Back to Journals » Research Reports in Clinical Cardiology » Volume 14
Clinical Profiles of Cardiovascular Diseases and Predictors of Outcome of Hospitalization in a Tertiary Teaching Hospital, Ethiopia: A Prospective Observational Study
Authors Hailu A , Gidey K, Mohamedniguss Ebrahim M , Berhane Y, Baraki TG , Hailemariam T, Negash A, Mesele H , Desta T, Tsegay H, Assefa M , Bayray A
Received 7 July 2023
Accepted for publication 19 September 2023
Published 29 September 2023 Volume 2023:14 Pages 69—83
DOI https://doi.org/10.2147/RRCC.S424830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Kones
Abraha Hailu,1 Kibreab Gidey,1 Mohamedawel Mohamedniguss Ebrahim,2 Yonas Berhane,1 Teklay Gebrehaweria Baraki,1 Tesfay Hailemariam,1 Ataklti Negash,1 Habtamu Mesele,1 Tekleab Desta,1 Haylsh Tsegay,1 Mulubirhan Assefa,3 Alemayehu Bayray3
1Department of Internal Medicine, School of Medicine, Mekelle University-College of Health Sciences, Mekelle, Tigray, Ethiopia; 2Department of Biostatistics, School of Public Health, Mekelle University-College of Health Sciences, Mekelle, Tigray, Ethiopia; 3Department of Epidemiology, School of Public Health, Mekelle University-College of Health Sciences, Mekelle, Tigray, Ethiopia
Correspondence: Abraha Hailu, Department of Internal Medicine, Cardiology Unit, School of Medicine, Mekelle University-College of Health Sciences, Mekelle, Tigray, Ethiopia, Tel +251911413363, Email [email protected]
Background: Documentation of cardiovascular diseases (CVDs) including major contributors to hospitalization and their outcomes in sub-Saharan Africa and Ethiopia is important to prioritize further research, prevention and treatment strategies.
Objective: This study aimed to describe the clinical profiles of CVD admissions, risk factors, and patient outcomes at Ayder Comprehensive Specialized Hospital (ACSH) in Tigray, Ethiopia.
Methods: Data of patients with the diagnosis of CVD was collected prospectively from Nov. 1, 2017 – Oct. 31, 2018 for patients admitted to medical wards and ICU of ACSH. Socio-demographic profiles, final admission diagnosis, duration of hospital stay and outcomes of hospitalization were variables recorded using a data abstraction checklist. Diseases were categorized using the World Health Organization’s International Classification of Diseases (ICD)-10 coding system. Data was analyzed using SPSS version 26.
Results: Of the 2084 admissions over 1 year period, 767 (36.5%) had CVDs. The mean age of the study population was 53.4 ± 19.4 years with 55% females. Leading CVD admissions were stroke (35%), Venous thromboembolism (17%), rheumatic heart disease (RHD) (16.2%), ischemic heart disease (IHD) (11.3%) and cardiomyopathy (CMP) (7.4%). Heart Failure (HF) was found in 300 (39.1%) patients with structural heart disease. The structural cardiac lesions in those with HF were RHD (36%), CMP (19%), IHD (18%) and Cor pulmonale (9%). The in-hospital mortality of all CVD admissions was 13.2%. Of the total 101 CVD deaths most were because of stroke (n=37, 36.6%) followed by IHD (18.8%). Hypertension was the most frequent risk factor (28.8%).
Conclusion: This study shows stroke to be the most common form of CVD and RHD to be the main reason for HF among hospital admissions in Northern Ethiopia, ACSH. Hypertension is the leading reported risk factor and hence the need to give high priority to hypertension and RHD control programs.
Keywords: cardiovascular disease, admissions, outcomes, sub-Saharan Africa, Ethiopia
Introduction
Documentation of CVDs including major contributors to hospitalization and their outcomes in sub-Saharan Africa (SSA) and Ethiopia is important to prioritize further research, prevention and treatment strategies. The considerable variation of CVD epidemiology not only among countries but even within country (urban-rural gradient) is attributable to nutritional, economic & the epidemiologic transition among others.1–3 Cardiovascular Diseases (CVDs) are the leading causes of death worldwide with above 70% in low and middle income nations.4 CVDs almost doubled over three decades (1990–2019) from 271 million to 523 million and the number of CVD related deaths increased from 12.1 million to 18.6 million in the same years as per the 2019 Global Burden of Diseases (GBD) study report.5 In sub-Saharan Africa (SSA), CVDs accounted for 38.5% of deaths due to Non Communicable Diseases from the report in GBD 2013 study and is expected to surpass Communicable related deaths by 2035.6–8
The few studies done in SSA on epidemiology of CVDs so far indicate hypertensive heart disease (HHD), RHD, cardiomyopathy (CMP), ischemic heart disease (IHD) and strokes with or without HF to be the leading disorders of CVDs.4,8 Limited data are available in SSA and within Ethiopia about CVDs and those available do have several limitations where studies are limited to institutional surveys or a small segment of the population in an urban area and also suffers from inconsistencies in definition of CVDs. For example, in some studies strokes and venous thrombosis are excluded from CVD reports while the WHO International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10) Version for 2010 groups these diseases under CVDs.9
In Ethiopia and other sub-Saharan African countries the few available hospital-based studies on CVDs have shown a steady increment.10–12 Many studies have used different and inconsistent ways of estimation and classification of CVDs leading sometimes to difficulty in knowing the exact disease burden and trends in a country/region and hence the need for use of the standardized definitions of CVDs.13 Ethiopia, the second most populous nation in Africa only has a few hospital-based CVD studies, WHO STEPS Survey reports or verbal autopsy based community studies which address CVD prevalence and CVD related deaths.14–16 These relatively recent studies report rheumatic heart disease, cardiomyopathies, hypertension and related disorders like hypertensive heart diseases, strokes and ischemic heart disease to be the dominant forms of adult CVDs in Ethiopia. Studies done in 1970–1990’s in Ethiopia showed CVDs constituting 4–13% of medical ward admissions.17,18
Because of lack of data specific to SSA, studies and recommendations from other populations have been used to inform cardiovascular disease prevention programs for SSA. Recommendations based on research outputs from elsewhere to SSA populations is not the best way to implement preventive and curative programs/policies.19 The lack of complete data is also accompanied by the lack of uniformity in defining and reporting types of CVDs in SSA and Ethiopia and inconsistent utilization of International Classification of Diseases.5,20 We did a prospective study using ICD-10 system of classification of diseases and we aimed at looking for the clinical profiles of CVDs, main complications and their risk factors and/or underlying causes and predictors of mortality in the CVD population admitted to Ayder University Hospital.
