Back to Journals » Therapeutics and Clinical Risk Management » Volume 18
Clinical Perspectives on Cardiac Rehabilitation After Heart Failure in Elderly Patients with Frailty: A Narrative Review
Authors Tsukakoshi D, Yamamoto S , Takeda S, Furuhashi K, Sato M
Received 9 June 2022
Accepted for publication 11 September 2022
Published 27 October 2022 Volume 2022:18 Pages 1009—1028
DOI https://doi.org/10.2147/TCRM.S350748
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Garry Walsh
Daichi Tsukakoshi,1 Shuhei Yamamoto,1 Shuhei Takeda,1 Keisuke Furuhashi,1 Masaaki Sato2
1Department of Rehabilitation, Shinshu University Hospital, Matsumoto, Japan; 2Division of Occupational Therapy, School of Health Sciences, Shinshu University, Matsumoto, Nagano, Japan
Correspondence: Shuhei Yamamoto, Department of Rehabilitation, Shinshu University Hospital, 3-1-1 Asahi, Matsumoto, Nagano, 390-8621, Japan, Tel +81-263-37-2836, Fax +81-263-37-2835, Email [email protected]
Abstract: The purpose of this narrative review is to examine rehabilitation modalities for patients with heart failure and Frailty who require comprehensive intervention. Ischemic heart disease is the leading cause of death worldwide, accounting for 16% of global mortality. Due to population growing and aging, the total number of heart failure patients continues to rise, a condition known as the heart failure pandemic. Furthermore, frailty has been associated with an increased risk for heart failure and increased morbidity and mortality. The 2021 update of the 2017 ACC expert consensus decision pathway for optimization of HF treatment has become more concerning, citing frailty as one of the 10 most important issues associated with heart failure with reduced ejection fraction (HFrEF). Frailty and heart failure share common pathological mechanisms and are associated with poor clinical outcomes. Most studies of frailty in patients with heart failure primarily focus on physical frailty, and associations between psycho-psychological and social factors such as cognitive dysfunction and social isolation have also been reported. These results suggest that a more comprehensive assessment of frailty is important to determine the risk in patients with heart failure. Therefore, mechanisms of the three domains, including not only physical frailty but also cognitive, psychological, spiritual, and social aspects, should be understood. In addition to interventions in these three domains, nutritional and pharmacological interventions are also important and require tailor-made interventions for the widely varied conditions associated with heart failure and frailty. Although several studies have shown a relationship between frailty and prognosis in patients with heart failure, interventions to improve the prognosis have not yet been established. Further information is needed on frailty intervention by a multidisciplinary team to improve the prognosis.
Keywords: frailty, heart failure, rehabilitation, cardiovascular diseases
Introduction
Ischemic heart disease is the leading cause of death worldwide, accounting for 16% of all deaths.1 Worldwide, it is estimated that approximately 64.3 million people are living with heart failure, and an estimated mortality rate of 13.5% at 1 year and 43.3% at 5 years.2 HF is not a single pathological diagnosis, but a clinical syndrome consisting of cardinal symptoms (eg breathlessness, ankle swelling, and fatigue) that may be accompanied by signs (eg elevated jugular venous pressure, pulmonary crackles, and peripheral oedema).3 Due to population growing and aging, the total number of HF patients continues to rise, a condition known as the HF pandemic.4 The 2021 update of the 2017 ACC expert consensus decision pathway for optimization of HF treatment has become more concerning, citing frailty as one of the 10 most important issues associated with HF with reduced ejection fraction (HFrEF).5
Frailty is recognized as an age-related clinical condition that is typically observed by a deterioration in the physiological capacity of several organ systems, and that causes an increased susceptibility to stressors.6 Frailty is generally recognized as a physical condition that precedes the development of a disability,7,8 but frailty and disability can coexist.9 With the rapid aging of society, the number of frailty elderly people is increasing, placing a heavy burden on the world’s health care systems.10 Frailty has been associated with an increased risk for HF and increases morbidity and mortality.11
Comprehensive treatment of HF and frailty, including not only physical but also cognitive, psychological, psycho-social, nutritional, and pharmacological factors, is essential. The purpose of this narrative review is to examine rehabilitation modalities for patients with HF and Frailty who require comprehensive intervention.
Frailty in HF
Frailty and HF share common pathological mechanisms and are associated with a worsening clinical outcome (Figure 1).12 In FRAIL-HF,13 a cohort study of 450 non-caring patients with worsening HF aged ≥70 years, the presence of frailty was closely associated with an increased risk of mortality, rehospitalization, and declined cardiac function at 1 year. In addition, a study examining the risk of heart failure rehospitalization in a conditional vulnerability model found that age, female sex, non-manual work, better medical compensation, longer QRS duration, and treatment with percutaneous coronary intervention increased the risk of heart failure rehospitalization.14 Furthermore, patients with HF and frailty more frequently complained of dyspnea, sleep disturbance, and depression than non-frail patients, and their quality of life is significantly impaired.15 In contrast, most studies on frailty in patients with HF have mainly focused on physical frailty,16 but not on cognitive dysfunction17 and social isolation.18,19 Those results indicate the importance of conducting a more comprehensive assessment of frailty to confirm the risk in patients with HF and are consistent with the publication from the heart failure association/European society of cardiology.20 Therefore, a more comprehensive assessment of frailty in patients with HF is needed to better understand the risk. To promote a comprehensive approach, incorporation vulnerability assessment into prognostic and treatment models for HF is strongly emphasized.21 In summary, three domains of HF include not only physical frailty but also cognitive, psychological, and social aspects.
 |
Figure 1 Commonalities between heart failure and frailty. Notes: Adapted from JACC Heart Fail, 7(12), Pandey A, Kitzman D, Reeves G. Frailty Is Intertwined With Heart Failure: Mechanisms, Prevalence, Prognosis, Assessment, and Management. 1001-1011, copyright (2019), with permission from Elsevier.12 |
Three Domains: FRAGILE-HF
We present the FRAGILE-HF trial22 that aimed to investigate the frequency of three domains of frailty in elderly patients with HF, the extent of their overlap, and the extent of their influence on death and rehospitalization. Of the 1180 hospitalized patients enrolled (median age 81 years, 57.4% male), physical frailty was found in 56.1%, social frailty in 66.4%, and cognitive decline in 37.1%. Of these three frailty domains, 13.5%, 31.4%, 36.9%, and 18.2% of all patients had 0, 1, 2, and 3 domains, respectively (Figure 2). These results indicate that 86.5% of elderly patients with HF had at least one frailty domain. During the follow-up period, the composite end-point occurred in 383 patients, and based on patients without evidence of frailty in any region, patients with one, two, and three regions of frailty were reported to be 1.38, 1.60, and 2.04 times more likely to have a composite end-point in a year, respectively (Figure 3). The assessment tools used in this trial are the cardiovascular health study (CHS) criteria, the questionnaire to define social frailty status (QSFS) of Makizako et al, and the Mini-Cog for the physical, social, and cognitive domains, respectively.
 |
Figure 2 Prevalence of impairment in each frailty domain in the total cohort and according to age category. Reprinted from Matsue Y, Kamiya K, Saito H, et al. Prevalence and prognosticimpact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILEHFcohort study. Eur J Heart Fail. 2020;22(11):2112-2119. © 2020 European Society of Cardiology.22 |
 |
Figure 3 Kaplan–Meier curves in FRAGILE-HF for (A) the combined endpoint of all-cause death and heart failure rehospitalization, and (B) all-cause death according to the number of frailty domains. Reprinted from Matsue Y, Kamiya K, Saito H, et al. Prevalence and prognosticimpact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILEHFcohort study. Eur J Heart Fail. 2020;22(11):2112-2119. © 2020 European Society of Cardiology.22 |
Comparison of Physical Frailty Assessment Tools
The Frailtools Project23 in five European countries (Spain, France, Italy, the United Kingdom, and Poland) conducted a comparative study of eight commonly used frailty assessment tools in older adults aged ≥75 years. Results showed the FRAIL scale (99.4%), SHARE-FI (98.3%), and GFST (95.0%) as the three most feasible scales. The three shortest average implementation times were clinical frailty scale (CFS) (24 s), GFST (72 s), and FRAIL scale (90 s). Agreement between tools was best for the CFS, and most of them were good.
Physical Frailty
Mechanism
The muscle hypothesis, shown in Figure 4, has been proposed as the cause of muscle weakness in patients with HF. First, decreased cardiac output reduces peripheral tissue perfusion, resulting in skeletal muscle atrophy. Furthermore, autonomic nervous system activity is impaired by increased catabolism due to increased production of inflammatory cytokines, increased oxidative stress, and immobility. All these factors induce skeletal muscle dysfunction, a veritable vicious cycle that results in further functional impairment. In other words, in addition to aging, low skeletal muscle perfusion in cardiovascular diseases (CVD) can easily lead to muscle weakness.
