Back to Journals » Clinical Ophthalmology » Volume 16
Clinical Results According to Inferior Oblique Manipulation in Patients with Inferomedial Blowout Fracture Involving the Orbital Strut
Authors Park J , Jo S, Choi HY
Received 24 October 2022
Accepted for publication 15 December 2022
Published 21 December 2022 Volume 2022:16 Pages 4263—4272
DOI https://doi.org/10.2147/OPTH.S394722
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Jungyul Park,1,2 Sunghyun Jo,1 Hee-Young Choi1,2
1Department of Ophthalmology, School of Medicine, Pusan National University Hospital, Busan, South Korea; 2Biomedical Research Institute, Pusan National University, Busan, South Korea
Correspondence: Hee-Young Choi, Department of Ophthalmology, School of Medicine, Biomedical Research Institute, Pusan National University, 179 Gudeok-Ro, Seo-Gu, Busan, 49241, Republic of Korea, Tel +82-51-240-7324, Fax +82-51-242-7341, Email [email protected]
Background: Detachment of the inferior oblique muscle may be necessary under certain circumstances to repair a large inferomedial orbital fracture involving the orbital strut. This study aimed to evaluate the outcomes of patients who underwent surgeries with and without inferior oblique muscle reattachment after its detachment to repair the orbital wall fractures.
Methods: Forty patients who underwent repair of combined floor and medial orbital wall fracture involving the orbital strut at a single tertiary institution between January 2014 and December 2020 were reviewed. Groups 1 and 2 comprised 20 patients each, who underwent surgery with inferior oblique muscle detachment without and with reattachment, respectively, and were followed up for at least 6 months postoperatively. Enophthalmos, Goldmann diplopia test, alignment test, ocular motility test, and orbital inferomedial angle ratio were the outcome measures.
Results: Statistically significant improvement was observed in ocular motility, diplopia, and enophthalmos postoperatively at the 1- and 6-month follow-up (p < 0.01). The mean postoperative inferomedial angle ratio (102.28 ± 10.62%) was improved significantly compared with the preoperative inferomedial angle ratio (115.61 ± 4.38%) (p = 0.004) in all patients. After surgery, inferior oblique muscle underaction was observed in seven and six patients in groups 1 and 2, respectively, which was associated with preoperative extraocular movement limitation and strabismus. Two patients showed diplopia in both groups at the last follow-up; they had inferior oblique muscle underaction but no enophthalmos.
Conclusion: Orbital fracture repair with or without inferior oblique muscle reattachment was clinically effective and safe; however, patients with preoperative strabismus and extraocular motility limitation should be informed of the increased risk of postoperative complications.
Keywords: orbital fracture, inferior oblique muscle, diplopia, strabismus, post-operative complications
Introduction
The floor and medial walls are the most commonly fractured areas of the orbit. Patients with extraocular muscle incarceration, double vision, hypoglobus, and marked orbital volume expansion require treatment.1–3 Combined fractures of the orbital floor and medial wall, in particular, those affecting the inferomedial orbital strut (IOS), are more likely to necessitate treatment due to increased risk of enophthalmos, strabismus, and even hypoglobus and diplopia.4 However, restoring the natural curves of the orbital wall without postoperative sequelae remains challenging, as the inferior oblique muscle (IOM) impedes access to the medial and inferior orbital wall.5–8 Although several procedures and techniques have been developed to increase the surgical view and treat these complicated fractures, no consensus on the ideal approach exists, and definite guidelines are lacking.