Methods and Materials
Study Area and Setting
This study was conducted in Northern Ethiopia’s largest tertiary hospital, Ayder Comprehensive Specialized Hospital (ACSH), in Mekelle University - College of Health Sciences located in Mekelle, 783 km north of Addis Ababa. Established in 2008, ACSH is a 500 bed public hospital rendering tertiary health care services to a catchment area of nearly 10 million.
The Internal Medicine department in ACSH has 116 beds and outpatient services with specialists and subspecialists in different units. The cardiology unit has five cardiologists, an outpatient cardiology center equipped with Echocardiography, Holter Blood pressure and ECG monitors, resting and stress ECG machines and a cardiac catheterization laboratory which was established in 2015.
Study Design and Data Collection
This study was part of the prospective observational study which was published in the international journal of general medicine which reported on the patterns of medical admissions to ACSH and hence the materials and methods with data collection, quality control and operational definitions were outlined in the previous publication.21 Demographic and clinical data on all patients (aged ≧18 years) admitted to Medical Wards and ICU at ACSH from November 1, 2017 – October 31, 2018 were collected and those with CVDs were separately analyzed. Data were collected using a structured questionnaire (see Annex) and data collectors were trained internal medicine residents. Patient was followed with continuous clinical evaluation, imaging and laboratory testing until final diagnosis was settled. The final diagnosis was clarified with comments from subspecialists from within the department and interdepartmental consultations and recorded at discharge. If a patient has more than one clinical condition, the main cause for the patients’ current admission was taken as final diagnosis. We made the primary discharge diagnosis as the main cause patient was hospitalized according to the definition of primary diagnosis in the WHO ICD-10 Version for 2010.9 Data collected were sociodemographic characteristics, main diagnosis, duration and outcomes of hospitalization.
Data Quality Control
During hospital stay and at discharge/death, data regarding each patient were recorded in the data collection format by internal medicine residents and interns assigned in respective wards. Each week the quality of the data collected was being cross checked and cleared by the team of investigators and compared to the ward’s discharge/death logs.
Operational Definitions
Heart failure (HF): diagnosed based on Echocardiographic evaluation in combination with clinical evaluation with Framingham study criteria.22
Hypertensive heart Disease (HHD): Diagnosed if there is concentric left ventricular hypertrophy, increased left atrial size, diastolic and/or systolic left ventricular (LV) dysfunction on echocardiography in patients with hypertension in the absence of alternative explanation. LV mass index and/or volumes were not routinely measured.
Ischemic heart disease: Diagnosed if there is typical angina or acute myocardial infarction and/or typical ECG abnormalities of acute myocardial infarction/myocardial ischemia, associated with cardiac regional wall motion abnormality as seen by 2D echocardiography or signs of coronary artery disease on invasive coronary angiography study.
Valvular heart disease, cardiomyopathies and other structural heart disease were diagnosed by the discretion of the echocardiographer (cardiologist) and based on standard guidelines on the management of valvular heart disease and world heart federation criteria to diagnose rheumatic heart disease.23
Final Diagnosis: is the diagnosis made by the treating team of investigators/treating physicians after proper history, physical examination, laboratory and imaging studies were followed up and reviewed at discharge.
Outcome of Hospitalization: is patient’s situation at the end of hospital stay which may be: death, discharge, left against medical advice (LAMA), referral or transfer to continue same treatment.
Complications
Hospitalization complications: Hospital acquired pneumonia (HAP), Aspiration Pneumonia, Acute Kidney Injury, UTI and others.
Complications of the disease progression: which may or may not lead to death unless treated.
Data Analysis
Was performed using SPSS version 26. Descriptive and inferential statistics were applied to the data. Categorical variables were described by using frequency, percentage, and graph. Continuous variables were described using an appropriate combination of measure of central tendency and measure of dispersion.
Independent predictors of death among admitted patients were identified by fitting multivariable logistic regression. Association between death and its predictors was reported using odds ratio with its 95% confidence interval and significance value (ie, p-value). Goodness of fit (GOF) of the multivariable model was assessed using Hosmer-Lemeshow test. Multicollinearity was also assessed using Variance Inflation Factor (VIF).
Ethical Considerations
Ethical clearance was obtained from the Mekelle University College of Health Sciences Institutional Review Board (Ref No. ERC 1136/2017). Verbal informed consent was obtained from patients and was acceptable and approved by the ethics committee. The study involves review of patient records while they were admitted and followed. Data was de-identified and stored in a password-secured computer. There was no cost to patients in the study and no compensation or consent was necessary to subjects as we were registering the findings from the patient chart before patients discharge. Our study complies with the principles of the Declaration of Helsinki.
Results
Sociodemographic Characteristics
Of the total 2084 medical ward and ICU admissions, 767 (36.8%) of them had CVDs and the admission pattern and predictors of death for all medical admissions to ACSH during the study period for all patients was published separately.21 The mean age was 53.4 ± 19.4 years (with the most represented age group being 65–74 years (20.2%), 60.9% were 55 years and above, 55% were females. Most were from Tigray (92%) and reside in urban and semi urban areas (56.2%) (See Table 1).
 |
Table 1 Sociodemographic Characteristics of CVD Admissions to ACSH, 2017–2018 |
Types of Cardiovascular Diseases and Underlying Causes for Heart Failure
Majority of patients with CVD were admitted due to stroke (n=272, 35%) followed by Venous thromboembolism (VTE) (n = 132, 17%), rheumatic heart disease (RHD) (n=124, 16.2%), ischemic heart disease (IHD) (n=87, 11.3%) and cardiomyopathy (CMP) (n=57, 7.4%). Majority of the stroke patients had ischemic origin (n=171, 62.9%). Atrial fibrillation was reported in 8.5% and heart failure in 300 of the CVD population (39.1%) (Table 2). Stroke constituted 13.1% of the total hospital admissions while Venous thromboembolism (VTE) was seen in 6.3% of the total admissions over the study period. Rheumatic heart disease, IHD, CMP and cor pulmonale constituted 6%, 4.2%, 2.7% and 1.8% of total admissions respectively.
 |
Table 2 Types of Cardiovascular Diseases in Patients Admitted to Medical Ward and ICU in ACSH, Nov 2017- Oct 2018 |
All patients (n=57, 100%) admitted with cardiomyopathy or pericardial disease (n=4, 100%) as their underlying cardiac structural lesion had symptomatic heart failure. While a significant majority of patients with hypertensive heart disease (91.7%), degenerative valvular heart disease (87.5%), and rheumatic heart disease (87.1%) had heart failure. The prevalence of heart failure was significantly different among the types of CVDs (p<0.001). Atrial fibrillation was present in 8.5% of CVD admissions and it was most frequently seen in patients with rheumatic heart disease (n=24, 19.4%) and ischemic stroke (n= 21, 12.3%) of the total CVDs. Within the Atrial fibrillation population, 36.9% of the cases were in patients with underlying rheumatic heart diseases (Table 3).
 |
Table 3 Types of Cardiovascular Diseases with Frequency Distribution for Heart Failure or Atrial Fibrillation in Patients Admitted to Medical Ward and ICU in ACSH, Nov 2017- Oct 2018 |
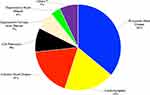
The leading (top 4) underlying causes of heart failure were RHD (36%) followed by CMP (19%), IHD (18%) and cor pulmonale (9%) (See Figure 1).
