Back to Journals » Clinical Ophthalmology » Volume 17
Clinical Performance of a New Trifocal IOL with a 7.0 mm Optical Zone
Authors Pastor-Pascual F, Orts-Vila P, Tañá-Sanz P, Tañá-Sanz S, Tañá-Rivero P
Received 11 August 2023
Accepted for publication 22 October 2023
Published 7 November 2023 Volume 2023:17 Pages 3397—3407
DOI https://doi.org/10.2147/OPTH.S435076
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Francisco Pastor-Pascual,1 Paz Orts-Vila,2 Pedro Tañá-Sanz,2 Santiago Tañá-Sanz,2 Pedro Tañá-Rivero2
1Cataract and Refractive Surgery Department, Oftalvist Valencia, Valencia, Spain; 2Cataract and Refractive Surgery Department, Oftalvist Alicante, Alicante, Spain
Correspondence: Pedro Tañá-Rivero, Oftalvist Alicante, Avda. Dénia 76, Alicante, 03013, Spain, Tel/Fax +34 965 14 15 00, Email [email protected]
Purpose: To evaluate the refractive and visual outcomes following cataract surgery and implantation of a diffractive trifocal intraocular lens (IOL) with a 7.0 mm optical zone.
Methods: A total of 23 patients who underwent bilateral implantation with the Triva-aXAY IOL were analyzed at 6 months post-surgery. The main outcome measures were refractive error, monocular and binocular uncorrected and corrected-distance visual acuity (UDVA, CDVA), uncorrected and corrected-distance intermediate visual acuity (UIVA, CDIVA) at 60 cm, uncorrected and corrected-distance near visual acuity (UNVA, CDNVA) at 40 cm, and binocular defocus curve. Patients also completed the Catquest-9SF questionnaire.
Results: All eyes were within ± 1.00D, and 91.30% of eyes within ± 0.50D, with a mean postoperative spherical equivalent of – 0.14± 0.29D. Similarly, 95.65% of patients showed a binocular UDVA ≥ 20/25, compared to 100% for CDVA, and the mean binocular UDVA and CDVA were 0.02± 0.06 and 0.00± 0.05 logMAR, respectively. At intermediate vision, 65.22% of patients showed a binocular UIVA ≥ 20/25, compared to 86.96% for CDIVA, and the mean binocular UIVA and CDIVA were 0.07± 0.06 and 0.06± 0.06 logMAR, respectively. At near, 95.65% of patients showed a binocular UNVA and CDNVA ≥ 20/25, with a mean binocular UDNVA and CDNVA of 0.04± 0.07 and 0.02± 0.05 logMAR, respectively. Finally, 95.65% of patients reported being quite satisfied to very satisfied with their vision and about 74% did not report any difficulty with their vision in their everyday life. Between 65.22% and 100% of patients reported no difficulty performing different tasks.
Conclusion: Our study shows good visual and refractive outcomes with high satisfaction in patients implanted with the Triva-aXAY IOL with a 7.0 mm optical zone.