Nunery et al6 described the “wraparound” technique for combined fractures in 2008 to span all fracture sites with a single implant. In this technique, IOM was preserved, and the implant was introduced inferiorly and maneuvered through the medial area using a “lariat”. The treatment was successful without serious complications. Nevertheless, skin incision, blind insertion, incomplete visualization of the fracture sites, 0.4-mm implant thickness, relatively small implant size to span combined fractures, and the presence or absence of IOS involvement are its limitations. In 2016, a study reporting ocular motility after repairing combined medial and inferior orbital wall fractures with IOM detachment and reattachment for retaining better visualization of fracture sites showed satisfactory results.9 This study examined 20 patients who underwent surgery with extended conjunctival incision with IOM disinsertion and IOM reattachment for combined orbital wall fractures. On the contrary, Raymond et al4 stated about surgery of combined orbital fractures involving the IOS with detachment of IOM in 16 patients. There were 5 out of 7 patients who improved diplopia, which was shown preoperatively. They also reported that there were no cases of new or worsened diplopia following surgery but no mentions about IOM functions after surgery.
Regardless of these studies, there is no definite guideline on manipulating IOM. Iatrogenic damage remains a concern to the IOM especially when a combined fracture involving IOS without diplopia or IOM dysfunction is operated.
Therefore, this study aimed to investigate the results according to the management of IOM in patients with combined medial and floor wall fractures involving IOS. We compared and evaluated the clinical outcomes with and without IOM reattachment in patients who underwent IOM disinsertion during the operation.
Subjects and Methods
A retrospective review was performed on 40 patients who underwent repair of combined floor and medial orbital wall fractures involving the IOS at a single tertiary institution, Pusan National University Hospital, Busan, South Korea, between January 2014 and December 2020. All patients provided written informed consent, and this study was conducted in accordance with the tenets of the Declaration of Helsinki. This study was approved by the Institutional Review Board of the Pusan National University Hospital (2203–001-112).
The indications for surgery included diplopia, ocular motility restriction within 30° of the primary position, enophthalmos more than 2 mm, and fractures involving at least half of the orbital floor and medial wall. Patients who underwent surgery with detachment of the IOM from the origin site and no reattachment were defined as group 1, and patients who underwent reattachment of the IOM to the origin site were defined as group 2. Patients having preoperative diplopia, strabismus, or thyroid eye diseases were excluded from the study to rule out any chance of diplopia caused by IOM manipulation. Patients with previous surgery for orbital trauma, less than 6 months of postoperative follow-up, and coexisting facial bone fractures were also excluded from this study.
Preoperative and Postoperative Assessment
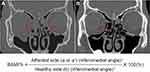
Preoperative evaluations included the visual acuity test; intraocular pressure measurement, mean time from injury to surgery, and enophthalmos evaluation; Goldmann diplopia test; ocular motility test; and IOM motility status assessment. Exophthalmos, Goldmann diplopia test, ocular alignment test, and ocular motility test results were evaluated at the initial examination before surgery and the 1- and 6-month postoperative follow-up examination. We defined “diplopia” as when a single binocular diplopia appearing within 30 degrees of the primary gaze. Enophthalmos was measured using Hertel exophthalmometry and described as a difference from the opposite side. The ocular motility test (ranging from 0 to 4) was performed through ductions, versions, and the Hess test. The ocular motility limitation was scored by the severity of limitation for upgaze, downgaze, adduction, and abduction. A score of 0 stated no limitation, −4 for 0% excursion, and −3 to −1 for 25% increments. We scored the most severe limitation in movement as the most affected muscle in cases of multiple extraocular muscle (EOM) involvement. IOM motility was evaluated using the Hess test during follow-up examinations 6 months postoperatively. IOM underaction was assessed by anomalous head tilting to the injured side, accompanying more pronounced hypotropia in upgaze with adduction than in the central or abduction position, which was confirmed by the Hess test. The inferomedial orbital angle (IMA) was measured in all patients on both sides of the orbit using orbital computed tomography (CT); the IMA ratio (IMAR)% was calculated pre- and postoperatively (Figure 1). The standard location for measuring the IMA is 9 mm below the level of the lateral orbital rim.10,11 The CT images were obtained with 1-mm fine cuts and reviewed by an experienced orbital surgeon (JYP).