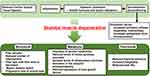 |
Figure 4 Cardiovascular disease and skeletal muscle degeneration. Notes: Adapted from Piepoli MF, Coats AJS. The “skeletal muscle hypothesis in heart failure” revised. Eur Heart J. 2013;34(7):486-488, by permission of Oxford University Press.134 |
Cardiac cachexia is a condition that results in weight loss in patients with HF24 and is characterized by anorexia, inflammation, insulin resistance, abnormal anabolism and catabolism, and anemia triggered by HF (Figure 5). In particular, intestinal edema due to HF directly causes anorexia, which leads to weight loss.25 Intestinal edema also leads to decreased digestive and absorption capacity of the gastrointestinal tract. Furthermore, patients with cardiac cachexia have reduced blood flow to the superior mesenteric artery, inferior mesenteric artery, and celiac artery compared to healthy participants and patients with HF without cardiac cachexia.26 Cardiac cachexia is closely associated with physical frailty in HF.
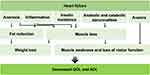 |
Figure 5 Concept of Cardiac Cachexia. Notes: Adapted from Clin Nutr, 27(6), Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. 793–799. Copyright 2008, with permission from Elsevier.24 |
Prevalence and Diagnosis
In the aforementioned FRAIL-HF,13 76% of patients with HF meet the physical frailty criteria, and to prevent frailty, the screening tool must first be understood. The screening tools for frailty widely varied (Table 1). Although no uniform standards have been established for diagnostic procedures, frailty screening is important to identify patients with HF and frailty and to provide multidomain interventions. We will present some of the most commonly and widely used tools, along with their prevalence.
 |
Table 1 List of Frailty Screening Tools |
The diagnostic methods in physical frailty can be broadly divided into the CHS criteria8 based on the Phenotype model by Fried et al and the frailty index27 based on the accumulated deficit model by Rookwood et al. Fried et al considered that five factors, shrinking, exhaustion, low activity, slowness, and weakness, become apparent in frailty (phenotypic model). The authors propose to evaluate these factors using alternative indices (unintentional weight loss, fatigue, physical activity, normal walking speed, and grip strength). This assessment method is called the CHS criteria as it was first used in the CHS. The criteria for the five alternative indices are as follows: frail, if three or more of the five items are met, and pre-frail, if one or two of the five items are met. Since this model does not include items associated with psycho-psychological and social frailty, it is used only to diagnose physical frailty and is the most widely used assessment method worldwide to date. In contrast, the frailty index based on the accumulated deficit model by Rookwood et al is a comprehensive assessment method based on the concept of comprehensive geriatric assessment. The frailty index is calculated by summing up the number of impairments in 70 items, including activities of daily living (ADLs), psychosocial risk factors, and geriatric syndromes. Considering its use in clinical situations and the application of previous studies in clinical practice, we believe that the CHS criteria should be used for physical frailty.
Self-reported questionnaires are easy to use in clinical practice regardless of facility. Yamada et al developed the frailty screening Index, a five-item self-reported questionnaire to predict disability in elderly patients.28 Nozaki et al reportedly evaluated whether the FRAIL scale questionnaire is consistent with the Fried criteria, can predict all-cause mortality, and reflects disability in patients with HF.29 Results showed that the FRAIL scale was moderately consistent with the Fried criteria, predicted all-cause mortality, and reflected clinically significant disabilities. The simplicity of the self-reported questionnaire, its reported validity and usefulness, and the fact that it does not require special instruments suggest that it will be used in the future.
For risk stratification and prognostic prediction in physical frailty of patients with HF, a multicenter prospective cohort of 2721 patients (median age: 76 [interquartile range 67–83] years, men: 60.5%) who were ambulatory at discharge was used. The FLAGSHIP study is instructive: usual walking speed of <0.98 m/s, 4 points; grip strength of <30.0 kg (men) or 17.5 kg (women), 5 points; PMADL-8 of ≥21, 2 points; SEW-7 of ≤20, 3 points and assigned a cutoff value and score for each indicator. Based on the total score, patients were classified into four categories: Category I, ≤3 points; Category II, 4–8 points; Category III, 9–13 points; and Category IV, 14 points. Results showed that the composite outcome (prevalence and cumulative incidence) of rehospitalization and death within 2 years increased with an increasing score. Remarkably, these results were used for Asian patients, excluding patients with dementia with mini mental state examination (MMSE) of ≤17 points.
The involvement of sarcopenia as a cause of physical frailty has also attracted attention, and its diagnostic criteria are suggestive. Rosenberg proposed the term sarcopenia, from the Greek sarco = muscle and penia = loss, for the decrease in skeletal muscle mass associated with aging, which had been the case for about 30 years.30 Subsequently, The European working group on sarcopenia in older people (EWGSOP)31 and the Asian working group for sarcopenia (AWGS)32 proposed diagnostic criteria for sarcopenia in 2010 and 2014, respectively. They were subsequently updated to EWGSOP233 and AWGS201934 in 2018 and 2019, respectively. The major change between the two is the diagnostic process. The first step is to screen the elderly for possible sarcopenia based on the SARC-F questionnaire (cutoff point ≥4) and clinical symptoms. It also provides clear cutoff points for measuring muscle strength and physical performance, which are commonly used in bed release. The cutoff points in the EWGSOP2 are grip strength <27 kg for men and <16 kg for women, chair stand test > 15 seconds, gait speed ≦ 0.8 m/s, short physical performance battery (SPPB) ≦ 8, timed-up and go test (TUG) ≧ 20 seconds, 400-m walk test ≧ 6 minutes. The cutoff points in AWGS2019 are grip strength for men < 28 kg women < 18 kg, chair stand test ≧ 12 seconds, gait speed ≦ 1.0 m/s, and SPPB ≦ 8. Although these are indicators of sarcopenia, they are simple indicators commonly used in rehabilitation settings and are useful in the assessment of physical frailty.
Cognitive and Psychological Frailty
Mechanism
Elderly patients with HF are thought to be more prone to cognitive dysfunction, leading to frailty.35,36 HF is often associated with CVD such as coronary artery disease and other complications including hypertension and diabetes, which can also be considered risk factors for cognitive dysfunction (Figure 6).37 Changes in the neuroendocrine axis, such as elevated cortisol and catecholamines in the blood and activation of the renin-angiotensin system associated with HF, also promote cognitive dysfunction.38 Decreased cardiac output due to HF directly results in reduced cerebral blood flow. Furthermore, age-related microvascular disorders and disrupted autoregulation of the cerebral blood flow associated with hypertension, diabetes mellitus, and other conditions accelerate the reduced cerebral blood flow. In particular, decreased blood flow to the ventral hippocampus, the brain region responsible for memory, is associated with cognitive dysfunction and depression severity in patients with HF.39
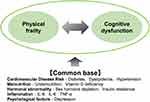 |
Figure 6 Commonalities between Physical Frailty and Cognitive dysfunction. Data from Kelaiditi et al.37 |
In addition to cognition, psychiatric symptoms of frailty include depression, apathy, and anxiety.40–43 Moreover, depression exacerbates sarcopenia via an extremely decreased activity level and elevated corticotropin-releasing factor adrenocorticotropic hormone levels.44 The presence of such psychiatric symptoms can exacerbate physical frailty.
Prevalence and Diagnosis
In the systematic review and meta-analysis of cognitive dysfunction and HF by Jane A Cannon et al, data from four prospective cohorts (n = 2513 patients) suggested a long-term cognitive decline in patients with HF compared to those without.45 Data showed that HF is associated with cognitive dysfunction in the long term. In a case-control study describing the presence or absence of HF (n = 4 articles, 1414 patients), the odds ratio for cognitive impairment in patients with HF was 1.67 (95% confidence interval [CI], 1.15–2.42). The prevalence of cognitive impairment in the HF cohort was 43% (95% CI, 30–55) (n = 26 studies, 4176 patients). Currently, cognitive frailty is defined by an international consensus group as meeting the following two criteria: (1) the presence of physical frailty and cognitive impairment and (2) the absence of Alzheimer’s disease or other dementia.37 Cognitive impairment in this context is defined as a clinical dementia rating of 0.5.