RHD, venous thrombosis and CMP were generally dominant before the age group 45–54 years and particularly the first two declined as age advances while CMP had its peak at age group 45–54 years. Stroke and degenerative valvular heart diseases sharply increased with age. Ischemic heart disease and cor pulmonale also steadily increased with age peaking at the age group 55–64 years (Figure 2).
Length of Hospitalization and Outcome
Most patients stayed less than 10 days and the median hospital stay was 8 days. The in-hospital mortality of all CVDs admissions was 13.2%. Majority (n=541, 83.4%) were discharged improved and 13.6% left against medical advice (LAMA) (Table 4). Of the total 101 CVD deaths most were because of stroke (n=37, 36.6%) followed by IHD (18.8%), CMP and Venous thrombosis (11.9% each) and RHD (8.9%). Out of the 300 patients with symptomatic heart failure, 50 patients died (17% in-hospital mortality) with Ischemic heart disease having the highest case fatality rate of 38% followed by CMP (24%) and RHD (14%).
 |
Table 4 Length of Stay and Outcome of Hospitalization in Medical Ward and ICU Admissions to ACSH, Nov 2017- Oct 2018 |
Cardiovascular Risk Factors
More than a third of patients (n= 276, 34.7%) had at least one CVD risk factor with 7.3% having two or more. Hypertension was the most frequent risk factor (28.8%) and was found in 52% of stroke cases and in 32% of IHD cases. Diabetes was the second most common type of CVD risk factor (9.6%) and was seen in 10.3% of stroke cases and in 31% of IHD cases. Dyslipidemia was found in 4.6% of the study population and was seen in 12.6% of IHD cases and 5.1% of stroke cases. Obesity was found in 3.7% and most were in those with IHD and CMP. Smoking and alcohol were rare, all those with alcohol as a risk factor were diagnosed with Cardiomyopathy (Figure 3).
 |
Figure 3 Cardiovascular risk factors in patients admitted to Medical ward and ICU in ACSH, Nov 2017- Oct 2018. |
Complications
Any form of complication occurred in 139 (18.1%) of the CVD population. The top three complications among all CVD admissions were aspiration pneumonia, hospital acquired pneumonia and cardiogenic shock contributing to 7.5%, 2.2% and 2.1% respectively (Figure 4). The major underlying causes (risk factors) in the 58 patients with aspiration pneumonia were found to be ischemic stroke (36.2%, n=58), hemorrhagic stroke (32.7), heart failure (13.8) and venous thromboembolism (5.2%). Half of those with cardiogenic shock (8 patients) had ischemic heart disease with 7 of them having Killip class IV ST Elevation Myocardial Infarction (STEMI) (contributing to 43.8% of the underlying cause of the cardiogenic shock). The remaining underlying causes of cardiogenic shock were RHD (18.2%), Pulmonary thromboembolism (12.5) and dilated cardiomyopathy (12.5%). The case fatality of cardiogenic shock was 75%.
 |
Figure 4 Complications of hospitalization and disease progression in patients admitted to Medical ward and ICU in ACSH, Nov 2017- Oct 2018, n=767. |
Causes and Predictors of Mortality
When the various causes of heart failure are combined (RHD, CMP, IHD etc), they accounted for 47 (48.8%) deaths. Stroke and pulmonary thromboembolism were responsible for 37 (38.1%) and 8 (6.2%) of total CVD deaths respectively. Of the heart failure related deaths, IHD was the leading cause contributing to 19 (40.4%) deaths followed by cardiomyopathy, cor pulmonale, and RHD which contributed to 11 (23.4%), 8 (17%), and 4 (8.5%) of heart failure deaths respectively. Majority of those with IHD and who died had acute coronary syndrome (n=12, 63.2%).
Predictors of death were examined by fitting binary logistic regression. During bivariate analysis, heart failure and complication were significantly related with death. But during multivariate analysis, only complication was found to be significantly related with mortality. Accordingly, in comparison to patients with no complications, the adjusted odds of death were 3.2, 5.0, and 6.1 times higher in patients with complications of hospitalization only, complication of disease progression only, and both complications of hospitalization and disease progression, respectively. The final multivariable logistic model was good fit for the data (Hosmer-Lemeshow test: χ2 (df=7, n=767) =4.2, p=0.521). There was no multicollinearity issue (maximum VIF= 1.07, mean VIF=1.03) (Table 5).
 |
Table 5 Predictors of Death Among Patients Admitted Due to Cardiovascular Diseases, ACSH, n=767 |
Discussion
This study found stroke to be the leading cause of CVD (cardiovascular disease) and RHD (rheumatic heart disease) to be the most common cause of heart failure among hospital admissions in Northern Ethiopia, ACSH. Hypertension was the leading CVD risk factor. Epidemiological transition’s role in disease burden in a given society is well reported. The variation in underlying pathologies, interaction with other organ systems, the segment of population affected and variations in the methodologies and definitions used may contribute to a difference in the epidemiology and burden of CVDs.24
Majority (60.9%) of the study population in our study has been 55 years and above and the mean age of our study population (53.4 years) is higher than from a study in Jimma University (mean age = 43.5 years) and much higher than another Ethiopian study from Black Lion Hospital (mean age = 31.63 years) which partly may be due to the fact that these studies have included young population in addition to population aging and increased life expectancy over the last few years.15,25 However, our study population appears younger compared to other reports from across Africa including the THESUS-HF study (mean age of 59 years), which is again less than those in the high income countries which has been more than 70 years.10,26,27 The age at death due to CVDs in SSA is 10–15 years younger than in western countries thus reducing workforce capacity and their positive contribution in the region’s economy.28
Most of the CVD population were females similar to a report from Addis Ababa and the heart of SOWETO study in South Africa even though the proportion of females was a bit lower (55%) in our study as opposed to 59% in both studies.15,29 Our finding is as opposed to findings from western countries where the proportion of males is dominant.30,31 Studies suggest that there are some differences between men and women in terms of prevalence, clinical presentation and outcomes of CVDs.