Keywords: large optic, diffractive, trifocal, intraocular lens, cataract
Introduction
One of the most promising recent advances in cataract surgery to correct presbyopia is to reduce spectacle dependence at different distances in order to provide patients with a full range of vision from distance to near vision. The increasing demand for good visual acuity at different distances has pushed manufacturers to develop multifocal intraocular lens (IOL) technology based on the initial designs from the late 1980s.1,2 Bifocal and trifocal IOLs, which split light into two or three different foci, have been proved useful for improving near and intermediate vision while maintaining distance visual acuity. Different peer-reviewed publications have pointed out that those patients implanted with trifocal models may achieve better intermediate vision than their counterparts implanted with bifocal models, while near and far vision, postoperative refraction or spectacle independence of bifocal models are similar to those of trifocal ones.3–5
Depending on their design properties and the optical technology used, patient outcomes after implantation may vary considerably. Some clinical data suggest that trifocal IOLs led to more photic disturbances (i.e halo and glare) than extended depth-of-focus IOLs6 since the light distribution between foci can increase these disturbances, but note that objective dysphotopsia is not reduced in extended depth-of-focus IOLs compared to trifocal IOLs.7 Most foldable IOLs for cataract surgery currently available on the market have an optical diameter of 6.0 mm, but the occurrence of photic phenomena might occur more frequently in patients with large pupils when the IOL optic diameter is only slightly smaller than the pupil size, thus causing optical distortions.8 Dysphotopsia, and the effect of IOL optic size, have been discussed such a lingering issue after cataract surgery.9 As such, the implantation of an IOL with a large optic might help to reduce these undesired optical images.10,11
A new trifocal IOL with a 7.0 mm optical zone, the Triva-aXAY IOL, has been recently made available to a few European surgeons in the context of a controlled market release phase (personal communication with the manufacturer HumanOptics Holding AG, Erlangen, Germany). The lens is the trifocal model of the Aspira-aXA.10,11
The aim of the current clinical study was to evaluate the postoperative visual acuity at different distances and refractive outcomes in cataract patients with bilateral implantation of this lens. Some additional measurements, such as the defocus curve and analysis of the patient’s satisfaction and quality-of-life, were also analysed.
Methods
This two-center prospective study was approved by the Ethics Committee of the Hospital Clínico San Carlos (Madrid, Spain) and the Regional Committee for Observational Prospective Studies (CAEPRO; Valencia, Spain). It was conducted in accordance with the tenets of the Declaration of Helsinki, and written informed consent was obtained from all patients prior to their enrollment in this study after explaining the possible consequences of the study to them.
The inclusion criteria were patients aged 50 years or older submitted to bilateral age-related cataract surgery and implanted with the Triva-aXAY IOL according to regular clinical practice, availability, willingness, and sufficient cognitive awareness to comply with examination procedures, preoperative corneal astigmatism ≤1.00 D and clear intraocular media other than cataract. The exclusion criteria were patients unable to comprehend the study requirements, irregular cornea (ie keratoconus, previous corneal refractive surgery, ocular anomalies or pathologies that could reduce visual function or postoperative IOL stability (ie severe amblyopia, macular degeneration), uncontrolled glaucoma, retinal detachment, macular degeneration or retinopathy and non-reactive pupils).
Intraocular Lens
All patients were implanted with the Triva-aXAY IOL model IOL. This is a one-piece trifocal diffractive posterior-chamber IOL with plate cut-out haptics. This IOL has an aspheric aberration-free anterior surface with a 3.5 mm central diffractive zone consisting of seven diffraction steps and an outer refractive zone of 2.5 mm, along with a posterior surface with 360° lens epithelial cell barrier. It has a total diameter of 11.0 mm and an optical zone diameter of 7.0 mm, thus providing an addition for near of +3.35D and +1.75D for intermediate distance. The lens is built in powers ranging from +10.00 to +30.00D in 0.50 D increments. It is made from hydrophilic acrylic with a UV-absorber, with a water content of 26% at 35°C, and contains a blue light filter that absorbs the high-energy portion of the light between 400 and 500 nm. Its Abbe number is 56. This IOL was granted CE-mark approval for use in May 2020.
Pre- and Postoperative Assessment
Preoperatively, patients underwent an extensive ophthalmologic examination, including slit-lamp examination, measurement of logMAR uncorrected and corrected-distance visual acuity (UDVA and CDVA), monocular manifest refraction, intraocular pressure (IOP) measurement, funduscopy, corneal topography with the Pentacam (Oculus Optikgeräte GmbH, Germany) and biometry with the IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany). The Barrett formula was used to calculate the IOL power.
