences and statements, typical of surgical egos, namely “my way is best”. The evidence from all of these comparative studies suggests that patients can be offered any of the various treatment options, with very similar results and side-effect profiles when Closure Fast™ is compared with other thermal techniques. The sales figures from the manufacturers and some of the cost-effectiveness studies also suggest that Closure Fast™ has an important place in the treatment of superficial venous disease.
While clinician preference determines what method is currently used, it seems that the scientific results show Closure Fast™ to be safe and efficacious with a low side-effect profile, with a cost-effective bonus included.
Disclosure
The authors have no conflicts of interest. They have never received funding from Covidien or VNUS technologies.
References
National Institute for health and Care Excellence (NICE). Radiofrequency Ablation of Varicose Veins. IPG8. London, UK: NICE; 2003. Available from: http://publications.nice.org.uk/radiofrequency-ablation-of-varicose-veins-ipg8/guidance. Accessed March 20, 2014. | |
Siribumrungwong B, Noorit P, Wilasrusmee C, Attia J, Thakkinstian A. A systematic review and meta-analysis of randomised controlled trials comparing endovenous ablation and surgical intervention in patients with varicose vein. Eur J Vasc Endovasc Surg. 2012;44:214–223. | |
Thomis S, Verbrugghe P, Milleret R, Verbeken E, Fourneau I, Herijgers P. Steam ablation versus radiofrequency and laser ablation: an in vivo histological comparative trial. Eur J Vasc Endovasc Surg. 2013;46:378–382. | |
Truckai C, Shadduck JH, inventors; Vnus Medical Technologies, Inc., original assignee. Electrosurgical instrument and method. United States patent US 7517349 B2. April 14, 2009. | |
Goode SD, Chowdhury A, Crockett M, et al. Laser and radiofrequency ablation study (LARA study): a randomised study comparing radiofrequency ablation and endovenous laser ablation (810 nm). Eur J Vasc Endovasc Surg. 2010;40:246–253. | |
Eklöf B, Perrin M. Review of randomized controlled trials comparing endovenous thermal and chemical ablation. Rev Vasc Med. 2014;2(1):1–12. | |
Goode SD, Kuhan G, Altaf N, et al. Suitability of varicose veins for endovenous treatments. Cardiovasc Intervent Radiol. 2009;32:988–991. | |
Jones L, Braithwaite BD, Selwyn D, Cooke S, Earnshaw JJ. Reprinted article “Neovascularisation is the principal cause of varicose vein recurrence: results of a randomised trial of stripping the long saphenous vein”. Eur J Vasc Endovasc Surg. 2011;42 Suppl 1:S57–S60. | |
Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366:1407–1409. | |
Milleret R, Huot L, Nicolini P, et al. Great saphenous vein ablation with steam injection: results of a multicentre study. Eur J Vasc Endovasc Surg. 2013;45:391–396. | |
Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg. 2011;98:1079–1087. | |
Lin JC, Nerenz DR, Migliore P, Young R, Shepard AD, Weaver WD. Cost analysis of endovenous catheter ablation versus surgical stripping for treatment of superficial venous insufficiency and varicose vein disease. J Vasc Surg Venous Lymphat Disord. 2014;2:98–103. | |
Proebstle TM, Alm J, Göckeritz O, et al. Three-year European follow-up of endovenous radiofrequency-powered segmental thermal ablation of the great saphenous vein with or without treatment of calf varicosities. J Vasc Surg. 2011;54:146–152. | |
García-Madrid C, Pastor Manrique JO, Sánchez VA, Sala-Planell E. Endovenous radiofrequency ablation (Venefit Procedure): impact of different energy rates on great saphenous vein shrinkage. Ann Vasc Surg. 2013;27:314–321. | |