Surgical Technique
The same surgical techniques were used in both groups, except for the manipulation of the IOM at the end of the surgery, as described previously.9
All procedures were performed under general anaesthesia. After inserting a corneal protector, transconjunctival and retro-caruncular incisions were made using electrocautery without lateral canthotomy. A transconjunctival incision was made 2 mm below the tarsal plate, the preseptal layer toward the inferior orbital rim was dissected, and an incision was made at the inferior orbital rim using electrocautery. The periosteum was separated from the bone using a double elevator to expose the fractured area of the inferior orbital wall. The prolapsed orbital tissues, including the inferior rectus, were lifted from the fracture site. The dissection was performed until the edge of the floor fracture could be seen laterally and posteriorly.
Posterior to the lacrimal crest, blunt dissection toward the medial wall behind the medial canthal tendon was performed vertically. The dissection was continued until the medial wall was identified, and the periosteal incision was made with electrocautery just behind the Horner’s muscle to expose the fractured medial orbital site. The prolapsed orbital tissues were lifted from the fracture site, and dissection was performed until the entire fracture site was visualized superiorly and posteriorly.
The dissection was extended from previous incisions inferomedially to the insertion site of IOM. IOM was directly identified and isolated using a Jameson muscle hook (Katena Inc., Denville, NJ, USA). IOM was detached from the origin with electrocautery in group 1, whereas double-arm 6–0 absorbable polyglactin sutures (Vicryl 6–0 Ethicon, Division of Johnson & Johnson Inc., Somerville, MA) were placed on the periosteum at the IOM origin and on the IOM approximately 2 mm away from the origin in group 2. Subsequently, IOM was severed using scissors. Subperiosteal dissection was performed until the total inferomedial fracture site was visualized posteriorly, and repositioning of the herniated orbital tissue was done. Prefabricated naturally curved polycaprolactone (PCL) (TnR Biofab, Gyeonggi, South Korea) and porous polyethylene (Medpor; Medtronic, USA) were implanted in the fracture site in groups 1 and 2, respectively. The PCL implant is fabricated to fit Asian orbits to span the total inferomedial wall; the Medpor implant was fashioned into L-shape manually and used to cover the total defect. After confirming the absence of restriction on the forced duction test, the conjunctival wound was closed using a single interrupted 6–0 absorbable polyglactin suture (Vicryl 6–0 Ethicon, Division of Johnson & Johnson Inc., Somerville, MA, USA) in group 1. IOM was reattached with the preplaced suture before conjunctival wound closure in group 2. Saline-soaked cottonoids were utilized for gentle elevation and manipulation of the herniated orbital tissues, and 1:100,000 epinephrine-soaked cottonoids were used for haemostasis in all patients. No bipolar cautery was used on the orbital tissues. Surgical success was defined as the absence of diplopia at the primary position or within 30° of gaze and the restoration of enophthalmos of less than 1 mm 6 months postoperatively.
Statistical Analyses
The normality of the data distribution was determined using the Kolmogorov–Smirnov test. Student’s t-test or the Mann–Whitney U-test was used to compare continuous data. Categorical variables were compared using the chi-square or Fisher’s exact test. The independent factors associated with IOM underaction at the last follow-up were identified using univariate and multivariate logistic regression analyses. The Statistical Package for the Social Sciences (SPSS) software (version 22.0; SPSS, Chicago, IL, USA) was used for all statistical analyses. Statistical significance was set at p < 0.05.
Results
Forty patients with combined orbital wall fractures involving the IOS were evaluated. There were 24 and 16 men and women, respectively, with a mean age of 42 ± 2.63 years (range, 15–77). The mean interval time from injury to surgery was 22.95 ± 4.09 days (range, 1–121). Seventeen and 23 patients had right and left orbital wall fractures, respectively. The mean follow-up visit was 9 ± 3.47 months (range, 6–17). Patients showed enophthalmos of –1.46 ± 0.25 (range, –5 to 3) mm compared with the normal side. EOM was evaluated using duction and version test, –1.1±0.17 (range, 0 to −4) limitation was observed, and 19 (47.5%) patients showed diplopia before surgery. The mean IMAR in the injured eye was 115.61 ± 4.38% (range, 43.26–188.02). The demographic and clinical characteristics of groups 1 and 2 are shown in Table 1.
 |
Table 1 Demographic and Clinical Characteristics of the Group 1 and Group 2 |
There were no differences in age, sex, preoperative visual acuity, preoperative intraocular pressure, presence and amount of enophthalmos, EOM limitation, diplopia, and IMAR between the groups. The mean interval time from injury to surgery was longer in group 2 than that in group 1 (28.79 ± 5.76 vs 17.40 ± 5.68, p = 0.024).