Postoperative examinations were performed at six months post-implantation. A standard ophthalmologic examination, including refraction and slit-lamp biomicroscopy, was performed. Visual acuities were measured using ETDRS charts, and specifically, monocular and binocular logMAR UDVA, CDVA, uncorrected distance intermediate visual acuity (UIVA), corrected distance intermediate visual acuity (CDIVA) at 60 cm, uncorrected distance near visual acuity (UNVA) and corrected distance near visual acuity (CDNVA) at 40 cm were measured. A binocular defocus curve was measured for each patient using the ETDRS chart positioned at 4 m, under photopic conditions, from +2.00D to –5.00D in 0.50D steps. Patients were asked to complete the Catquest-9SF questionnaire,12 which has been validated in a Spanish population for trifocal IOLs.13 All data are shown as the mean ± standard deviation and ranges. Complications and adverse events were also recorded during the study.
Sample Size and Statistical Analysis
The sample size estimated for this “pilot” study was calculated according to Sullivan,14 using the highest standard deviation of the monocular visual acuity defocus curve derived from a previous sample of 16 eyes implanted with the Triva-aA lens, which shares the same diffractive optic as the Triva-aXA lens but with a different platform (6.0 mm optic/C-loop haptics versus 7.0 mm optic/plate cut-out haptics, respectively). With a standard deviation of 0.24 logMAR at +2.00D defocus, a confidence interval of 95% and a maximum tolerated margin of error of 0.10 logMAR; a minimum of 22 patients was required, considering a drop-out rate of approximately 15% after the 6-month follow-up [N= [(1.96 × 0.24)/0.1]2=22].
Results
A total of 46 eyes from 23 consecutive patients were enrolled in this study. Table 1 shows the demographics for the patients included in the study and some preoperative measurements obtained. The mean age was 70.87±5.35 years (range 60 to 80 years), with 17 patients being female (73.91%) and six male (26.08%). There were no complications in any of the cases during surgery and follow-up.
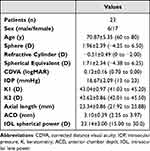 |
Table 1 Demographic Characteristics and Preoperative Measurements of Participants Shown as Means, Standard Deviations (SD) and Ranges |
Standard graphs for reporting refractive and visual acuity outcomes were constructed.15 For the efficacy of the procedure, Figure 1 was plotted. This figure shows the cumulative postoperative monocular and binocular UDVA and CDVA, UIVA and CDIVA, and UNVA and CDNVA, respectively, at six months post-surgery. Table 2 shows the detailed mean visual acuity outcomes for monocular and binocular conditions at the three distances evaluated. At distance (Figure 1, top), 69.57% of patients showed a binocular UDVA of 20/20 or better compared to 91.30% for CDVA. These percentages increased to 95.65% and 100% for a cumulative value of 20/25 or better, respectively. The postoperative mean values for binocular UDVA and CDVA were 0.02±0.06 and 0.00±0.05 logMAR, respectively (about 20/20). At intermediate vision (60 cm, Figure 1, middle), 26.09% of patients showed a binocular UIVA of 20/20 or better compared to 30.43% for CDIVA. These percentages increased to 65.22% and 86.96% for a cumulative value of 20/25 or better, respectively. The postoperative mean values for binocular UIVA and CDIVA were 0.07±0.06 and 0.06±0.06 logMAR, respectively. At near (Figure 1, bottom), 52.17% of patients showed a binocular UNVA of 20/20 or better compared to 65.22% for CDIVA. These percentages increased to 95.65% for a cumulative value of 20/25 or better. The postoperative mean values for binocular UNVA and CDNVA were 0.04±0.07 and 0.02±0.05 logMAR, respectively. Figure 2 shows the change in lines of visual acuity between the postoperative monocular and binocular UDVA and CDVA at six months post-surgery. All eyes and patients showed the same or better UDVA than CDVA.