Top five causes of total CVD admission were stroke followed by venous thrombosis, rheumatic heart disease, ischemic heart disease, cardiomyopathy and cor pulmonale. While in those with structural heart diseases, the leading causes of admissions due to heart failure was rheumatic heart disease followed by cardiomyopathies, ischemic heart disease, cor pulmonale and degenerative valve diseases. This study shows CVD admissions to have contributed to more than a third (36.5%) of the total medical admissions which is consistent with previous studies showing the steady rise of CVD admissions in SSA nations as evidenced by the contributions of CVD admissions increasing from 3.9% of medical admissions in 1950–59 to nearly 20% in the decade 2000–2010.20,32 More recent studies from Ethiopia also show the rise of CVDs, increasing from 21.7% in 1981/82 to 58% in 2011/12, as major reasons for medical ICU admissions.12,33 A literature review in 2001 has reported that CVDs contributed 7–10% of all medical admissions and our study shows up to five times increment in the proportion of CVDs among medical admissions.34
This study found stroke to be the leading reason for CVDs admission (35.7%) consistent with the global burden of diseases, injuries and risk factors 2010 study on epidemiology of CVDs in SSA, but the subtype of stroke was dominantly ischemic in our finding contrary to this report where hemorrhagic stroke was reportedly dominant.24 Stroke was followed by RHD (16.2%) in our finding similar to other studies done in hospitals and community in Ethiopia which reported RHD usually in the top three causes of morbidity and mortality.12,15 In previous studies done in Ethiopia, RHD has been the leading cause of CVD even though there is a change in pattern with ischemic heart disease and hypertension and related disorders like hypertensive heart disease, stroke and others steadily increasing.17,35 A study done in 2017 in six main referral hospitals in Ethiopia showed RHD, Congenital heart diseases and hypertensive heart diseases to be the top three causes of cardiovascular disorders but this study did not include stoke/thrombosis cases in its report and included children of all ages (those <13 years of age constituted 23.6% of the study population) and was an outpatient based study in contrast to our study.14 In a study done in our setting, Mekelle University six years earlier which was echocardiography based study and in ACSH outpatient cardiology unit, showed that valvular heart diseases were leading echocardiographically detected cardiac abnormalities with RHD dominating in the young.36 The study from Ethiopia, Jimma University out patient cardiac clinic which also reported RHD to be the leading cause of cardiac diseases did not include strokes or venous thrombosis cases, unlike our study.25 A retrospective 30 year CVD trend analysis study done in Addis Ababa black lion hospital, Ethiopia, which included pulmonary embolism cases showed stroke to be a third cause of medical ICU admission after acute coronary syndrome and heart failure.12
Among heart failure patients, the leading underlying cause in our study has been found to be RHD (36%) corroborating the systematic review of heart failure epidemiology in Ethiopia in 2018 which reported that valve diseases (dominated by RHD) constituted 29.2–81% causes of heart failure.33 A study from St. Paul Hospital in Addis Ababa reported that RHD constituted 30% of acute heart failure cases while a black lion hospital study also showed that 81.3% of patients with advance heart failure (NYHA class III–IV) were having Valvular lesions with majority being RHD.11,15 A study from South west Ethiopia also has reported RHD as the leading cause of HF (32.8%) followed by hypertensive heart diseases (HHD) (24.8%). Our finding was similar to a study done in Cameroon where rheumatic heart disease was the leading causes of heart failure particularly in the young.37 This is contrary to studies from Nigeria and South Africa, Ghana and Burkina Faso where hypertensive heart disease was the leading cause of heart failure.26,38–40 In the Nigerian study, RHD was a third cause of HF.26 In general, still RHD continues to dominate the cause of Heart failure in Ethiopia and a considerable number of SSA nations driven by low community awareness, poor socioeconomic status, overcrowding and poor accessibility and availability of medical service predisposing communities to a high burden of rheumatic fever and RHD.41 As the cost of cardiac surgery in Ethiopia is about 11,000 USD currently which majority of patients in Ethiopia with RHD cannot afford, it is important to focus on the primordial, primary and secondary prevention activities of RHD in parallel to tertiary level care including availing cardiac surgery for those affected.42
In sub-Saharan Africa, reports since the mid of 20th century show that hypertensive heart disease (HHD) ranks in the top three as a cause of heart failure (HF).43 This study shows HHD to be a less frequent cause of CVD admission, contributing to only 4%, even less than cor pulmonale which contributed to 9% of HF admissions. The fact that cor pulmonale is an important cause of HF in SSA has been addressed in previous studies.44 The unexpectedly low prevalence of HHD in our study could be partly due to underdiagnosis on echocardiographic evaluations but the ongoing existence and continued dominance of CVDs of post infectious complications like RHD, cardiomyopathy and cor pulmonale is worth noting.44,45 Even though our study shows hypertension to be a not so common reason for heart failure admissions it is the leading risk factor for overall CVDs. In line with our study’s finding, there is a study in 2011 from Cameroon where hypertension was a not so dominant cause of heart failure.37 In more recent studies particularly from western Africa, HHD is reportedly the leading cause of HF in contrast to our finding where HHD contributed to only 1.6% of total CVDs.10,26,39 Studies from South Africa and Nigeria have indicated the high prevalence of hypertensive heart disease as major cause of HF.46,47 The variation could also emanate from variation in epidemiologic transition, our study population being majority rural and semiurban or could arise from the definition and identification of HHD with HF. But again, it is worth noting that in this study, hypertension was the leading risk factor for CVDs, present in 28.9% of the total CVD population and in more than half of stroke and more than one third of IHD population.