Table 2 summarises the overall functional characteristics, including diplopia, enophthalmos, EOM limitation, and IOM weakening postoperatively. Diplopia was significantly better 1 and 6 months after surgery compared with that in the initial visits (p = 0.014 and p = 0.047, respectively). Nineteen cases of diplopia at the initial examination improved significantly, with four cases improving 6 months postoperatively (p = 0.047). In group 1, 12 patients with preoperative diplopia improved, five and two patients remained diplopia 1 and 6 months postoperatively (p > 0.05, not shown in the table). In group 2, seven patients with preoperative diplopia improved, four and two patients remained postoperative diplopia 1 and 6 months after surgery, respectively (p > 0.05, not shown in the table). There were no statistical differences between the two groups. Enophthalmos significantly improved 1 and 6 months postoperatively (p < 0.001 and p = 0.013, respectively). The number of cases with enophthalmos was significantly lesser in group 1 than that in group 2, 6 months postoperatively. EOM limitation was observed until 1 month postoperatively (p = 0.068); a significant improvement was observed 6 months postoperatively (p < 0.001). Group 2 showed –1.05 ± 0.62 limitation, which was more significantly restricted compared with that of group 1; however, both groups showed significantly better ocular motility compared with that at the initial visit 6 months postoperatively (group 1, p < 0.001; group 2, p = 0.004; not shown in the table) and no difference was observed between the two groups. At the final examination, IOM weakening was observed in seven (35%) and six (30%) patients in groups 1 and 2, respectively (p = 1.0).
 |
Table 2 Comparison of Functional Defects After Surgery Between Group 1 and Group 2 |
Figure 1 shows a representative case of IMA in an injured orbit pre and postoperatively. Figure 2 shows the changes in the IMA ratio pre and postoperatively. All patients, including both groups, significantly recovered the natural IMA compared with that of the normal orbit (total, p = 0.004; group 1, p < 0.001; group 2, p < 0.001).
Table 3 shows the results of the univariate and multivariate logistic regression analyses associated with IOM weakening at the final follow-up. Univariate logistic regression analysis showed that preoperative EOM limitation, diplopia, and strabismus were significantly associated with IOM weakening (p = 0.004, p = 0.002, and p = 0.001 for horizontal strabismus, p = 0.025 for vertical strabismus, and p = 0.0043 for combined strabismus, respectively). To multivariate analysis, covariates were selected of which p-values under 0.1. After adjusting for confounding factors, multivariate analysis showed that preoperative EOM limitation and strabismus were associated with IOM weakening at the final follow-up examination (p = 0.005; p = 0.016 for horizontal, p = 0.023 for vertical, and p = 0.048 for combined strabismus, respectively). In terms of diplopia at the last follow-up, no factor was related to remnant diplopia postoperatively according to the univariate and multivariate logistic regression analyses (not shown in the table).
 |
Table 3 Factors Associated with Inferior Oblique Weakening at 6 Months After Surgery Investigated by Univariate and Multivariable Logistic Regression Analysis |
Table 4 shows the characteristics of the patients with diplopia at the last follow-up. Two patients (50%) had their IOM detached, whereas the remaining two (50%) had their IOM reattached. Except for one patient, who was referred more than 3 months after trauma due to systemic problems, three other patients (75%) had their operations approximately 2 weeks after trauma. All patients had diplopia at the primary position or within 30° of gaze, strabismus, and EOM limitation of more than −1 preoperatively. The horizontal strabismus was recovered postoperatively, and there was no case of new-onset diplopia. However, approximately −2 to −1 EOM limitation and IO weakening remained in all patients at the last visit.