 |
Table 2 Visual Acuity Outcomes (logMAR) of Patients Implanted with the Triva-aXAY Intraocular Lens Shown as Means, Standard Deviations (SD) and Ranges at 6 Months of Follow-Up |
For predictability, Figure 3 (top) shows the histogram of postoperative spherical equivalent (SE) refraction relative to the intended target refraction. The highest percentage of eyes (47.83%) was for the range ±0.13D, followed by 30.43% for the –0.50 to –0.14D range. All eyes were within ±1.00D and 91.30% of eyes within ±0.50D. The mean postoperative SE was –0.14±0.29D (ranging from –0.75 to 0.75D). Figure 3 (bottom) shows the distribution of the postoperative refractive cylinder. Specifically, 82.61% of eyes showed a value ≤0.50D and all eyes a value ≤1.00D. The mean postoperative refractive cylinder was –0.28±0.35D, ranging from 0.00 to –1.00D.
 |
Figure 3 Histogram of the postoperative spherical equivalent refraction (D), top, and refractive cylinder (D), bottom, at 6 months post-surgery. |
Figure 4 shows the mean high-contrast photopic binocular defocus curve. The values reported show three peaks, one at the expected distance focus (0.00D of vergence), one at the intermediate focus (–1.50D of vergence) and the last one at the near focus (–3.00D of vergence). Two smooth valleys can be observed between the three peaks.
Patients were asked to complete the Catquest-9SF questionnaire, which comprises several items, namely global daily life difficulty, global vision satisfaction and a group of several items related to day-to-day activities. Almost all patients (95.65%) reported being quite satisfied to very satisfied with their vision at present, with a mean score of 3.30±0.55, and about 74% did not report any difficulty with their vision in their everyday life, while about 26% reported finding some difficulties (mean score 3.74±0.44, see Table 3). With regard to difficulties carrying out different tasks, in general between 65.22% and 100% of patients reported no difficulty performing the tasks defined in Catquest-9SF. Table 3 shows the patient-reported difficulties with their vision assessed using the Catquest-9SF questionnaire in greater detail.
 |
Table 3 Outcomes of Patient Reported Difficulties with Their Vision Assessed Using the Catquest-9SF Questionnaire |
Discussion
Different systematic reviews and meta-analyses have analyzed the visual and refractive outcomes of several trifocal IOLs available on the market with different optical designs.3–6 These publications confirmed the good visual performance of patients implanted with trifocal lenses, especially for intermediate distances. A Bayesian network meta-analysis considering 27 studies of randomized clinical trials with 2605 patients has recently concluded that, for patients considering a multifocal IOL due to presbyopia, bilateral implantation of a trifocal IOL might be an optimal option without compromising distant visual acuity.16 The current clinical study aimed to assess the refractive and visual outcomes at different distances following cataract surgery and implantation of the new trifocal Triva-aXAY IOL with a 7.0 mm optical zone.
Since no previous studies have been published with the Triva-aXAY IOL, we cannot compare our findings with previous outcomes, although we can perform a comparison with other studies that analyzed other diffractive trifocal IOLs. Note that, in addition to the different optical design, it is challenging to compare our study with previous ones using other lenses because of the differences in study design and different samples and follow-ups.