The in-hospital mortality may vary based on the age of the population studied and clinical characteristics among others. The in-hospital mortality of CVD admissions in this study was similar to the 12.4% mortality reported from Nigeria but was lower than the 21.9% report from Tanzania.48,49 In-hospital mortality was also lower in our study as compared to a study done in Addis Ababa (24.4%) but higher than the 9.2% mortality reported in a study done in Cameron.37 The reason for the higher rate from Addis Ababa may be due to the clinical characteristics of the patients as they included solely acutely decompensated heart failure population.11 In acute decompensation of HF, deaths as high as 27% at four weeks follow up and 46% at one year follow up was reported from France.27 A study from Nigeria in 2007 reported a mortality of 67% among advanced HF admissions.26 Mortality of Heart failure in Africa has been reported as 9–12.5%.43 The mean age at death for RHD in our study was 53 years and again this is different from previous reports in Ethiopia with most RHD patients dying before 30 years of age as has been reported in one study by Oli K. and Asmera J. the mean age at death was 26 years.50
To the best of our knowledge, the prevalence of venous thromboembolism (VTE) is not well documented in Ethiopia except for a few studies on risk factors and even in SSA.51,52 In SSA, the prevalence of venous thrombosis has been reported to be 2.4–9.6% in postoperative cases, and 380–448 per 100000 births/year in pregnant/postpartum mothers. Venous thromboembolism is in the top three causes of death globally from CVD following ischemic heart disease (IHD) and stroke.53 This is in line with our finding of VTE being the second most common cause of CVD admission and contributing to 6.3% of all admissions. The prevalence of VTE is higher in our study than a in a study done in Jimma University, Ethiopia which showed a prevalence of 2.2% (221 out of the 10,080 admissions).54 This difference could partly be because our study has also included pulmonary embolism cases as opposed to the limitation in the report from Jimma where they did not use Pulmonary CT angiography to support the diagnosis while this imaging modality was available in our set up.
Ischemic heart disease has been steadily increasing in SSA including Ethiopia although still of a lesser burden when compared to other CVDs in Ethiopia and SSA.44,55 Ischemic heart disease has been of low prevalence in Ethiopia as evidenced by a study done in 1980’s in the country’s largest hospital, Tikur Anbessa, where IHD constituted to 0.35% of medical admissions while the finding of our study shows a 12 times increment in the contribution of IHD to medical admissions in Northern Ethiopia.56 Our finding is another evidence for the increasing burden of ischemic heart disease in hospitalized patients in Ethiopia contributing 4.2% of total medical admissions, 11.3% of total CVD admissions and has been found to be a third cause of heart failure admissions similar to studies in Addis Ababa done recently.11,12,15,33,57 A recent prospective study from Jimma and Ambo University hospitals in Ethiopia has shown that IHD is the leading cause of HF even though they have excluded causes of right sided heart failure and they did not report details of the diagnostic methods they used other than mentioning that the diagnosis was based on echocardiography reports.58 The study from Jimma and Ambo University hospitals were done in a setting where there was no coronary angiography, nevertheless, the study gives clues to the rise of IHD in Ethiopia but needs to be replicated in a rigorously conducted study with detailed diagnostic methodologies of IHD including clinical details, echocardiography, resting and Exercise ECG, CT Coronary angiography and invasive coronary angiography which unfortunately are lacking in most settings in Ethiopia and other SSA.
In SSA, the contributions of chronic diseases like HIV / AIDS, schistosomiasis and tuberculosis to cor pulmonale has been there, unfolding more but again neglected and is a double burden to the non-communicable disease care.46,59 Considerable proportion of heart failure patients in our study (9% of total CVD admissions and 1.8% of total medical admissions) had cor pulmonale albeit lower than reported from the heart failure in Soweto studies.38,60 The prevalence of pulmonary hypertension has been much lower than our finding in a study done using a retrospectively collected data from January 2001 to December 2012 at Tikur Anbessa hospital in the capital Addis Ababa which was 0.5% of all CVDs and 2% among those with advanced NYHA class III–IV HF.15 Again, the prevalence of cor pulmonale in our study is more than two times higher than the 3.8% prevalence reported among CVDs in the study done in Jimma University in Ethiopia in 2008.25 It is thought from previous reports and clinical experience that cor pulmonale develops mostly following pulmonary tuberculous complications like pulmonary fibrosis and bronchiectasis, possible indoor air pollution, schistosomiasis or pulmonary arterial hypertension related with HIV/AIDS.60 An echocardiography based report from ACSH in 2014 shows that pulmonary hypertension of any etiology was diagnosed in 30.6% of the 1028 echocardiograms even though the most common cause would understandably be due to left heart failure but it is indicative of the burden of potential burden of right heart failure in the Ethiopian setting and hence the need for further study on the burden of cor pulmonale and/or right side heart failure in Ethiopia and SSA.36
Atrial Fibrillation was present in 8.5% of CVDs and most were in patients with rheumatic heart disease (36.9%) and ischemic stroke (32.3%). This is less than the prevalence reported in the Gulf acute heart failure registry (Gulf CARE) and in Nigerian study where AF was seen in 14% and 20.7% respectively, even though the patients from the Nigerian study had advanced heart failure26,61 The prevalence of atrial fibrillation in our study is similar, albeit a little higher in our case, to a study from Ghana where atrial fibrillation was present in 7.1% of the study population and the dominant underlying structural cardiac abnormality was RHD in 45% in the Ghanaian study and 36.9% in our study.62
Registry data from the Euro-Heart failure survey, the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF) and the acute decompensated national registry (ADHERE) from the United States and Europe has shown that the in hospital mortality of patients with acute heart failure varied from 4–7%.63,64
The documentation of CVD risk factors and implementation of control strategies have contributed in the reduction of mortality seen in developed nations.22,28 Hypertension has been a significant contributor for the burden of CVDs in SSA and globally which is consistent with our finding.65,66 Hypertension and Diabetes were the most prevalent risk factors particularly in those with stroke and ischemic heart diseases similar to reports from Addis Ababa, Ethiopia.14,67 In a study done in 300 patients with coronarography proven ischemic heart disease (IHD) in Addis Ababa, Ethiopia, the leading risk factors of IHD were dyslipidemia, hypertension and diabetes seen in 63%, 61.2% and 41% of patients respectively whereas in our study hypertension, diabetes and dyslipidemia were found in 32%, 31% and 4.6% of the IHD population.57 The reason for the low prevalence of dyslipidemia in our IHD patients may be due to the limitation of lipid profile work up due to financial restrictions. The fact that stroke has been the main cause of hospital CVD admission with hypertension, diabetes, obesity, dyslipidemia, smoking and alcohol being important risk factors reported indicates the relevance of these risk factors as also reported in previous studies in other parts of Ethiopia, SSA & western countries.2,68
The top three CVD related causes of mortality in our study were heart failure (48.8%), stroke (38.1%) and pulmonary thromboembolism (6.2%). In a similar study in Nigeria consisting of CVD admissions, stroke followed by heart failure were the leading causes of death. This difference could be explained by the high prevalence of hypertension (reported in 55.7%) in the Nigerian study population.48 Of the heart failure related deaths, IHD was the leading cause contributing to 19 (40.4%) deaths. RHD contributed only for 4 (8.5%) of heart failure deaths, even if it was the most common cause of heart failure admission; and this can be partly explained by the younger age population of RHD.