Two expert meetings were held to develop a conceptual framework for frailty, and a consensus document was produced. Therefore, psychological frailty was defined as a decline in cognition, mood, and coping.46 However, international definitions of psychological and mental frailty have not yet been established. Shimada et al conducted a longitudinal study of psychological frailty, defined as the coexistence of physical frailty (J-CHS criteria) and depressed mood (≥5 on the geriatric depression rating scale), in Japanese community-dwelling elderly people.47 Their results showed that the hazard ratio of elderly people with physical frailty alone to be certified for long-term care was 1.69, whereas that for the elderly people with psychological frailty was as high as 2.24. Another study of 179 frailty elderly Italian subjects, age 65 years and older, with a Montreal Cognitive Assessment (MoCA) score of less than 26 points, found a strong and significant correlation between MoCA score and the 5-m walking speed test (r 0.877; p < 0.001).48
Social Frailty
Mechanism
Social frailty is the most unexplored concept among the three domains. To date, no standardized definition of social frailty has been proposed; however, it has been conceptualized as the loss or risk of losing social support, activities, and resources needed to meet social needs.49 Social interactions are critical to human health. Previous studies have shown that social frailty is associated with physical functioning, cognition, depression, and prediction of mortality.50 Exercise intolerance and depression are common factors associated with HF and social frailty. HF induces exercise intolerance and depression and may lead to disconnection from society because of reduced opportunities to go out and interact with others. This can result in social frailty, with the loss of social activities needed to meet social needs. Because social activities often require the integration of higher-order physical and mental abilities than basic activities of daily living, social frailty may occur relatively early during the frail process. Recently, the prevailing view is that social frailty is caused by the interaction of independent factors in four areas summarized by Bunt et al in their scoping review: meeting basic social needs, social resources, social behaviors and activities, and general resources.51
Prevalence and Diagnosis
Saito et al reported that among the 148 patients with HF (80 ± 8 years, 51% male), 73 (49%) were socially isolated.19
As mentioned above, social frailty is the most unexplored concept among the three domains. Therefore, there is no consensus on assessing social frailty. A systematic review that examined assessment tools for social frailty reported 27 assessment tools that include at least one or more social questions.52 However, as of 2018, tools focusing solely on social frailty only include the following: the social frailty phenotype,53 the QSFS54 of Makizako et al, and the social frailty index.55
In patients with HF, the QSFS of Makizako et al is frequently used. In the sub-analysis of FRAGILE-HF,56 the QSFS was also used, showing that 825 (66.5%) of 1240 participants had social frailty, which was associated with all-cause mortality and rehospitalization at 1 year post-discharge. The QSFS is recommended for the assessment of social frailty in patients with HF due to multiple reports and its simplicity with only five questions. Conversely, studies using the Tilburg frailty indicator,57 a comprehensive tool including physical, psychological, and social aspects, have reported that social frailty in elderly patients with HF negatively affects self-care, and therefore, different uses should be considered for different purposes.
Intervention Strategies for Patients with HF and Frailty
Various interventions for frailty have been proposed (Table 2). As mentioned above, frailty is composed of three domains, and a wide range of interventions are required. Although intervention strategies associated with physical frailty have dominated the reports, nutritional, cognitive, psycho/psychological/cognitive, and pharmacologic interventions are also important. Here we review the intervention strategies, particularly in patients with HF.
 |
Table 2 Interventions in Elderly Frail Patients with Heart Failure |
Physical Intervention
The goals of exercise therapy are to improve subjective symptoms and exercise tolerance, improve life expectancy, improve the quality of life, and reduce cardiac events. These goals have been achieved with exercise therapy. However, the majority of studies on exercise therapy in HF were obtained from studies on chronic HF and HFrEF. The HF-ACTION trial,58 a large clinical trial funded by the National Institutes of Health and enrolling 2331 ambulatory patients with HF (LVEF 35%, NYHA class II–IV) from 82 centers, randomized patients to be treated with a combination of standard medical therapy and aerobic exercise and found that combination of both therapies was effective at 30 months. The quality of life was compared between the two groups. Results showed a greater improvement in the quality of life in the exercise group. Furthermore, adjustment for background factors showed a lower risk of all-cause mortality and hospitalization and cardiovascular mortality and rehospitalization. ExTraMACH study59 has shown that exercise therapy improves survival and hospitalization rates. On the other hand, the ExTraMACH II study,60 which increased the number of randomized controlled trials (RCTs) analyzed to 18, including 3912 patients, to account for cluster (or trial-level) data characteristics, showed no significant effects on the risk of death or hospitalization. The study using individual participant data in the HF-ACTION trial reported that physical activity was a predictor of death and rehospitalization,61 whereas the ExTraMACH II study did not provide physical activity data; therefore, future analysis is warranted.
Unlike the studies described herein, we present the REHAB-HF trial,62 comprising 53% of patients having HFpEF. In the REHAB-HF trial, the intervention was started early after hospitalization for HF, and rehabilitation in the outpatient and home settings was also introduced at the early stage. Interventions are divided into four domains, such as muscle strength and balance, and the rehabilitation content is determined in detail based on the patient’s level. The main outcome, SPPB, improved by 1.5 (0.9–2.0) points in the intervention group over the control group. However, since this RCT included 349 out of 27,300 patients who met the criteria, whether results can be truly applied in clinical practice remains to be elucidated.
Exercise therapy primarily consists of aerobic exercise and resistance training. In recent years, whether aerobic exercise or resistance training should be recommended for elderly patients with HF and frailty remains controversial. First, aerobic exercise is recommended based on the following reasons: 6 months of aerobic exercise (3 months of supervised exercise and 3 months monitored by telephone after) for patients with HF aged ≥70 years has been associated with a 6-min walking distance (6MWD), ADL score, risk of rehospitalization, and quality of life.63 Aerobic exercise in CVD also improves the vascular endothelial function, promotes vascular remodeling and angiogenesis, improves autonomic balance, and is myocardioprotective.64 Compared with resistance training, aerobic exercise has a greater maximal oxygen uptake (VO2 max) improvement65–67 (Table 3). Collectively, HFrEF and HFpEF have been reported to have different improvement mechanisms in exercise tolerance; HFrEF has been attributed to improvements in cardiac pump function, vascular function, and muscle fiber and mitochondrial function, whereas HFpEF is attributed to increased oxygen extraction capacity (arteriovenous) in the peripheral skeletal muscle.68 However, the duration of aerobic exercise intervention in all studies has been notably long, ie several months. Considering the improvement mechanism, the manifestation of effects takes time, and some believe that it is not suitable for elderly patients with HF who are seeking happiness in the present study. Above all, most elderly people are not athletes in the real world and most elderly patients with HF have frailty, which often precludes effective aerobic exercise at 60% of the maximal load. Moreover, the OptimEx-Clin study69 conducted at five centers in Germany and Norway also reported no significant differences in high-intensity interval training or medium-intensity continuous training compared to guideline-based physical activity for patients with HFpEF, which brings into focus the importance of resistance training. A systematic review and meta-analysis of resistance training in patients with HF demonstrated that it produces statistically significant improvements in peak VO2 and 6MWD measurements without harming the cardiac structure and function.70 Early resistance training can be safely performed even after STEMI, and quality of life improvements, exercise tolerance, and endothelial function have been observed.5,71,72 Based on these points, resistance training can be easily introduced to elderly patients with HF and frailty at various risks. Furthermore, resistance training has been reported to improve not only muscle strength but also muscle quality, such as inter- and intramuscular coordination, which may be effective against sarcopenia.73 To date, we have discussed patients who underwent either aerobic exercise or resistance training. Resistance training in addition to exercise is known to improve cardiopulmonary function and quality of life,74 and we believe that elderly patients with HF with a wide range of exercise tolerance and comorbidities need tailor-made interventions based on the discussed knowledge.
 |
Table 3 Effects of Aerobic Exercise and Resistance Training |
Nutritional Intervention
Nutritional disorders are characteristic findings of sarcopenia and cachexia in physical frailty. In HF, hyponutrition develops due to anorexia, increased physical inactivity and resting energy demands, intestinal edema due to congestion, and circulatory failure. As mentioned, weight loss in patients with HF is known as cardiac cachexia. The prognosis for cardiac cachexia is extremely poor, with a reported 3-year survival rate of 50%.75 Therefore, nutritional intervention and assessment are important. Nutrition assessment tools include the prognostic nutritional index), subjective global assessment, controlling nutritional status, nutritional risk index), geriatric nutritional risk index (GNRI), mini nutritional assessment), mini nutritional assessment-short form), and the above tools. The global leadership initiative on malnutrition (GLIM) is a tool used to assess malnutrition. In a systematic review that evaluated the prediction of all-cause mortality in HF using nutrition tools other than GLIM, HRs for all-cause mortality were MNA (HR = 2.62, 95% CI 1.11–6.20, P = 0.03] and MNA-SF (HR = 1.94, 95% CI 1.40–2.70, P < 0.001) were reported to be the best.76 Among them, the GLIM criteria, released in 2018, are the world’s first international criteria for diagnosing undernutrition, with four societies from Europe, the United States, Asia, and South America participating in their development, and are an evaluation index for evaluation. Compared to the widely used GNRI, the GLIM criteria are partly recommended because they do not include serum albumin, which is greatly affected by inflammation and volume overload in HF. The following information is a summary of the study results.