In our study, we obtained good monocular and binocular visual acuity outcomes at different distances. The mean values are shown in Table 2. At distance, 69.57% of patients showed a binocular UDVA ≥20/20 compared to 91.30% for CDVA (see Figure 1, top). These percentages increased to 95.65% and 100% for a cumulative value of ≥20/25, respectively. The mean values for binocular UDVA and CDVA were 0.02±0.06 and 0.00±0.05 logMAR, respectively. Alfonso et al17 analyzed a large sample of 102 patients implanted with the aspheric trifocal diffractive AT LISA tri 839MP IOL (Carl Zeiss Meditec AG) at six months post-surgery. Similarly to us, they found that all patients achieved a binocular CDVA ≥20/25. In another study, Alfonso et al18 evaluated 40 patients implanted bilaterally with the AcrySof IQ PanOptix IOL (Alcon Labs) at six months post-surgery. They found that all patients achieved a binocular CDVA ≥20/25 and about 80% a CDVA ≥20/20. In our series, the postoperative mean values for binocular UDVA and CDVA were 0.02±0.06 and 0.00±0.05 logMAR, respectively. Our results are in agreement with those of other authors using other lenses. For example, Lapid-Gortzak et al19 compared the PanOptix and AT LISA IOLs in 93 and 89 patients at 4–6 months. They found a binocular mean UDVA of 0.014±0.098 and 0.003±0.112 logMAR for the PanOptix and AT LISA groups, respectively. Similarly, Torky et al20 assessed the visual performance of patients implanted bilaterally with the PanOptix (26 patients) and AT LISA (27 patients) IOLs at six months post-surgery. They found a mean CDVA of –0.06 and –0.08 logMAR for both groups, respectively. Our results also support the safety of the procedure since all eyes and patients showed the same or better UDVA than CDVA (Figure 2). At intermediate vision, 26.09% of patients showed a binocular UIVA ≥20/20 compared to 30.43% for CDIVA (see Figure 1, middle). These percentages increased to 65.22% and 86.96% for a cumulative value of ≥20/25, respectively. In their study, Alfonso et al17 found 39.6% of patients implanted with the AT LISA with a binocular CDIVA ≥20/25 at 60 cm and about 30% of patients implanted with the PanOptix IOL.18 Our mean values for binocular UIVA and CDIVA were 0.07±0.06 and 0.06±0.06 logMAR, respectively. Ferreira et al21 assessed 30 patients implanted with the PanOptix IOL and 30 patients with the FineVision POD F (PhysIOL s.a.) at three months post-surgery. They found a binocular CDIVA at 66 cm of 0.00 logMAR or better in 83.3% and 73.3% of patients in the PanOptix and FineVision groups, respectively. Lapid-Gortzak et al19 found values of 0.049±0.127 and 0.116±0.125 logMAR for UIVA (60 cm) in their study, and Torky et al20 reported a mean UIVA (60 cm) of 0.00 and 0.16 logMAR for the PanOptix and AT LISA groups, respectively. At near (see Figure 1, bottom), 52.17% of patients showed a binocular UNVA ≥20/20 compared to 65.22% for CDIVA. These percentages increased to 95.65% for a cumulative value ≥20/25. Alfonso et al17,18 found 86.1% and about 85% of patients with a binocular CDNVA ≥20/25 with the AT LISA and the PanOptix IOLs, respectively. Ferreira et al21 reported that a value of 0.00 logMAR of binocular CDNVA was achieved in 83.3% and 76.7% of patients in the PanOptix and FineVision groups, respectively. In our series, the mean values for binocular UNVA and CDNVA were 0.04±0.07 and 0.02±0.05 logMAR, respectively. Lapid-Gortzak et al19 found mean values of 0.082±0.103 and 0.136±0.13 logMAR for UNVA for the PanOptix and AT LISA IOLs, respectively; and Torky et al20 0.00 and –0.01 logMAR for the same groups, respectively.
With regard to the predictability of the procedure, Figure 4 shows the distribution of the postoperative SE and refractive cylinder. The mean postoperative SE and refractive cylinder were –0.14±0.29D and –0.28±0.35D, respectively. Alfonso et al17 found a mean postoperative sphere and cylinder of –0.06±0.21D and –0.09±0.21D, respectively, for the AT LISA IOL. Similarly, for the PanOptix IOL, Alfonso et al18 found a mean postoperative sphere and cylinder of 0.03±0.33D and −0.18±0.28D, respectively, and a mean SE of –0.06±0.33D. They also found 55% of eyes with an SE of ±0.13D and 22.50% of eyes between –0.50 and –0.14D. In our study, we found similar mean values and distribution, with the highest percentage of eyes (47.83%) for the range ±0.13D, followed by 30.43% for the range –0.50 to –0.14D. Torky et al20 reported slightly higher SE values of –0.31±0.37 and –0.23±0.42D for the PanOptix and AT LISA IOLs, respectively.