To tackle HF and stroke, implementation of prevention strategies on their most common causes and risk factors (RHD and hypertension) is of paramount importance. In studies done in communities affected by RHD the awareness on sore throat, acute rheumatic fever and RHD has been reported to be low.69,70 One of the challenges in the prevention and control of RHD is the availability of Benzathine Penicillin G (BPG) and the fear of adverse events following the injection of BPG by health professionals as well as patients71,72 In a systematic review of data from 33 surveys, the prevalence of hypertension has been reported to be 30% in SSA and among these 27% were aware of their hypertensive status, 18% were on treatment, and only ≈7% had controlled blood pressure.73,74 Hence, it is important to raise these low level of awareness, prevention and treatment of RHD and hypertension to prevent complications that emanate from sore throat and high blood pressure and obviously needs immense mobilization of resources to the needy.
Limitations and Strengths
The present study has some limitations. This is only a single center study as well as both medical ward and ICU admissions are reported together. Being a study done only in a tertiary hospital, it may have inherent weakness in its applicability to the larger community and hence the need for multicenter studies. However, in the absence of robust community based studies, this hospital based study can help as a source of evidence on the CVDs patterns in the community it serves and the eastern Africa region in particular and SSA in general. Heart failure was classified based on the ventricular systolic function and other modalities like Cardiac MRI, SPECT, Cardiac CT and Brain natriuretic peptide were not used in this study as they were not available. This study was limited to in-hospital and there was no follow up for a period of time after discharge. The documentation of CHA2DS2-Vasc score in patients with atrial fibrillation is important and is lacking in our study and we recommend future studies to include this important risk score.
However this study is the only of its kind in the region and being a prospective study it lays a firm ground for further studies. All patients with stroke had brain imaging (CT Scan or MRI) and all heart failure patients with ECG and Echocardiography as well as when indicated, exercise stress and/or cardiac catheterization were done.
Focused research in CVDs in a prospective design at multinational and multisite level is required to properly document the actual burden CVDs in Ethiopia and SSA to recommend appropriate interventions.
Conclusion
This study has revealed the dominance of stroke as the leading form of CVD with hypertension as the most prevalent risk factor and the persistence of RHD as the top cause of heart failure in Northern Ethiopia, ACSH. A high priority should be given to prevent stroke and RHD and their risk factors to forestall the CVD epidemic in the future. Multisite, multi country, prospective cohort studies are required to clarify the CVD epidemiology, risk factors, prognosis and implement preventive and treatment strategies in Ethiopia and SSA.
Acknowledgment
We are grateful to staff of the medical ward as well as ICU nurses and residents for their support towards the success of this study and the patients. We are also thankful to Mekelle University - College of health sciences, Ayder University Hospital.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Gersh BJ, Sliwa K, Mayosi BM, Yusuf S. Novel therapeutic concepts: the epidemic of cardiovascular disease in the developing world: global implications. Eur Heart J. 2010;31(6):642–648. doi:10.1093/eurheartj/ehq030
2. Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol. 2020;76(25):2982–3021. doi:10.1016/j.jacc.2020.11.010
3. Zühlke L, Sliwa K, Naidoo P, et al. Cardiovascular medicine and research. Nat Rev Cardiol. 2019;16(11):642–644. doi:10.1038/s41569-019-0269-z
4. Keates AK, Mocumbi AO, Ntsekhe M, Sliwa K, Stewart S. Cardiovascular disease in Africa: epidemiological profile and challenges. Nat Publ Gr. 2017;14(5):273–293. doi:
5. Brodmann M, Cahill TJ, Hil DP, et al. Global burden of cardiovascular diseases. J Am Coll Cardiol. 2020;76(25):2982–3021.
6. Nyirenda MJ. Non-communicable diseases in sub-Saharan Africa: understanding the drivers of the epidemic to inform intervention strategies. Int Health. 2016;8(3):157–158. doi:10.1093/inthealth/ihw021
7. Mensah GA, Roth GA, Sampson UKA, et al. Mortality from cardiovascular diseases in sub-Saharan Africa, 1990–2013: a systematic analysis of data from the Global Burden of Disease Study 2013. Cardiovasc J Afr. 2015;26(2):S6–S10. doi:10.5830/CVJA-2015-036
8. Ntusi NAB, Mayosi BM. Epidemiology of heart failure in sub-Saharan Africa. Expert Rev Cardiovasc Ther. 2009;7(2):160–180. doi:10.1586/14779072.7.2.169
9. World Health Organization. International statistical classification of diseases and related health problems, 10th revision ICD-10: tabular list. World Health Organization; 2016:332–345. Available from: http://www.who.int/classifications/icd/icdonlineversions/en/.
10. Damasceno A, Mayosi BM, Sani M, et al. The causes, treatment, and outcome of acute heart failure in 1006 Africans from 9 countries. Arch Intern Med. 2012;172(18):1386–1394. doi:10.1001/archinternmed.2012.3310
11. Woldeyes E, Zewdu H, Abera H, et al. Clinical characteristics and in hospital outcome of acute heart failure: a five-year experience at a tertiary care hospital in Ethiopia. Ethiopian Med J. 2020;58(1):21–28.
12. Araya Giday EW, Weldeyes E. Trends in cardiovascular disease over time: a 30-year retrospective analysis of medical-ICU admissions. Ethiop Med J. 2015;53(3):133–139.
13. Kariuki JK, Stuart-shor EM, Leveille SG, Hayman LL, Israel B, Medical D. Methodological challenges in estimating trends and burden of cardiovascular disease in Sub-Saharan Africa. Cardiol Res Pract. 2015;2015. doi:10.1155/2015/921021
14. Yadeta D, Guteta S, Alemayehu B, et al. Spectrum of cardiovascular diseases in six main referral hospitals of Ethiopia. Heart Asia. 2017;9(1):1–5. doi:10.1136/heartasia-2016-010766
15. Abdissa SG, Oli K, Feleke Y, Goshu DY, Begna DM, Tafese A. Spectrum of cardiovascular diseases among Ethiopian patients at Tikur Anbessa Specialized University Teaching Hospital, Addis Ababa. Ethiop Med J. 2014;52(1):9–17.