 |
Table 4 Characteristics of the Patients Showing Diplopia at the Final Follow-Up |
Discussion
Cases with extensive orbital fractures of the medial and inferior orbital walls involving rupture of the IOS are uncommon and challenging; however, restoring the broken wall to its original shape is essential. The cornerstone procedure for treating combined medial and inferior orbital wall fractures involving the IOS is the manipulation of the IOM. Detaching IOM permits a thorough examination of the fracture site on the inner aspects of the orbital wall and the application of an appropriately preshaped or L-shaped fabricated implant to restore the lost natural curve of the orbital wall.9,12 Moreover, reattachment following IO detachment facilitated great surgical results. We reported satisfactory surgical outcomes following combined orbital wall fractures with IO reattachment.9 Rodriguez et al13 have also reported remarkable surgical outcomes employing an extended transcaruncular technique with reattachment of IO in patients having medial wall fractures. Although the surgical results with IO reattachment showed great results, there are still minor concerns including IO underaction and diplopia. Alameddine et al12 observed that 13.3% of patients who had their IOM reattached experienced transient new-onset diplopia, with all achieving recovery. Rodriguez et al13 reported transient diplopia after surgery through IOM reattachment. In contrast, in our previous study, 10% of patients who underwent IOM reattachment experienced binocular double vision, which did not resolve.9 The function of IOM has not been properly described in these previous reports. In this study, we found evidence of IOM underaction in the ocular motility and Hess test in six (30%) patients who underwent IOM reattachment.
Similarly, in case of IO detachment and passive reattachment to its origin, seven (35%) patients showed IOM underaction 6 months postoperatively. There was no difference in the outcome of IOM movement depending on how IOM was handled. There are some studies about IOM underaction following IOM detachment without reattachment. Shorr et al14 who described movement following IOM detachment reported that IOM underaction persisted in some patients postoperatively. Iatrogenic IOM palsy has been documented by Tiedemann et al.15 In cases with diplopia, several studies in which IOM was permitted to attach spontaneously reported double vision; most cases were transient, as in this study.4,16,17 In our study, IOM underaction was not always correlated with diplopia, with 4 out of 13 patients showing diplopia at the last follow-up. The inflammation and accompanying injuries after severe trauma made it difficult to evaluate the function of IOM preoperatively. Nevertheless, we recognized that patients with postoperative diplopia already had diplopia, strabismus, and EOM limitation preoperatively.
In conclusion, transient diplopia after surgery was mostly recovered regardless of IOM management, and IOM underaction was observed in approximately 30% of patients in both groups at the final follow-up.
In the event of EOM limitation, restriction of movement was detected to a greater extent when reattaching IOM 1 month postoperatively (Table 2). However, it recovered to a similar level in both groups at 6 months. It is hypothesized that IOM manipulation during the cutting and reattaching procedure in group 2 may cause muscle paresis and inflammation and induce initial limitation of eye movement.15
To summarize these results about IOM underaction, diplopia, and EOM limitation, both surgical skills were equally remarkable and showed similar postoperative sequelae.