The defocus curve in Figure 4 shows that the maximum visual acuity value was obtained at a vergence of 0D, corresponding to the far focus. A second peak was found at –1.50D of vergence, corresponding to the intermediate focus, and a third peak at –3.00D of vergence, corresponding to the near focus. Between +1.00D and –3.50D of vergence, the curve showed a wide range of useful vision, with visual acuity values higher than 0.2 logMAR. Our outcomes therefore suggest that the creation of a third intermediate focus does not involve a reduction in the other two main foci (distance and near focus). These visual acuity values may be considered suitable to obtain a high level of spectacle independence. In their study with the PanOptix IOL, Alfonso et al18 found a binocular defocus curve with one peak at the expected distance focus with the best visual acuity (0D), followed by a depression with a reduced visual acuity (about 1D, 1 m), subsequently improving (about 2D, 50 cm). Similarly, for their PanOptix and AT LISA IOL groups, Torky et al20 achieved a visual acuity of 0.3 logMAR or better, with defocus levels ranging from –2.50D to 0D. The best results for the PanOptix IOL group were obtained at a defocus of 0D and –2.00D, simulating a distance and 50 cm, with values of –0.04 and 0.01 logMAR, respectively. For the AT LISA group, the best visual acuity (–0.07 logMAR) was obtained with a defocus of 0D, progressively decreasing with negative defocus up to a second peak of good acuity at –2.5D (0.07 logMAR).
The results of the Catquest-9SF questionnaire showed that 95.65% of patients reported being quite satisfied to very satisfied with their vision after ¡surgery. Our results agree with those found by Lapid-Gortzak et al19 in this regard. In this study, patients were asked to respond either “yes” or “no” to the question “Given your current postoperative vision, if you had to do it all over again would you have the same lens implanted?”. These authors found that most patients in both groups (PanOptix IOL 96%; AT LISA IOL 97%) responded “yes” to the question, thus showing the high level of patient satisfaction. With regard to difficulties carrying out different tasks in our study, in general between 65.22% and 100% of patients reported no difficulty performing the different tasks assessed (see Table 3). Torky et al20 found that about 90% of patients with the PanOptix and AT LISA IOLs were spectacle-independent for far, intermediate and near vision.
As discussed previously, there are no previous publications with this lens, although it is of interest to discuss some articles published using the monofocal version of the lens with the same optical diameter as regards to the stability of the lens when implanted. In this sense, Wendelstein et al9 evaluated the rotational stability, tilt and decentration of the monofocal Aspira-aXA IOL with an optical diameter of 7.0 mm when implanted in 74 eyes using intraoperative and slit-lamp images (for IOL rotation), and Scheimpflug imaging (for IOL tilt and decentration) at 1 week, 1 month and 4 months. At the latest follow-up, IOL rotation was within 5.0° in 85% of the eyes (n=40) and within 10.0° in 98% (n=46), and the IOL vertical and horizontal tilt referenced to the pupillary axis was, on average, <1.5° in both eyes (n=54; maximum 5.85°). These authors found that decentration in both meridians was on average <0.10 mm in both eyes (maximum 0.30 mm), and concluded that this lens showed good and stable positioning within the capsular bag over a four-month period. They also pointed out that the four-point non-angulated haptic design combined with the 7.0 mm optic provides a large surface contact between the lens and the capsular bag. In non-toric IOLs is also important to assess the rotational stability in order to study how the physical shape and haptic design (friction between the haptic and the capsular bag) are important factors that may affect its stability. Schrecker et al22 analyzed the same lens with a large follow-up period (1.5 years) on a sample of 55 eyes, finding that the lens was stable over the postoperative course as decentration was <0.02 mm and tilt <5.5°, with a median rotation of 1.8° within the first postoperative week, which was not significantly different from the rotation between surgery and at 1.5 years (median 1.4°). They concluded that the lens shows good position stability in the capsular bag, thus highlighting the advantages of a 7.0 mm optic in the diagnostics and treatment of peripheral retinal pathologies.