16. Fenta EH, Sisay BG, Gebreyesus SH, Endris BS. Trends and causes of adult mortality from 2007 to 2017 using verbal autopsy method, Addis Ababa, Ethiopia. BMJ Open. 2021;11(11):1–8. doi:10.1136/bmjopen-2020-047095
17. Maru M. The changing pattern of cardiovascular diseases in Ethiopia. East Afr Med J. 1993;70(12):772–776.
18. Abraham G. Pattern of cardiovascular diseases among adult hospitalized Ethiopians. Ethiop Med J. 1982;20:63–68.
19. Kengne AP, Ntyintyane LM, Mayosi BM. Review Article A systematic overview of prospective cohort studies of cardiovascular disease in sub-Saharan Africa. Cardiovasc J Afr. 2012;23(2):103–112. doi:10.5830/CVJA-2011-042
20. Etyang AO, Scott G, Anthony J, et al. Medical causes of admissions to hospital among adults in Africa: a systematic review Anthony. Glob Heal Action. 2013;6(1):19090. doi:10.3402/gha.v6i0.19090
21. Hailu A, Gidey K, Ebrahim MM, et al. Patterns of medical admissions and predictors of mortality in ayder comprehensive Specialized Hospital, Northern Ethiopia: a prospective observational study. Int J Gen Med. 2023;16:243–257. doi:10.2147/IJGM.S385578
22. McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285(26):1441–1446. doi:10.1056/nejm19711223285260
23. Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation. 2014;129(23):2440–2492. doi:10.1161/CIR.0000000000000029
24. Moran A, Forouzanfar M, Sampson U, Chugh S, Feigin V, Mensah G. ScienceDirect the epidemiology of cardiovascular diseases in Sub-Saharan Africa: the Global Burden of Diseases, Injuries and Risk Factors 2010 Study. Prog Cardiovasc Dis. 2013;56(3):234–239. doi:10.1016/j.pcad.2013.09.019
25. Habte B, Alemseged F, Tesfaye D. The pattern of cardiac diseases at the Cardiac Clinic of Jimma University Specialised Hospital, South West Ethiopia. Ethiop J Health Sci. 2011;20(2). doi:10.4314/ejhs.v20i2.69435
26. Familoni OB, Olunuga TO, Olufemi BW. A clinical study of pattern and factors affecting outcome in Nigerian patients with advanced heart failure. Cardiovasc J Afr. 2007;18(5):308–311.
27. Zannad F, Mebazaa A, Juillière Y, et al. Clinical profile, contemporary management and one-year mortality in patients with severe acute heart failure syndromes: the EFICA study. Eur J Heart Fail. 2006;8(7):697–705. doi:10.1016/j.ejheart.2006.01.001
28. Amusa GA. Cardiovascular disease: a Global Epidemic extending into Sub-Saharan Africa. A Review of Literature. Jos J Med. 2002;6(2):6–12.
29. Sliwa K, Wilkinson D, Hansen C, et al. Spectrum of heart disease and risk factors in a black urban population in South Africa (the Heart of Soweto Study): a cohort study. Lancet. 2008;371(9616):915–922. doi:10.1016/S0140-6736(08)60417-1
30. Vancheri F, Tate AR, Henein M, et al. Time trends in ischaemic heart disease incidence and mortality over three decades (1990–2019) in 20 Western European countries: systematic analysis of the Global Burden of Disease Study 2019. Eur J Prev Cardiol. 2022;29(2):396–403. doi:10.1093/eurjpc/zwab134
31. Fox CS, Evans JC, Larson MG, Kannel WB, Levy D. Temporal trends in coronary heart disease mortality and sudden cardiac death from 1950 to 1999: the Framingham Heart Study. Circulation. 2004;110(5):522–527. doi:10.1161/01.CIR.0000136993.34344.41
32. Oyoo GO, Ogola EN. Clinical and socio demographic aspects of congestive heart failure patients at Kenyatta National Hospital, Nairobi. East Afr Med J. 1999;76(1):23.
33. Tsega TA, Demissei BG. A systematic review of epidemiology, treatment and prognosis of heart failure in adults in Ethiopia. J Cardiovasc Med. 2018;19(3):91–97. doi:10.2459/JCM.0000000000000617
34. Mendez GF, Cowie MR. The epidemiological features of heart failure in developing countries: a review of the literature. Int J Cardiol. 2001;80(2–3):213–219. doi:10.1016/S0167-5273(01)00497-1
35. Mamo Y, Oli K. Trends of acute myocardial infarction admissions over a decade, Tikur Anbessa Hospital. Ethiop Med J. 2001;39(3):193–202.
36. Adem A, Abebe S, Hailu A, Feleke B, Berhe M, Atsibeha MDV. Heart diseases in North Ethiopia pattern of echocardiographic abnormalities among adult cardiac patients - an experience from Ayder hospital of Mekelle University. Ethiop Med J. 2014;52(4):173–183.
37. Tantchou Tchoumi JC, Ambassa JC, Kingue S, et al. Occurrence, aetiology and challenges in the management of congestive heart failure in sub-Saharan Africa: experience of the Cardiac Centre in Shisong, Cameroon. Pan Afr Med J. 2011;8688:1–7.
38. Stewart S, Wilkinson D, Hansen C, et al. Predominance of heart failure in the heart of Soweto study cohort: emerging challenges for urban African communities. Circulation. 2008;118(23):2360–2367. doi:10.1161/CIRCULATIONAHA.108.786244
39. Appiah LT, Sarfo FS, Agyemang C, et al. Current trends in admissions and outcomes of cardiac diseases in Ghana. Clin Cardiol. 2017;40(10):783–788. doi:10.1002/clc.22753
40. Mandi DG, Bamouni J, Yaméogo RA, et al. Spectrum of heart failure in sub-saharan Africa: data from a tertiary hospital-based registry in the eastern center of Burkina Faso. Pan Afr Med J. 2020;36:1–11. doi:10.11604/pamj.2020.36.30.19321
41. Gemechu T, Mahmoud H, Parry EHO, Phillips DIW, Yacoub MH. Community-based prevalence study of rheumatic heart disease in rural Ethiopia. Eur J Prev Cardiol. 2017;24(7):717–723. doi:10.1177/2047487316687104
42. Zilla P, Yacoub M, Zühlke L, et al. Global unmet needs in cardiac surgery. Glob Heart. 2018;13(4):293–303. doi:10.1016/j.gheart.2018.08.002
43. Bloomfield GS, Barasa FA, Doll JA, Velazquez EJ. Heart failure in Sub-Saharan Africa. Curr Cardiol Rev. 2013;9(2):157–173. doi:10.2174/1573403X11309020008
44. Mocumbi AO. Lack of focus on cardiovascular disease in sub-Saharan Africa. Cardiovasc Diagn Ther. 2012;2(1):74–77. doi:10.3978/j.issn.2223-3652.2012.01.03
45. Opie LH, Mayosi BM, Sa FCP. Cardiovascular Disease in Sub-Saharan Africa. Circulation. 2005;112(23):3536–3540. doi:10.1161/CIRCULATIONAHA.105.597765
46. Mayosi BM. Contemporary trends in the epidemiology and management of cardiomyopathy and pericarditis in sub-Saharan Africa. Heart. 2007;93(10):1176–1183. doi:10.1136/hrt.2007.127746
47. Onwuchekwa AC, Asekomeh GE. Pattern of heart failure in a Nigerian teaching hospital. Vasc Health Risk Manag. 2009;5:745–750. doi:10.2147/vhrm.s6804
48. Ansa VO, Ekott JU, Bassey EO. Profile and outcome of cardiovascular admissions at the University of Uyo Teaching Hospital, Uyo-a five year review. Niger J Clin Pract. 2008;11(1):22–24.