Nutritional therapy for patients with HF is not yet well established, and even the 2020 meta-analysis reported a few valid articles. Thus, further RCTs are needed to accumulate evidence.77 A few representative trials are presented below. The PICNIC study78 showed that a 6-month personalized nutritional support provided by specialists reduced the HF-related 1-year mortality and rehospitalization rates compared to a control group. The study patients had a mean age of 79.2 years, BMI of 25.2 kg/m2, and included HFpEF. Notably, a sub-analysis of patients with and without renal dysfunction (GFR, <60 mL/min/1.73 m2) showed that the study was effective even in patients with renal dysfunction who were considered protein limited. The second is the EFFORT trail79 for patients with HF at risk for low nutrition. The median age was 78.8 years and a Nutritional Risk Screening 2002 (NRS-2002) score of ≥3 was the inclusion criterion. Patient-specific nutritional support included interventions, such as energy intake, protein (1.2–1.5 g/kg/day), multivitamins, and disease adjustments. Results showed a low risk of death and cardiovascular events within 30 days. Evidence on energy requirements of elderly patients with HF is lacking, and further accumulation of evidence is essential.
Several reports have been made in recent years on the quality of protein. Compared to vegetable proteins, animal proteins are more efficiently utilized, contain important nutrients, and are expectedly effective in preventing and improving muscle strength and muscle mass loss.80 On the other hand, animal protein foods contain high saturated fatty acids and cholesterol levels.81 Increased animal protein intake has also been reportedly associated with total mortality, death from CVD, cancer death,82 and diabetes occurrence.83,84 Conversely, vegetable proteins contain phytochemicals and have been shown to have antioxidant benefits.85 Protein selection should be tailored to the patient’s cardiovascular and renal function and diabetes mellitus. In a review article on HF and diabetes mellitus (DM), he states that coronary microvascular inflammation with endothelial dysfunction is considered the common denominator of HFpEF, DM, and obesity and can explain the synergistic relationship between the pathogenesis of HFpEF, DM and obesity. He also states that lifestyle modification is very effective in improving the quality of life of patients with asymptomatic diastolic dysfunction and HFpEF. Therefore, we believe that patients with HFpEF presenting with DM and renal insufficiency need not only nutritionally therapy but also additional exercise therapy.86
Finally, we present a study reporting that L-arginine, a semi-essential amino acid involved in many biological processes, enhances the effects of cardiac rehabilitation.87 l-arginine is the substrate used by nitric oxide (NO) synthase to produce NO and is known to have beneficial effects on the endothelium that promote vasodilation, it is known to inhibit inflammation and improve physical function. In this previous study, patients were assigned, with a 2:1 ratio, to add to their standard therapy one bottle containing 1.66 g of L-arginine or one bottle of identical aspect apart from not containing L-arginine, twice a day orally for 3 weeks. Patients completed the 6-Minute Walk Test (6MWT) and were evaluated with the Borg Modified 0–10 Cognitive Exertion Evaluation (BRPE) before and at the end of treatment. 75 patients who received L-arginine and 35 patients who received placebo successfully completed the study. The 6MWT distance was significantly increased in the L-arginine group compared to both baseline and placebo (P < 0.0001). In addition, there was a significant improvement in BRPE in the L-arginine group, but not in the placebo group. These results indicate that L-arginine enhances response to cardiac rehabilitation regardless of age, gender, baseline functional capacity, or comorbidities. Thus, reports of improved therapeutic outcomes with various nutrients, not limited to energy intake and protein inoculation, have been increasing in recent years, and their clinical application is expected.
Psycho-Psychological and Cognitive Interventions
Genetic factors are known to increase the risk of dementia. However, a study examining the extent of offsetting by lifestyle factors reported that a healthy lifestyle with regular exercise, combined with non-smoking, a healthy diet, and moderate alcohol intake, was associated with a lower risk of dementia, regardless of the genetic risk.88 As shown in this report, the effects of combined interventions on the cognitive decline are often tested. Some of the most representative trials are described below. First, the effects of a multidomain intervention (diet, exercise, cognition, and vascular risk management) and regular health advice were tested using a neuropsychological test battery as an outcome in 1260 Finnish patients aged 60–77 years at risk for dementia. The FINGER trial89 showed that it was effective in maintaining or improving cognitive function. In contrast, the pre-DAVA trial,90, a cluster RCT of 3526 Dutch subjects aged 70–78 years that tested the effects of nurse lifestyle guidance (smoking, diet, physical activity, weight, and blood pressure) every 4 months, showed no effects on cognitive function. Moreover, no cognitive function effect was found in the MAPT trial91 that tested the effects of Supplement ω3 and group sessions (cognitive training, physical activity, and nutrition) in 1680 patients without dementia aged ≥70 years in France and Monaco. The FINGER trial, the only trial with positive effects, involved a very high frequency of interventions by physiotherapists, nurses, nutritionists, and psychologists during the 2-year intervention period, and the content was very rich, making it difficult to generalize to the real world. We would like to note this point.
Focusing on multidomain interventions, we now consider the extent of a single exercise intervention in benefiting the cognitive function. First, the DAPA trial92 that examined the effects of a moderate- to high-intensity aerobic and strength exercise training program in 494 patients with dementia with a mean age of 77 years, concluded that only improvements in physical fitness were observed, without cognitive function effects. On the other hand, a meta-analysis of 36 studies comprising patients aged ≥50 years, excluding studies comprising patients with neurological and psychiatric disorders, found that aerobic exercise, strength training, multi-component training, and tai chi all improved cognitive function.93 These two studies differ in terms of whether participants are patients with dementia or elderly in general. Patients who already have dementia may have had difficulty learning and generalizing exercise in the first place and therefore may not have been able to successfully approach the cognitive function. For patients who already have cognitive decline, interventions such as the FINGER trial that forcefully stimulate the activity of external factors may be a good idea. Since high activity is known to have a positive effect on brain capacity and Aβ, not only the amount of exercise during the intervention but also to increase it in daily life should be managed.94,95 For the cognitive function of elderly patients with mild cognitive impairment or dementia, the amount of exercise and cognition, such as in the dual-task, should be increased. Some meta-analyses have found combined exercise and cognitive training interventions, such as the dual-task, to be effective for cognitive function in elderly patients with mild cognitive impairment or dementia; therefore, incorporating these interventions is an option for patients with cognitive decline.96
In summary, the effectiveness of exercise therapy alone versus multiple interventions including nutritional guidance varies depending on the patient and the method used. The trend was that in elderly patients without cognitive decline, even exercise therapy alone was likely effective because the content of the instruction could be generalized in daily life. Conversely, in patients with declined cognitive, the content of instruction cannot be generalized in daily life; thus, they should be forced to exercise through external factors. Since lifestyle guidance, including diet and nutrition, is not easily habit-forming, regular exercise therapy externally provided is considered most reliable for elderly patients with dementia. Combining intervention of exercise and cognitive training, eg, a dual task, could be provided within the exercise therapy.
Social Intervention
Social frailty worsens physical and cognitive functioning and depression and predicts mortality.50,97 The prevalence and prognostic impact of weak social networks in patients with HF has been addressed in previous studies; however, results have been inconsistent.98 Although this is an area that seems to summarize all frailty areas, it is the least studied area, and only a few RCTs reported effective intervention methods. Previous studies have shown that those without physical frailty but with social frailty have a higher risk of subsequently developing physical frailty.99 Furthermore, the mortality rate has been reportedly lower if the patient has physical frailty but not social frailty than those with both; thus, intervention for social frailty is important.100 Here, we present an approach that may be effective.
Eighty pre-frail and frail adults aged ≥65 years (mean age 83) in a community study in Australia showed that non-professional, home-based physical training, nutrition, and social support interventions were effective for malnutrition and frailty.101 In this study, non-professional visits by volunteers were made twice a week for 12 weeks and focused on social aspects that activated the cognitive function, such as encouraging excursions and conversation exchanges. Remarkably, social support alone effectively improves malnutrition and frailty in this study. Intervention studies focusing on supervised group exercise have also reported that group activities may reduce or resolve social frailty by promoting social interaction and physical factors.102 Participation in gatherings that promote social interaction, such as group-based social support programs (eg, salon activities), has been found to strengthen social connections and reduce the incidence of disability.103,104
Therefore, the promotion of a population approach that does not cause social disconnection is believed to be the preferred coping method. If social disconnection has already occurred, social connections can be re-established using the latest technology, specifically video calls and social networking services, among others. Other exercises using virtual reality (VR) have also been investigated and have been reported to significantly enhance physical and cognitive functions.105 Other studies have examined tablet-based applications and exercises that combine VR and stationary bikes for frail elderly people.106,107 The TELEREH-HF trial, which examined the effects of hybrid comprehensive telerehabilitation, consisting of remote monitoring of training at home in patients with HF, significantly improved the peak oxygen consumption and quality of life.108 As these technologies become more widespread, social disconnection can be solved by allowing patients to dine with friends or go for a walk in the comfort of their own homes. Some studies have reported that satisfaction with significant activities, including a wide range of activities from daily life to work, social life, and leisure time activities important, enjoyable, and rewarding for the individual patient, is associated with social frailty. During the acute phase and hospitalization, patients tend to focus only on physical and cognitive functions; however, early acquisition of meaningful activities beyond these functions may help correct social frailty.