One of the possible additional benefits of using a 7.0 mm optic IOL is that it allows for a wide posterior capsulotomy and permits peripheral retinal visualization, which may be valuable if retinal treatment with laser or vitreoretinal surgery is needed. In this sense, Borkenstein and Borkenstein23 assessed six myopic patients with posterior segment disease implanted with the lens at 10 weeks post-phacoemulsification and intravitreal injection. They found that, during surgery and postoperative examinations, the wide IOL optic permitted an enhanced view of the fundus, and that the IOLs remained stable after implantation, especially during intravitreal injection at the end of surgery. These authors did not observe any IOL displacement or shift and showed that implantation of this lens enables a wide view of the fundus during and after surgery, with no additional risks or negative effects, and may also reduce the risk of dysphotopsia in cases of lens decentration in large capsular bags.
And, in relation to possible dysphotopsia, Bonsemeyer et al11 analyzed 120 eyes of 86 patients with 57 eyes receiving the Aspira-aXA IOL (7.0 mm and plate-haptics) and 63 eyes with the Aspira-aA IOL (6.0 mm and C-loop haptics, HumanOptics Holding AG, Erlangen, Germany). They found, in relation to positive dysphotopsia, that there was a statistically significant difference between both groups at 1 month postoperatively, with a lower incidence in the group with the Aspira-aXA lenses (31.6% versus 52.4%). They found a reduction of cases with follow-up not being statistically significant at 3 and 12 months, but there was a 2.4-fold reduction in the group with the Aspira-aXA lens compared with the Aspira-aA lens. For negative dysphotopsia, the pattern was similar with a lower incidence in the group with the Aspira-aXA lens (5.4%, vs 20.6%). Also, the difference was no longer statistically significant at 3- and 12-months post-surgery, but in the last follow-up, there were no cases with the Aspira-aXA lens and 2 cases with the Aspira-aXA lens. These authors finally concluded that the Aspira-aXA lens with 7.0 mm optic diameter and plate haptics reduced both positive and negative dysphotopsia.
Finally, we should consider the limitations of our study. First, despite the sample size calculation, our sample is relatively small and further studies involving larger samples are desirable. Second, we have evaluated the outcomes of patients implanted with the Triva-aXAY IOL, and no comparison with a control monofocal or other trifocal group was carried out. Previous literature on the monofocal version of this lens, and other trifocal diffractive lenses, was used to discuss the outcomes found. Finally, our study analyzed the outcomes at six months, and a longer follow-up would give more information about the performance of the lens, specifically for the patient satisfaction and photic phenomena, which are likely to improve due to neuroadaptation with time.
Conclusion
To summarize, the present clinical study confirms that the diffractive Triva-aXAY IOL with a 7.0 mm optical zone is a successful trifocal IOL, as supported by several refractive and visual metrics obtained in our sample of patients. The high percentage of satisfied patients when this lens is implanted suggests that this model seems to be an excellent option to be considered in patients aiming to be spectacle-independent at different distances. Note that this is the first study to assess the visual and refractive performance of this IOL, therefore additional clinical studies should be carried out with larger samples and longer follow-ups. We consider that future studies should also be carried out with the toric version of this model, focusing on astigmatism predictability and the rotational stability at different times post-surgery.
Data Sharing Statement
Data are not available for sharing.
Funding
This study was funded by HumanOptics Holding AG (Erlangen, Germany).