Enophthalmos did not affect IOM weakening or diplopia; however, enophthalmos was observed in a significantly higher proportion of patients in group 2 than that in group 1; its severity was statistically significant, possibly due to the PCL pre-shaped implant manufactured to fit the Asian orbital structure, which enabled restoration to a suitable and natural shape in group 1. Restoration of the right curvature of the inner orbit, rather than simple restoration of the angle of the IMA, is a more effective technique for restoring enophthalmos. Moreover, compared to 1 month, the enophthalmos advanced by 0.7–1.0 mm 6 months postoperatively. Thus, when the extent of the fracture is large, surgery should be performed with the goal of overcorrection by approximately 1 mm in comparison with the fellow eye.9,12
Diplopia occurred in patients with IOM weakening. No factors related to diplopia were discovered in this study; however, we found important factors associated with IOM weakening, such as preoperative strabismus and preoperative EOM limitation. This suggests that the larger the prior muscular injury, the more difficult it is for the IO muscle to return to its previous position and function, regardless of the form of surgery. There are three basic mechanisms by which trauma damages the extraocular muscles: muscle involvement in orbital wall fractures, muscle contusion, and traumatic disinsertion or laceration of the extraocular muscles. Muscle involvement in orbital wall fractures can be caused by two different mechanisms: muscle incarceration18 or flap tear of the rectus muscle, as described by Ludwig in 2001.19 Contusion of the extraocular muscle results from the impact of an object on the surface of the extraocular muscle without perforating any structures. Edema or hematoma forms in the belly of the muscle after trauma, restricting its function.20 Lastly, when it comes to traumatic disinsertion, the muscle capsule is linked to the check ligaments and intermuscular membrane, and it cannot retract deeply into the orbit; the muscle is typically reattached behind the normal insertion position. Thus, the muscular function is maintained.20 We speculate that IOM disinsertion with a periosteal elevator permits IOM to reposition spontaneously after the procedure, a mechanism similar to the last basic mechanism described above. Although it is not possible to explain the exact mechanism of damage in each case, two theories have been suggested based on these mechanisms: first, there is significant damage to the muscles and additional IOM manipulation, including disinsertion or reattachment with sutures. These factors together prohibit IOM from returning to its original position and prevent the recovery of its function even after IOM is reattached. Second, serious trauma to IOM preoperatively eventually impairs the recovery of IOM function postoperatively, regardless of the surgical technique and IO manipulation. Strabismus, EOM limitation, and diplopia (univariate analysis) were significantly observed in patients with IOM weakening preoperatively, which supports this theory. According to Tiedemann et al,15 a patient with diplopia and strabismus presenting with diplopia and ocular movement disorder preoperatively supported this theory. Comprehensively, even though there was no preoperative factor associated with postoperative diplopia, it may be due to preoperative dysfunction of the extraocular muscle. It is predicted with meticulous preoperative examination, especially strabismus and EOM limitation test.
We investigated the long-term alterations in ocular motility, especially in IOM, diplopia, and enophthalmos, which occur when IOM is detached without reattachment and when IOM is reattached. Although there was a significant difference in the timing of surgery in the demographic characteristics of the two groups, this is not a well-studied factor that affects the results in which we are interested. In addition, it was not identified as a factor related to final IOM weakening or diplopia. Additionally, considering newly published findings on a new paradigm for surgical timing21 or reports demonstrating favourable outcomes in diplopia or enophthalmos 14 days after injury, the difference in surgical time does not appear to substantially influence the outcome.22–28 However, in 2002, Hossal and Beatty29 reported that older age and longer mean time intervals negatively affected postoperative diplopia, especially in cases of combined fractures. In addition, surgery 14 days after injury was associated with more diplopia and maintenance of enophthalmos. The degree of damage was severe in our study, and the number of cases of strabismus and double vision was slightly greater than that of general cases. While interpreting the results, the timing between surgery and trauma was relatively long in group 2, which might negatively affect the final results.
Our study has several limitations due to different implant types being used in the groups and different timings of surgery. However, as we believe non-absorbable and absorbable implants are great candidates for any size of orbital fracture, it does not undermine our key results and the purpose of this study. In terms of surgical timing, although the difference in surgical time does not appear to substantially influence the surgical outcomes based on previous studies,22–28 it is necessary to consider that there is a slight chance of underestimating the results in group 2.29
In conclusion, both surgical techniques had a comparable effect on IOM and showed successful surgical results with improvement of diplopia, ocular movement, strabismus, enophthalmos, and IMA. Owing to the simplicity and speed of the procedure, IOM disinsertion without reattachment may also be a good choice for surgeons unfamiliar with the extraocular muscles. In addition, when we consider one patient with diplopia in group 1 who underwent surgery on the 121st day after trauma, this surgical technique without reattachment of IOM is not necessarily inferior in terms of the postoperative side effects.