Pharmacological Intervention
Effective drugs for HF have been developed over the years. In the 2022 AHA/ACC/HFSA guideline for the management of HF: executive summary, each drug is listed with a high recommendation class and evidence level.109 The ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure, revised in 2021 for the first time in five years, revised the treatment algorithm for HFrEF. The new algorithm includes angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor-neprilysin inhibitors (ARNIs), beta-blockers, mineralocorticoid receptor antagonists (MRAs), sodium-glucose co-transporter 2 inhibitors (SGLT-2Is), fluid retention improving loop Diuretics were positioned as drugs that should be administered to all patients.3 Here, we will focus on the impact of drugs on frailty including the fantastic four (ARNIs, beta-blockers, MRAs, and SGLT2Is), which have been increasingly investigated in recent years.110
A study investigating the effects of ARNIs on physical frailty as assessed by a modified version of Fried’s frailty phenotype in patients with advanced HFrEF on the heart transplant waiting list found not only physical frailty but also improved 6MWT and VO2 max and NT-pro-BNP111 and improved depressive symptoms in the same patients.112 In those with HFrEF, ACE-Is and angiotensin receptor blocker to ARNIs, along with 6MWT, significantly improved depression, anxiety symptoms, and functional status.113 In frail elderly patients with diabetes mellitus and HFpEF, the study of SGLT2Is showed significant improvements in the 5-m walk test and cognitive function.114
We have discussed the efficacy of pharmacotherapy in patients with HFrEF, and the ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure also state that “there are no large RCTs that have met their primary endpoints, and no treatment currently has demonstrated reliable efficacy for patients with HFpEF.” However, the EMPEROR-Preserved trial showing the efficacy of empagliflozin in HFpEF was published in ESC 2021, although not in time for the guideline revision process.115 Empagliflozin reduced the combined risk of cardiovascular death, hospitalization for heart failure, and emergency/urgent heart failure requiring intravenous treatment (432 versus 546 patients [empagliflozin versus placebo, respectively]; hazard ratio, 0.77 [95% CI, 0.67–0.87]; P<0.0001). In addition, Empagliflozin reduced the total number of heart failure hospitalizations that required intensive care (hazard ratio, 0.71 [95% CI, 0.52–0.96]; P=0.028) and the total number of all hospitalizations that required a vasopressor or positive inotropic drug (hazard ratio, 0.73 [95% CI, 0.55–0.97]; P=0.033).
As mentioned above, although pharmacologic interventions are effective in preventing frailty, multiple drug use in HF is a risk factor for frailty. The frailty and comorbid elderly population are at utmost risk due to the prevalence of polypharmacy is increasing rapidly and their resistance to all diseases and infections is impaired.
It is also essential to identify frail older individuals with polypharmacy; a study reported a significant correlation between the frailty index score and both potentially inappropriate prescribing and adverse drug reactions in hospitalized elderly patients.116 Polypharmacy is defined as “the use of six or more multiple drugs”, and polypharmacy is known to be common in patients with HF, with a significantly higher risk of adverse events.117–123 One of the reasons why polypharmacy is common in patients with HF is that appropriate polypharmacy occurs due to medical necessity. In 2022, six drugs are recommended by the AHA/ACC/HFSA guideline for the management of HF: executive summary, and the addition of drugs for comorbid conditions could result in multiple drug combinations.107 Another reason is the high incidence of inappropriate polypharmacy due to unnecessary prescriptions for elderly patients. The frailty and comorbid elderly population is at utmost risk due to the prevalence of polypharmacy is increasing rapidly and their resistance to all diseases and infections is impaired.124 To evaluate the medication appropriateness in 231 elderly patients with HF attending the University of Michigan in the United States, 50–70% of prescriptions were inappropriate.125 In a study on the number of drug prescriptions and taste sensitivity in patients with HF, the polypharmacy group with ≥8 prescriptions had a significantly higher rate of all four types of taste disorder complications and a significantly lower energy intake rate.126 These results suggest that polypharmacy can lead to poor nutrition, frailty, and cachexia. Thus, not only physicians and pharmacists but also nurses and rehabilitation-related professionals should evaluate the daily dietary intake, weight changes, and physical function changes and make efforts to detect polypharmacy.
Conclusion
To introduce evidence and well-known studies, the explanation was divided by domain. However, because frailty spans multiple domains, interventions should not be divided into exercise, nutrition, and drugs, but rather, interventions should further be evaluated to multiple domains such as psycho-psychological, cognitive, and social to compensate for deficiencies. This is because we believe that such tailor-made interventions are more necessary than single interventions for the various symptoms associated with heart failure and frailty. Several studies have shown a relationship between frailty and HF prognosis; however, interventions that improve prognosis remain unclear. Future information regarding interventions aimed at improving the prognosis for multidomain frailty is needed.
Abbreviations
ADLs, activities of daily living; ARNI, angiotensin receptor-neprilysin inhibitors; CFS, Clinical Frailty Scale; CHS, Cardiovascular Health Study; CVD, cardiovascular diseases; DAPA, Dementia and Physical Activity; EFFORT, Effect of Early Nutritional Therapy on Frailty, Functional Outcomes and Recovery of Undernourished Medical Inpatients Trial; FINGER, The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability; EMPEROR-Preserved, Empagliflozin Outcome Trial in Patients with Chronic Heart Failure with Preserved Ejection Fraction; GLIM, Global Leadership Initiative on Malnutrition; GNRI, Geriatric nutritional risk index; HF, heart failure; HFpEF, Heart failure with preserved ejection fraction; HFrEF, Heart Failure with Reduced Ejection Fraction; MAPT, Multidomain Alzheimer Preventive Trial; MMSE, Mini Mental State Examination; QSFS, Questionnaire to define Social Frailty Status; RCT, randomized controlled trials; VR, virtual reality.
Disclosure
The authors report no conflicts of interest in this work.
References
1. The top 10 causes of death [homepage on the Internet]. WHO | World Health Organization; 2019. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
2. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: a systematic review and meta-analysis. Eur J Heart Fail. 2019;21(11):1306–1325.3. doi:10.1002/ejhf.1594
3. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599–3726. doi:10.1093/eurheartj/ehab368
4. Shimokawa H, Miura M, Nochioka K, Sakata Y. Heart failure as a general pandemic in Asia. Eur J Heart Fail. 2015;17(9):884–892. doi:10.1002/ejhf.319
5. Maddox TM, Januzzi JL, Januzzi JL, et al.; Writing Committee. 2021 Update to the 2017 ACC expert consensus decision pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810. doi:10.1016/j.jacc.2020.11.022.
6. Dent E, Martin FC, Bergman H, Woo J, Romero-Ortuno R, Walston JD. Management of frailty: opportunities, challenges, and future directions. Lancet. 2019;394(10206):1376–1386. doi:10.1016/S0140-6736(19)31785-4
7. Junius-Walker U, Onder G, Soleymani D, et al. The essence of frailty: a systematic review and qualitative synthesis on frailty concepts and definitions. Eur J Intern Med. 2018;56:3–10.
8. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156.
9. Cheung JTK, Yu R, Wu Z, Wong SYS, Woo J. Geriatric syndromes, multimorbidity, and disability overlap and increase healthcare use among older Chinese. BMC Geriatr. 2018;18(1):147.