49. Maro E, Kaushik R. The role of echocardiography in the management of patients with congestive heart failure. “Tanzanian experience” Abstract. Cent Afr J Med. 2009;55(5):35–40. doi:10.4314/cajm.v55i5-8.63638
50. Oli K, Asmera J. Rheumatic heart disease in Ethiopia: could it be more malignant? Ethiop Med J. 2004;42(1):1–8.
51. Gebremedhin A, Shamebo M. Deep venous thrombosis in a University Teaching Hospital, Addis Ababa, Ethiopia. East Afr Med J. 1998;75(7):432–435.
52. Danwang C, Temgoua MN, Agbor VN, Tankeu AT, Noubiap JJ. Epidemiology of venous thromboembolism in Africa: a systematic review. J Thromb Haemost. 2017;15(9):1770–1781. doi:10.1111/jth.13769
53. Goldhaber SZ, Morrison RB. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379(9829):1835–1846. doi:10.1016/S0140-6736(11)61904-1
54. Amare H, Getachew A. Deep vein thrombosis in a tertiary hospital from Ethiopia. Thromb Res. 2021;198:17–18. doi:10.1016/j.thromres.2020.11.019
55. Mensah GA. Ischaemic heart disease in Africa. Heart. 2008;94(7):836–843. doi:10.1136/hrt.2007.136523
56. Fikreyesus Y, Bahta Y. Myocardial infarction in the Tikur Anbessa Teaching Hospital. A five-year review. Ethiop Med J. 1989;27(2):55–61.
57. Shashu BA, Ayele MA. Original article The pattern of coronary artery diseases as diagnosed by coronary angiography and the outcome of Percutaneous Coronary Intervention (PCI) in Ethiopia. Ethiop J Heal Dev. 2007;28:11–16.
58. Beri B, Fanta K, Bekele F, Bedada W. Management, clinical outcomes, and its predictors among heart failure patients admitted to tertiary care hospitals in Ethiopia: prospective observational study. BMC Cardiovasc Disord. 2023;23(1):1–10. doi:10.1186/s12872-022-03008-7
59. Mayosi BM, Wiysonge CS, Ntsekhe M, et al. Clinical characteristics and initial management of patients with tuberculous pericarditis in the HIV era: the Investigation of the Management of Pericarditis in Africa (IMPI Africa) registry. BMC Infect Dis. 2006;9:1–9.
60. Stewart S, Mocumbi AO, Carrington MJ, Pretorius S, Burton R, Sliwa K. A not-so-rare form of heart failure in urban black Africans: pathways to right heart failure in the Heart of Soweto Study cohort. Eur J Heart Fail. 2011;13(10):1070–1077. doi:10.1093/eurjhf/hfr108
61. Sulaiman K, Panduranga P, Al-zakwani I, et al. Clinical characteristics, management, and outcomes of acute heart failure patients: observations from the Gulf acute heart failure registry (Gulf CARE). Eur J Heart Fail. 2015;17(4):374–384. doi:10.1002/ejhf.245
62. Amoah AGB, Kallen C. Aetiology of heart failure as seen from a national cardiac referral centre in Africa. Cardiology. 2000;93(1–2):11–18. doi:10.1159/000006996
63. Cleland JGF, Swedberg K, Follath F, et al. The EuroHeart Failure survey programme - A survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–463. doi:10.1016/S0195-668X(02)00823-0
64. Yancy CW, Abraham WT, Albert NM, et al. Quality of Care of and Outcomes for African Americans Hospitalized With Heart Failure. Findings From the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure) Registry. J Am Coll Cardiol. 2008;51(17):1675–1684. doi:10.1016/j.jacc.2008.01.028
65. Yuyun MF, Sliwa K, Kengne AP, Mocumbi AO, Bukhman G. Cardiovascular diseases in sub-saharan Africa compared to high-income countries: an epidemiological perspective. Glob Heart. 2020;15(1):1–18. doi:10.5334/gh.403
66. Paolo F, Michelle C, Miller A. Cardiovascular disease and hypertension in sub-Saharan Africa: burden, risk and interventions. Intern Emerg Med. 2016;11(3):299–305. doi:10.1007/s11739-016-1423-9
67. Adem A, Demis TFY, Feleke Y. Trend of diabetic admissions in Tikur Anbessa and St. Paul’s University Teaching Hospitals from January 2005-December 2009, Addis Ababa, Ethiopia. Ethiop Med J. 2011;49(3):231–238.
68. Shiferaw F, Letebo M, Misganaw A, et al. Non-communicable Diseases in Ethiopia: disease burden, gaps in health care delivery and strategic directions. Ethiop J Heal Dev. 2018;32(3):1–12.
69. Nalubwama H, Ndagire E, Sarnacki R, et al. Community perspectives on primary prevention of rheumatic heart disease in Uganda. Glob Heart. 2022;17(1):5. doi:10.5334/gh.1094
70. Hailu A, Tsega T, Gebregziabher T, et al. Community awareness of sore throat and rheumatic heart disease in Northern Ethiopia. Sudan Hear J. 2020;7(3):1–25.
71. Sanyahumbi A, Ali S, Benjamin IJ, et al. Penicillin reactions in patients with severe rheumatic heart disease: a presidential advisory from the American Heart Association. J Am Heart Assoc. 2022;11(5):1–9. doi:10.1161/JAHA.121.024517
72. Wyber R, Johnson TD, Patel B. Supply of benzathine penicillin G: the 20-year experience in Australia. Aust N Z J Public Health. 2015;39(6):506–508. doi:10.1111/1753-6405.12415
73. Minja NM, Nakagaayi D, Aliku T, et al. Cardiovascular diseases in Africa in the twenty-first century: gaps and priorities going forward. Front Cardiovasc Med. 2022;9:1–20. doi:10.3389/fcvm.2022.1008335
74. Ataklte F, Erqou S, Kaptoge S, Taye B, Echouffo-Tcheugui JB, Kengne AP. Burden of undiagnosed hypertension in sub-saharan Africa: a systematic review and meta-analysis. Hypertension. 2015;65(2):291–298. doi:10.1161/HYPERTENSIONAHA.114.04394
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.