Patients with preoperative strabismus and EOM limitation should be informed of the increased risk of postoperative complications. Both surgical techniques may result in approximately 30% of cases of IOM underaction.
No factors associated with diplopia were identified in this study, and all patients who developed diplopia had IOM weakness. This information provides sufficient insight into the factors related to IOM weakening. Although the sample size was small and this study was retrospective, we believe it can provide critical information to surgeons who encounter difficulty with IOM handling. We aim to confirm the impact of manipulating IOM in detail in our future studies, which will include two groups with IO disinsertion and non-disinsertion.
Data Sharing Statement
Data are available upon request.
Ethics Approval and Informed Consent
Institutional Review Board Statement: The study was conducted in accordance with the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Pusan National University Hospital (IRB No. 2203-001-112).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Consent for Publication
Informed consent for publication was obtained from the patients in this study, as potentially medical information may be included in this article.
Funding
There is no funding to report.
Disclosure
The authors declare that they have no competing or conflicts of interests.
References
1. Burnstine MA. Clinical recommendations for repair of orbital facial fractures. Curr Opin Ophthalmol. 2003;14(5):236–240. doi:10.1097/00055735-200310000-00002
2. Dortzbach RK. Orbital floor fractures. Ophthalmic Plast Reconstr Surg. 1985;1(2):149–152. doi:10.1097/00002341-198501020-00014
3. Harris G. Orbital blow-out fractures: surgical timing and technique. Eye. 2006;20(10):1207–1212. doi:10.1038/sj.eye.6702384
4. Cho RI, Davies BW. Combined orbital floor and medial wall fractures involving the inferomedial strut: repair technique and case series using preshaped porous polyethylene/titanium implants. Craniomaxillofac Trauma Reconstr. 2013;6:161–170. doi:10.1055/s-0033-1343785
5. Mici E, Calvo A, Cicciù M, et al. Complex orbital fractures: three-dimensional planning and combined surgical approach. J Craniofac Surg. 2018;29(7):1965–1968. doi:10.1097/SCS.0000000000005022
6. Nunery WR, Tao JP, Johl S. Nylon foil “wraparound” repair of combined orbital floor and medial wall fractures. Ophthalmic Plast Reconstr Surg. 2008;24:271–275. doi:10.1097/IOP.0b013e3181788de8
7. Scolozzi P, Momjian A, Heuberger J, et al. Accuracy and predictability in use of AO three-dimensionally preformed titanium mesh plates for posttraumatic orbital reconstruction: a pilot study. J Craniofac Surg. 2009;20:1108–1113. doi:10.1097/SCS.0b013e3181abb44b
8. Su GW, Harris GJ. Combined inferior and medial surgical approaches and overlapping thin implants for orbital floor and medial wall fractures. Ophthalmic Plast Reconstr Surg. 2006;22:420–423. doi:10.1097/01.iop.0000242163.03589.0e
9. Ahn JH, Jung JH, Choi HY. Ocular motility after repair of combined medial and inferior orbital wall fractures with extended conjunctival incision with inferior oblique reattachment. J Craniofac Surg. 2016;27:1312–1315. doi:10.1097/SCS.0000000000002695
10. Moon SJ, Lee WJ, Roh TS, et al. Sex-related and racial variations in orbital floor anatomy. Arch Craniofac Surg. 2020;21:219–224. doi:10.7181/acfs.2020.00143
11. Zhang T, Young S, Lang SS, et al. Prebending of prefabricated orbital implants: towards improved orbital angle symmetry post craniofacial trauma surgery. J Craniofac Surg. 2022;33(3):740–743. doi:10.1097/SCS.0000000000008107