Isobe JH, Sentell KC, Nichols LA, Simms CS. Twelve-month experience using reprocessed ClosureFast radiofrequency catheters. J Vasc Surg Venous Lymphat Disord. 2014;2:115–116. | |
Tolva VS, Cireni LV, Bianchi PG, Lombardo A, Keller GC, Casana RM. Radiofrequency ablation of the great saphenous vein with the ClosureFAST™ procedure: mid-term experience on 400 patients from a single centre. Surg Today. 2013;43:741–744. | |
Nordon IM, Hinchliffe RJ, Brar R, et al. A prospective double-blind randomized controlled trial of radiofrequency versus laser treatment of the great saphenous vein in patients with varicose veins. Ann Surg. 2011;254:876–881. | |
Bisang U, Meier TO, Enzler M, Thalhammer C, Husmann M, Amann-Vesti BR. Results of endovenous ClosureFast treatment for varicose veins in an outpatient setting. Phlebology. 2012;27:118–123. | |
Zuniga JM, Hingorani A, Ascher E, et al. Short-term outcome analysis of radiofrequency ablation using ClosurePlus vs ClosureFast catheters in the treatment of incompetent great saphenous vein. J Vasc Surg. 2012;55:1048–1051. | |
Roos MT, Borger van der Burg BL, Wever JJ. Pain perception during and after VNUS ClosureFAST™ procedure. Phlebology. 2011;26:209–212. | |
Shepherd AC, Gohel MS, Brown LC, Metcalfe MJ, Hamish M, Davies AH. Randomized clinical trial of VNUS® ClosureFAST™ radiofrequency ablation versus laser for varicose veins. Br J Surg. 2010;97:810–818. | |
Almeida JI, Kaufman J, Göckeritz O, et al. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: a multicenter, single-blinded, randomized study (RECOVERY study). J Vasc Interv Radiol. 2009;20:752–759. | |
Dermody M, O’Donnell TF, Balk EM. Complications of endovenous ablation in randomized controlled trials. J Vasc Surg Venous Lymphat Disord. 2013;1:427–436. e1. | |
Holdstock JM, Marsh P, Whiteley MS, Price BA. It is possible to cause damage to a laser fibre during delivery of tumescent anaesthesia for endovenous laser ablation (EVLA). Eur J Vasc Endovasc Surg. 2008;36:473–476. | |
Rudarakanchana N, Berland TL, Chasin C, Sadek M, Kabnick LS. Arteriovenous fistula after endovenous ablation for varicose veins. J Vasc Surg. 2012;55:1492–1494. | |
Jacobs CE, Pinzon MM, Orozco J, Hunt PJ, Rivera A, McCarthy W. Deep venous thrombosis after saphenous endovenous radiofrequency ablation: is it predictable? Ann Vasc Surg. 2014;28(3):679–685. | |
Sufian S, Arnez A, Labropoulos N, Lakhanpal S. Incidence, progression, and risk factors for endovenous heat-induced thrombosis after radiofrequency ablation. J Vasc Surg Venous Lymphat Disord. 2013;1:159–164. | |
Sadek M, Kabnick LS, Rockman CB, et al. Increasing ablation distance peripheral to the saphenofemoral junction may result in a diminished rate of endothermal heat-induced thrombosis. J Vasc Surg Venous Lymphat Disord. 2013;1:257–262. | |
Harlander-Locke M, Jimenez JC, Lawrence PF, Derubertis BG, Rigberg DA, Gelabert HA. Endovenous ablation with concomitant phlebectomy is a safe and effective method of treatment for symptomatic patients with axial reflux and large incompetent tributaries. J Vasc Surg. 2013;58:166–172. | |
Kuhlmann A, Prenzler A, Hacker J, Graf von der Schulenburg JM. Impact of radiofrequency ablation for patients with varicose veins on the budget of the German statutory health insurance system. Health Econ Rev. 2013;3:9. | |
Carroll C, Hummel S, Leaviss J, et al. Clinical effectiveness and cost-effectiveness of minimally invasive techniques to manage varicose veins: a systematic review and economic evaluation. Health Technol Assess. 2013;17:i–xvi, 1–141. | |
Perrin M. Endovenous radiofrequency ablation of saphenous vein reflux. The VNUS Closureprocedure with ClosureFAST. An updated review. Int Angiol. 2010;29:303–307. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.