10. Ilinca S, Calciolari S. The patterns of health care utilization by elderly Europeans: frailty and its implications for health systems. Health Serv Res. 2015;50(1):305–320. doi:10.1111/1475-6773.12211
11. Yang X, Lupón J, Vidán MT, et al. Impact of frailty on mortality and hospitalization in chronic heart failure: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7(23):e008251. doi:10.1161/JAHA.117.008251
12. Pandey A, Kitzman D, Reeves G. Frailty is intertwined with heart failure: mechanisms, prevalence, prognosis, assessment, and management. JACC Heart Fail. 2019;7(12):1001–1011. doi:10.1016/j.jchf.2019.10.005
13. Vidán MT, Blaya-Novakova V, Sánchez E, Ortiz J, Serra-Rexach JA, Bueno H. Prevalence and prognostic impact of frailty and its components in non-dependent elderly patients with heart failure. Eur J Heart Fail. 2016;18(7):869–875. doi:10.1002/ejhf.518
14. Tian J, Yan J, Zhang Q, et al. Analysis of re-hospitalizations for patients with heart failure caused by coronary heart disease: data of first event and recurrent event. Ther Clin Risk Manag. 2019;15:1333–1341. doi:10.2147/TCRM.S218694
15. Denfeld QE, Winters-Stone K, Mudd JO, Hiatt SO, Lee CS. Identifying a relationship between physical frailty and heart failure symptoms. J Cardiovasc Nurs. 2018;33(1):E1–E7. doi:10.1097/JCN.0000000000000408
16. Singh M, Stewart R, White H. Importance of frailty in patients with cardiovascular disease. Eur Heart J. 2014;35(26):1726–1731. doi:10.1093/eurheartj/ehu197
17. Patel A, Parikh R, Howell EH, Hsich E, Landers SH, Gorodeski EZ. Mini-cog performance: novel marker of post discharge risk among patients hospitalized for heart failure. Circ Heart Fail. 2015;8(1):8–16. doi:10.1161/CIRCHEARTFAILURE.114.001438
18. Friedmann E, Thomas SA, Liu F, et al. Relationship of depression, anxiety, and social isolation to chronic heart failure outpatient mortality. Am Heart J. 2006;152(5):
19. Saito H, Kagiyama N, Nagano N, et al. Social isolation is associated with 90-day rehospitalization due to heart failure. Eur J Cardiovasc Nurs. 2019;18(1):16–20.
20. Vitale C, Jankowska E, Hill L, et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur J Heart Fail. 2019;21(11):1299–1305. doi:10.1002/ejhf.1611
21. Rich MW, Chyun DA, Skolnick AH, et al. Knowledge gaps in cardiovascular care of the older adult population: a scientific statement from the American Heart Association, American College of Cardiology, and American Geriatrics Society. J Am Coll Cardiol. 2016;67(20):2419–2440. doi:10.1016/j.jacc.2016.03.004
22. Matsue Y, Kamiya K, Saito H, et al. Prevalence and prognostic impact of the coexistence of multiple frailty domains in elderly patients with heart failure: the FRAGILE-HF cohort study. Eur J Heart Fail. 2020;22(11):2112–2119. doi:10.1002/ejhf.1926
23. Oviedo-Briones M, Ár L, Carnicero JA, et al. A comparison of frailty assessment instruments in different clinical and social care settings: the Frailtools project. J Am Med Dir Assoc. 2021;22(3):
24. Evans WJ, Morley JE, Argilés J, et al. Cachexia: a new definition. Clin Nutr. 2008;27(6):793–799. doi:10.1016/j.clnu.2008.06.013
25. Valentova M, von Haehling S, Bauditz J, et al. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37(21):1684–1691. doi:10.1093/eurheartj/ehw008
26. Sandek A, Swidsinski A, Schroedl W, et al. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64(11):1092–1102. doi:10.1016/j.jacc.2014.06.1179
27. Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hébert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353(9148):205–206. doi:10.1016/S0140-6736(98)04402-X
28. Yamada M, Arai H. Predictive value of frailty scores for healthy life expectancy in community-dwelling older Japanese adults. J Am Med Dir Assoc. 2015;16(11):
29. Nozaki K, Kamiya K, Hamazaki N, et al. Validity and utility of the questionnaire-based FRAIL scale in older patients with heart failure: findings from the FRAGILE-HF. J Am Med Dir Assoc. 2021;22(8):1621–1626.e2. doi:10.1016/j.jamda.2021.02.025
30. Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(5 Suppl):990S–991S. doi:10.1093/jn/127.5.990S
31. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412–423. doi:10.1093/ageing/afq034
32. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95–101. doi:10.1016/j.jamda.2013.11.025
33. Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31. doi:10.1093/ageing/afy169
34. Chen LK, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi:10.1016/j.jamda.2019.12.012
35. Butrous H, Hummel SL. Heart failure in older adults. Can J Cardiol. 2016;32(9):1140–1147. doi:10.1016/j.cjca.2016.05.005
36. Heckman GA, McKelvie RS, Rockwood K. Individualizing the care of older heart failure patients. Curr Opin Cardiol. 2018;33(2):208–216. doi:10.1097/HCO.0000000000000489
37. Kelaiditi E, Cesari M, Canevelli M, et al. Cognitive frailty: rational and definition from an (I.A.N.A./I.A.G.G.) international consensus group. J Nutr Health Aging. 2013;17(9):726–734. doi:10.1007/s12603-013-0367-2
38. Ampadu J, Morley JE. Heart failure and cognitive dysfunction. Int J Cardiol. 2015;178:12–23. doi:10.1016/j.ijcard.2014.10.087
39. Suzuki H, Matsumoto Y, Ota H, et al. Hippocampal blood flow abnormality associated with depressive symptoms and cognitive impairment in patients with chronic heart failure. Circ J. 2016;80(8):1773–1780.
40. Parrotta I, Maltais M, Rolland Y, et al. The association between apathy and frailty in older adults: a new investigation using data from the Mapt study. Aging Ment Health. 2020;24(12):1985–1989. doi:10.1080/13607863.2019.1650890
41. Ayers E, Shapiro M, Holtzer R, Barzilai N, Milman S, Verghese J. Symptoms of apathy independently predict incident frailty and disability in community-dwelling older adults. J Clin Psychiatry. 2017;78(5):e529–e536. doi:10.4088/JCP.15m10113
42. Soysal P, Veronese N, Thompson T, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. 2017;36:78–87. doi:10.1016/j.arr.2017.03.005
43. Zhao W, Zhang Y, Liu X, et al. Comorbid depressive and anxiety symptoms and frailty among older adults: findings from the West China health and aging trend study. J Affect Disord. 2020;277:970–976. doi:10.1016/j.jad.2020.08.070
44. Amitani M, Asakawa A, Amitani H, Inui A. Control of food intake and muscle wasting in cachexia. Int J Biochem Cell Biol. 2013;45(10):2179–2185. doi:10.1016/j.biocel.2013.07.016
45. Cannon JA, Moffitt P, Perez-Moreno AC, et al. Cognitive impairment and heart failure: systematic review and meta-analysis. J Card Fail. 2017;23(6):464–475. doi:10.1016/j.cardfail.2017.04.007
46. Gobbens RJJ, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. Towards an integral conceptual model of frailty. J Nutr Health Aging. 2010;14(3):175–181. doi:10.1007/s12603-010-0045-6
47. Shimada H, Lee S, Doi T, Bae S, Tsutsumimoto K, Arai H. Prevalence of psychological frailty in Japan: NCGG-SGS as a Japanese National Cohort Study. J Clin Med. 2019;8(10):E1554. doi:10.3390/jcm8101554
48. Mone P, Gambardella J, Lombardi A, et al. Correlation of physical and cognitive impairment in diabetic and hypertensive frail older adults. Cardiovasc Diabetol. 2022;21(1):10. doi:10.1186/s12933-021-01442-z
49. Keshvani N, Pandey A. Beyond physical impairment: the role of social frailty in heart failure. J Am Heart Assoc. 2021;10(17):e022187. doi:10.1161/JAHA.121.022187
50. Ma L, Sun F, Tang Z. Social frailty is associated with physical functioning, cognition, and depression, and predicts mortality. J Nutr Health Aging. 2018;22(8):989–995. doi:10.1007/s12603-018-1054-0
51. Bunt S, Steverink N, Olthof J, van der Schans CP, Hobbelen JSM. Social frailty in older adults: a scoping review. Eur J Ageing. 2017;14(3):323–334. doi:10.1007/s10433-017-0414-7
52. Bessa B, Ribeiro O, Coelho T. Assessing the social dimension of frailty in old age: a systematic review. Arch Gerontol Geriatr. 2018;78:101–113. doi:10.1016/j.archger.2018.06.005
53. Garre-Olmo J, Calvó-Perxas L, López-Pousa S, de Gracia Blanco M, Vilalta-Franch J. Prevalence of frailty phenotypes and risk of mortality in a community-dwelling elderly cohort. Age Ageing. 2013;42(1):46–51. doi:10.1093/ageing/afs047
54. Makizako H, Shimada H, Tsutsumimoto K, et al. Social frailty in community-dwelling older adults as a risk factor for disability. J Am Med Dir Assoc. 2015;16(11):
55. Teo N, Gao Q, Nyunt MSZ, Wee SL, Ng TP. Social frailty and functional disability: findings from the Singapore longitudinal ageing studies. J Am Med Dir Assoc. 2017;18(7):