12. Alameddine RM, Tsao JZ, Ko AC, et al. Incidence of diplopia after division and reattachment of the inferior oblique muscle during orbital fracture repair. J Craniomaxillofac Surg. 2018;46(8):1247–1251. doi:10.1016/j.jcms.2018.05.026
13. Rodriguez J, Galan R, Forteza G, et al. Extended transcaruncular approach using detachment and repositioning of the inferior oblique muscle for the traumatic repair of the medial orbital wall. Craniomaxillofac Trauma Reconstr. 2009;2(1):35–40. doi:10.1055/s-0029-1202598
14. Shorr N, Baylis HI, Goldberg RA, et al. Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology. 2000;107:1459–1463. doi:10.1016/S0161-6420(00)00241-4
15. Tiedemann LM, Lefebvre DR, Wan MJ, et al. Iatrogenic inferior oblique palsy: intentional disinsertion during transcaruncular approach to orbital fracture repair. J AAPOS. 2014;18(5):511–514. doi:10.1016/j.jaapos.2014.06.005
16. de Haller R, Imholz B, Scolozzi P. Pseudo-Brown syndrome: a potential ophthalmologic sequela after a transcaruncular–transconjunctival approach for orbital fracture repair. J Oral Maxillofac Surg. 2012;70(8):1909–1913. doi:10.1016/j.joms.2012.03.015
17. Malhotra R, Saleh GM, de Sousa J-L, et al. The transcaruncular approach to orbital fracture repair: ophthalmic sequelae. J Craniofac Surg. 2007;18(2):420–426. doi:10.1097/scs.0b013e31803384c2
18. Wei LA, Durairaj VD. Pediatric orbital floor fractures. J AAPOS. 2011;15(2):173–180. doi:10.1016/j.jaapos.2011.02.005
19. Ludwig IH, Brown MS. Strabismus due to flap tear of a rectus muscle. Trans Am Ophthalmol Soc. 2001;99:53–62.
20. Krarup J, de Decker W. Catalog of direct eye muscle injuries. Klin Monbl Augenheilkd. 1982;181:437–443. doi:10.1055/s-2008-1055268
21. Kersten RC, Vagefi MR, Bartley GB. Orbital “blowout” fractures: time for a new paradigm. Ophthalmology. 2018;125:796–798. doi:10.1016/j.ophtha.2018.02.014
22. Amrith S, Almousa R, Wong WL, et al. Blowout fractures: surgical outcome in relation to age, time of intervention, and other preoperative risk factors. Craniomaxillofac Trauma Reconstr. 2010;3(3):131–136. doi:10.1055/s-0030-1262955
23. Beigi B, Khandwala M, Gupta D. Management of pure orbital floor fractures: a proposed protocol to prevent unnecessary or early surgery. Orbit. 2014;33:336–342. doi:10.3109/01676830.2014.902475
24. Ben Simon GJ, Syed HM, McCann JD, et al. Early versus late repair of orbital blowout fractures. Ophthalmic Surg Lasers Imaging Retina. 2009;40(2):141–148. doi:10.3928/15428877-20090301-05
25. Dal Canto AJ, Linberg JV. Comparison of orbital fracture repair performed within 14 days versus 15 to 29 days after trauma. Ophthalmic Plast Reconstr Surg. 2008;24(6):437–443. doi:10.1097/IOP.0b013e31818aac9b
26. Roncević R, Stajcić Z. Surgical treatment of posttraumatic enophthalmos: a study of 72 patients. Ann Plast Surg. 1994;32(3):288–294. doi:10.1097/00000637-199403000-00011
27. Scawn RL, Lim LH, Whipple KM, et al. Outcomes of orbital blow-out fracture repair performed beyond 6 weeks after injury. Ophthalmic Plast Reconstr Surg. 2016;32(4):296–301. doi:10.1097/IOP.0000000000000511
28. Shin KH, Baek SH, Chi M. Comparison of the outcomes of non-trapdoor-type blowout fracture repair according to the time of surgery. J Craniofac Surg. 2011;22(4):1426–1429. doi:10.1097/SCS.0b013e31821cc2cd
29. Hossal BM, Beatty RL. Diplopia and enophthalmos after surgical repair of blowout fracture. Orbit. 2002;21(1):27–33. doi:10.1076/orbi.21.1.27.2598
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.