56. Jujo K, Kagiyama N, Saito K, et al. Impact of social frailty in hospitalized elderly patients with heart failure: a FRAGILE-HF registry subanalysis. J Am Heart Assoc. 2021;10(17):e019954. doi:10.1161/JAHA.120.019954
57. Gobbens RJJ, Van assen MALM, Luijkx KG, Wijnen-Sponselee MT, Schols JMGA. The Tilburg Frailty Indicator: psychometric properties. J Am Med Dir Assoc. 2010;11(5):344–355. doi:10.1016/j.jamda.2009.11.003
58. O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi:10.1001/jama.2009.454
59. Piepoli MF, Davos C, Francis DP, Coats AJS; ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ. 2004;328(7433):189. doi:10.1136/bmj.328.7441.711-b
60. Taylor RS, Walker S, Smart NA, et al. Impact of exercise-based cardiac rehabilitation in patients with heart failure (ExTraMATCH II) on mortality and hospitalisation: an individual patient data meta-analysis of randomised trials. Eur J Heart Fail. 2018;20(12):1735–1743. doi:10.1002/ejhf.1311
61. Keteyian SJ, Leifer ES, Houston-Miller N, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. J Am Coll Cardiol. 2012;60(19):1899–1905. doi:10.1016/j.jacc.2012.08.958
62. Kitzman DW, Whellan DJ, Duncan P, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl J Med. 2021;385(3):203–216. doi:10.1056/NEJMoa2026141
63. Antonicelli R, Spazzafumo L, Scalvini S, et al. Exercise: a “new drug” for elderly patients with chronic heart failure. Aging. 2016;8(5):860–872. doi:10.18632/aging.100901
64. Fiuza-Luces C, Santos-Lozano A, Joyner M, et al. Exercise benefits in cardiovascular disease: beyond attenuation of traditional risk factors. Nat Rev Cardiol. 2018;15(12):731–743. doi:10.1038/s41569-018-0065-1
65. Williams MA, Haskell WL, Ades PA, et al. Resistance exercise in individuals with and without cardiovascular disease: 2007 update: a scientific statement from the American Heart Association Council on clinical cardiology and council on nutrition, physical activity, and metabolism. Circulation. 2007;116(5):572–584. doi:10.1161/CIRCULATIONAHA.107.185214
66. Smart N, Marwick TH. Exercise training for patients with heart failure: a systematic review of factors that improve mortality and morbidity. Am J Med. 2004;116(10):693–706. doi:10.1016/j.amjmed.2003.11.033
67. Jakovljevic DG, Donovan G, Nunan D, et al. The effect of aerobic versus resistance exercise training on peak cardiac power output and physical functional capacity in patients with chronic heart failure. Int J Cardiol. 2010;145(3):526–528. doi:10.1016/j.ijcard.2010.04.060
68. Tucker WJ, Lijauco CC, Hearon CM, et al. Mechanisms of the improvement in peak VO2 with exercise training in heart failure with reduced or preserved ejection fraction. Heart Lung Circ. 2018;27(1):9–21. doi:10.1016/j.hlc.2017.07.002
69. Mueller S, Winzer EB, Duvinage A, et al. Effect of high-intensity interval training, moderate continuous training, or guideline-based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2021;325(6):542–551. doi:10.1001/jama.2020.26812
70. Fisher S, Smart NA, Pearson MJ. Resistance training in heart failure patients: a systematic review and meta-analysis. Heart Fail Rev. 2021;3:1–8.
71. Arthur HM, Gunn E, Thorpe KE, et al. Effect of aerobic vs combined aerobic-strength training on 1-year, post-cardiac rehabilitation outcomes in women after a cardiac event. J Rehabil Med. 2007;39(9):730–735.
72. Vona M, Codeluppi GM, Iannino T, Ferrari E, Bogousslavsky J, von Segesser LK. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation. 2009;119(12):1601–1608.
73. Piepoli MF, Conraads V, Corrà U, et al. Exercise training in heart failure: from theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur J Heart Fail. 2011;13(4):347–357.
74. Hollings M, Mavros Y, Freeston J, Fiatarone Singh M. The effect of progressive resistance training on aerobic fitness and strength in adults with coronary heart disease: a systematic review and meta-analysis of randomised controlled trials. Eur J Prev Cardiol. 2017;24(12):1242–1259.
75. Anker SD, Ponikowski P, Varney S, et al. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349(9058):1050–1053.
76. Hu Y, Yang H, Zhou Y, et al. Prediction of all-cause mortality with malnutrition assessed by nutritional screening and assessment tools in patients with heart failure: asystematic review. Nutr Metab Cardiovasc Dis. 2022;25(7):1799–1806.
77. Habaybeh D, de Moraes MB, Slee A, Avgerinou C. Nutritional interventions for heart failure patients who are malnourished or at risk of malnutrition or cachexia: a systematic review and meta-analysis. Heart Fail Rev. 2021;26(5):1103–1118.
78. Bonilla-Palomas JL, Gámez-López AL, Castillo-Domínguez JC, et al. Nutritional intervention in malnourished hospitalized patients with heart failure. Arch Med Res. 2016;47(7):535–540.
79. Hersberger L, Dietz A, Bürgler H, et al. Individualized nutritional support for hospitalized patients with chronic heart failure. J Am Coll Cardiol. 2021;77(18):2307–2319.
80. Bradlee ML, Mustafa J, Singer MR, Moore LL. High-protein foods and physical activity protect against age-related muscle loss and functional decline. J Gerontol a Biol Sci Med Sci. 2017;73(1):88–94.
81. Halbesma N, Bakker SJL, Jansen DF, et al. High protein intake associates with cardiovascular events but not with loss of renal function. J Am Soc Nephrol. 2009;20(8):1797–1804.
82. Fung TT, van Dam RM, Hankinson SE, Stampfer M, Willett WC, Hu FB. Low-carbohydrate diets and all-cause and cause-specific mortality: two cohort studies. Ann Intern Med. 2010;153(5):289–298.
83. Kurotani K, Nanri A, Goto A, et al. Red meat consumption is associated with the risk of type 2 diabetes in men but not in women: a Japan public health center-based prospective study. Br J Nutr. 2013;110(10):1910–1918.
84. Sluijs I, Beulens JWJ, van der A DL, Spijkerman AMW, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care. 2010;33(1):43–48.
85. Carlsen MH, Halvorsen BL, Holte K, et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 2010;9:3.
86. Jankauskas SS, Kansakar U, Varzideh F, et al. Heart failure in diabetes. Metabolism. 2021;125:154910.
87. Mone P, Izzo R, Marazzi G, et al. L-arginine enhances the effects of cardiac rehabilitation on physical performance: new insights for managing cardiovascular patients during the COVID-19 pandemic. J Pharmacol Exp Ther. 2022;381(3):197–203.
88. Lourida I, Hannon E, Littlejohns TJ, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437.
89. Rosenberg A, Ngandu T, Rusanen M, et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: the FINGER trial. Alzheimers Dement. 2018;14(3):263–270.
90. Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6-year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster-randomised controlled trial. Lancet. 2016;388(10046):797–805.
91. Andrieu S, Guyonnet S, Coley N, et al. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol. 2017;16(5):377–389.
92. Lamb SE, Sheehan B, Atherton N, et al. Dementia And Physical Activity (DAPA) trial of moderate to high intensity exercise training for people with dementia: randomised controlled trial. BMJ. 2018;361:k1675.
93. Northey JM, Cherbuin N, Pumpa KL, Smee DJ, Rattray B. Exercise interventions for cognitive function in adults older than 50: a systematic review with meta-analysis. Br J Sports Med. 2018;52(3):154–160.
94. Tan ZS, Spartano NL, Beiser AS, et al. Physical activity, brain volume, and dementia risk: the Framingham study. J Gerontol a Biol Sci Med Sci. 2017;72(6):789–795.
95. Rabin JS, Klein H, Kirn DR, et al. Associations of physical activity and β-amyloid with longitudinal cognition and neurodegeneration in clinically normal older adults. JAMA Neurol. 2019;76(10):1203–1210.
96. Karssemeijer EGA, Aaronson JA, Bossers WJ, Smits T, Olde Rikkert MGM, Kessels RPC. Positive effects of combined cognitive and physical exercise training on cognitive function in older adults with mild cognitive impairment or dementia: a meta-analysis. Ageing Res Rev. 2017;40:75–83.
97. Tsutsumimoto K, Doi T, Makizako H, et al. Association of social frailty with both cognitive and physical deficits among older people. J Am Med Dir Assoc. 2017;18(7):603–607.
98. Huynh QL, Saito M, Blizzard CL, et al. Roles of nonclinical and clinical data in prediction of 30-day rehospitalization or death among heart failure patients. J Card Fail. 2015;21(5):374–381.
99. Makizako H, Shimada H, Doi T, et al. Social frailty leads to the development of physical frailty among physically non-frail adults: a four-year follow-up longitudinal cohort study. Int J Environ Res Public Health. 2018;15(3):E490.
100. Yamada M, Arai H. Social frailty predicts incident disability and mortality among community-dwelling Japanese older adults. J Am Med Dir Assoc. 2018;19(12):1099–1103. doi:10.1016/j.jamda.2018.09.013
101. Luger E, Dorner TE, Haider S, Kapan A, Lackinger C, Schindler K. Effects of a home-based and volunteer-administered physical training, nutritional, and social support program on malnutrition and frailty in older persons: a randomized controlled trial. J Am Med Dir Assoc. 2016;17(7):
102. Tarazona-Santabalbina FJ, Gómez-Cabrera MC, Pérez-Ros P, et al. A multicomponent exercise intervention that reverses frailty and improves cognition, emotion, and social networking in the community-dwelling frail elderly: a randomized clinical trial. J Am Med Dir Assoc. 2016;17(5):426–433. doi:10.1016/j.jamda.2016.01.019
103. Saito T, Kai I, Takizawa A. Effects of a program to prevent social isolation on loneliness, depression, and subjective well-being of older adults: a randomized trial among older migrants in Japan. Arch Gerontol Geriatr. 2012;55(3):539–547. doi:10.1016/j.archger.2012.04.002
104. Hikichi H, Kondo N, Kondo K, Aida J, Takeda T, Kawachi I. Effect of a community intervention programme promoting social interactions on functional disability prevention for older adults: propensity score matching and instrumental variable analyses, JAGES Taketoyo study. J Epidemiol Community Health. 2015;69(9):905–910. doi:10.1136/jech-2014-205345
105. Htut TZC, Hiengkaew V, Jalayondeja C, Vongsirinavarat M. Effects of physical, virtual reality-based, and brain exercise on physical, cognition, and preference in older persons: a randomized controlled trial. Eur Rev Aging Phys Act. 2018;15(1):10. doi:10.1186/s11556-018-0199-5
106. Pedroli E, Cipresso P, Greci L, et al. An immersive motor protocol for frailty rehabilitation. Front Neurol. 2019;10:1078. doi:10.3389/fneur.2019.01078
107. Pedroli E, Cipresso P, Greci L, et al. A new application for the motor rehabilitation at home: structure and usability of bal-app. IEEE Trans Emerg Top Comput. 2021;9(3):1290–1300. doi:10.1109/TETC.2020.3037962
108. Piotrowicz E, Pencina MJ, Opolski G, et al. Effects of a 9-week hybrid comprehensive telerehabilitation program on long-term outcomes in patients with heart failure: the Telerehabilitation in Heart Failure Patients (TELEREH-HF) randomized clinical trial. JAMA Cardiol. 2020;5(3):300–308. doi:10.1001/jamacardio.2019.5006
109. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA guideline for the management of heart failure: executive summary: a Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;3:101161CIR0000000000001062.
110. Bauersachs J. Heart failure drug treatment: the fantastic four. Eur Heart J. 2021;42(6):681–683. doi:10.1093/eurheartj/ehaa1012
111. Cacciatore F, Amarelli C, Maiello C, et al. Sacubitril/valsartan in patients listed for heart transplantation: effect on physical frailty. ESC Heart Fail. 2020;7(2):757–762. doi:10.1002/ehf2.12610
112. Cacciatore F, Amarelli C, Maiello C, et al. Effect of Sacubitril-Valsartan in reducing depression in patients with advanced heart failure. J Affect Disord. 2020;272:132–137. doi:10.1016/j.jad.2020.03.158
113. Dereli S, Kılınçel O, Çerik İB, Kaya A. Impact of sacubitril/valsartan treatment on depression and anxiety in heart failure with reduced ejection fraction. Acta Cardiol. 2020;75(8):774–782. doi:10.1080/00015385.2020.1730577
114. Mone P, Lombardi A, Gambardella J, et al. Empagliflozin improves cognitive impairment in frail older adults with type 2 diabetes and heart failure with preserved ejection fraction. Diabetes Care. 2022;1:dc212434.
115. Packer M, Butler J, Zannad F, et al. Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation. 2021;144(16):1284–1294. doi:10.1161/CIRCULATIONAHA.121.056824
116. Cooper JA, Cadogan CA, Patterson SM, et al. Interventions to improve the appropriate use of polypharmacy in older people: a Cochrane systematic review. BMJ Open. 2015;5(12):e009235. doi:10.1136/bmjopen-2015-009235
117. Goyal P, Bryan J, Kneifati-Hayek J, et al. Association between functional impairment and medication burden in adults with heart failure. J Am Geriatr Soc. 2019;67(2):284–291. doi:10.1111/jgs.15654
118. Gnjidic D, Hilmer SN, Blyth FM, et al. Polypharmacy cutoff and outcomes: five or more medicines were used to identify community-dwelling older men at risk of different adverse outcomes. J Clin Epidemiol. 2012;65(9):989–995. doi:10.1016/j.jclinepi.2012.02.018
119. Fried TR, O’Leary J, Towle V, Goldstein MK, Trentalange M, Martin DK. Health outcomes associated with polypharmacy in community-dwelling older adults: a systematic review. J Am Geriatr Soc. 2014;62(12):2261–2272. doi:10.1111/jgs.13153
120. Goyal P, Mangal S, Krishnaswami A, Rich MW. Polypharmacy in heart failure: progress but also problem. Am J Med. 2021;134(9):1071–1073. doi:10.1016/j.amjmed.2021.05.007
121. Mastromarino V, Casenghi M, Testa M, et al. Polypharmacy in heart failure patients. Curr Heart Fail Rep. 2014;11(2):212–219. doi:10.1007/s11897-014-0186-8
122. Unlu O, Levitan EB, Reshetnyak E, et al. Polypharmacy in older adults hospitalized for heart failure. Circ Heart Fail. 2020;13(11):e006977. doi:10.1161/CIRCHEARTFAILURE.120.006977
123. Denny RM, Hummel SL. Heart failure medical management in 2020: searching for the right polypharmacy. Circ Heart Fail. 2020;13(11):e007779. doi:10.1161/CIRCHEARTFAILURE.120.007779
124. Rahman S, Singh K, Dhingra S, et al. The double burden of the COVID-19 pandemic and polypharmacy on geriatric population - public health implications. Ther Clin Risk Manag. 2020;16:1007–1022. doi:10.2147/TCRM.S272908
125. Brinker LM, Konerman MC, Navid P, et al. Complex and potentially harmful medication patterns in heart failure with preserved ejection fraction. Am J Med. 2021;134(3):374–382. doi:10.1016/j.amjmed.2020.07.023
126. Kinugasa Y, Nakayama N, Sugihara S, et al. Polypharmacy and taste disorders in heart failure patients. Eur J Prev Cardiol. 2020;27(1):110–111. doi:10.1177/2047487319856717
127. Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur J Heart Fail. 2012;14(9):1041–1049. doi:10.1093/eurjhf/hfs096
128. Gharacholou SM, Sloane R, Cohen HJ, Schmader KE. Geriatric inpatient units in the care of hospitalized frail adults with a history of heart failure. Int J Gerontol. 2012;6(2):112–116. doi:10.1016/j.ijge.2012.01.012
129. Kamiya K, Sato Y, Takahashi T, et al. Multidisciplinary cardiac rehabilitation and long-term prognosis in patients with heart failure. Circ Heart Fail. 2020;13(10):e006798. doi:10.1161/CIRCHEARTFAILURE.119.006798
130. Mudge AM, Pelecanos A, Adsett JA. Frailty implications for exercise participation and outcomes in patients with heart failure. J Am Geriatr Soc. 2021;69(9):2476–2485. doi:10.1111/jgs.17145
131. Pulignano G, Del Sindaco D, Di Lenarda A, et al. Usefulness of frailty profile for targeting older heart failure patients in disease management programs: a cost-effectiveness, pilot study. J Cardiovasc Med. 2010;11(10):739–747. doi:10.2459/JCM.0b013e328339d981
132. Witham MD, Gray JM, Argo IS, Johnston DW, Struthers AD, McMurdo MET. Effect of a seated exercise program to improve physical function and health status in frail patients > or = 70 years of age with heart failure. Am J Cardiol. 2005;95(9):1120–1124. doi:10.1016/j.amjcard.2005.01.031
133. Yun S, Enjuanes C, Calero-Molina E, et al. Effectiveness of telemedicine in patients with heart failure according to frailty phenotypes: insights from the iCOR randomised controlled trial. Eur J Intern Med. 2022;96:49–59. doi:10.1016/j.ejim.2021.09.021
134. Piepoli MF, Coats AJS. The “skeletal muscle hypothesis in heart failure” revised. Eur Heart J. 2013;34(7):486-488. doi:10.1093/eurheartj/ehs463
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