Disclosure
Dr Francisco Pastor-Pascual reports grants from Alcon Labs, Hoya Surgical AG, BVI, and Carl Zeiss Meditech, outside the submitted work. Dr Pedro Tañá-Rivero reports grants from Alcon Labs, HOYA Surgical, BVI, Carl Zeiss Meditec, and AST Products Inc, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Keates RH, Pearce JL, Schneider RT. Clinical results of the multifocal lens. J Cataract Refract Surg. 1987;13(5):557–560. doi:10.1016/S0886-3350(87)80114-1
2. Hansen TE, Corydon L, Krag S, Thim K. New multifocal intraocular lens design. J Cataract Refract Surg. 1990;16(1):38–41. doi:10.1016/S0886-3350(13)80871-1
3. Shen Z, Lin Y, Zhu Y, Liu X, Yan J, Yao K. Clinical comparison of patient outcomes following implantation of trifocal or bifocal intraocular lenses: a systematic review and meta-analysis. Sci Rep. 2017;7(1):45337. doi:10.1038/srep45337
4. Yang JJ, Liu QP, Li JM, Qin L. Comparison of visual outcomes with implantation of trifocal versus bifocal intraocular lens after phacoemulsification: a Meta-analysis. Int J Ophthalmol. 2018;11(3):484–492.
5. Jin S, Friedman DS, Cao K, et al. Comparison of postoperative visual performance between bifocal and trifocal intraocular Lens based on randomized controlled trails: a meta-analysis. BMC Ophthalmol. 2019;19(1):78. doi:10.1186/s12886-019-1078-1
6. Zhong Y, Wang K, Yu X, Liu X, Yao K. Comparison of trifocal or hybrid multifocal-extended depth of focus intraocular lenses: a systematic review and meta-analysis. Sci Rep. 2021;11(1):6699. doi:10.1038/s41598-021-86222-1
7. Escandón-García S, Ribeiro FJ, McAlinden C, Queirós A, González-Méijome JM. Through-focus vision performance and light disturbances of 3 new intraocular lenses for presbyopia correction. J Ophthalmol. 2018;2018:6165493. doi:10.1155/2018/6165493
8. Bournas P, Drazinos S, Kanellas D, Arvanitis M, Vaikoussis E. Dysphotopsia after cataract surgery: comparison of four different intraocular lenses. Ophthalmologica. 2007;221(6):378–383. doi:10.1159/000107496
9. Werner L. Dysphotopsia, a lingering issue after cataract surgery: effect of IOL optic size. J Cataract Refract Surg. 2022;48(1):1–2. doi:10.1097/j.jcrs.0000000000000864
10. Wendelstein J, Laubichler P, Fischinger I, et al. Rotational stability, tilt and decentration of a new IOL with a 7.0 mm Optic. Curr Eye Res. 2021;46(11):1673–1680. doi:10.1080/02713683.2021.1929329
11. Bonsemeyer MK, Becker E, Liekfeld A. Dysphotopsia and functional quality of vision after implantation of an intraocular lens with a 7.0 mm optic and plate haptic design. J Cataract Refract Surg. 2022;48(1):75–82. doi:10.1097/j.jcrs.0000000000000735
12. Kabanovski A, Hatch W, Chaudhary V, et al. Validation and application of Catquest-9SF in various populations: a systematic review. Surv Ophthalmol. 2020;65(3):348–360. doi:10.1016/j.survophthal.2019.12.002
13. Lundström M, Llovet F, Llovet A, et al. Validation of the Spanish Catquest-9SF in patients with a monofocal or trifocal intraocular lens. J Cataract Refract Surg. 2016;42(12):1791–1796. doi:10.1016/j.jcrs.2016.10.011
14. Sullivan L. Power and Sample Size Determination; 2020. Boston University School of Public Health.
15. Reinstein DZ, Archer TJ, Srinivasan S, et al. Standard for Reporting Refractive Outcomes of Intraocular Lens-Based Refractive Surgery. J Refract Surg. 2017;33(4):218–222. doi:10.3928/1081597X-20170302-01
16. Cho JY, Won YK, Park J, et al. Visual outcomes and optical quality of accommodative, multifocal, extended depth-of-focus, and monofocal intraocular lenses in presbyopia-correcting cataract surgery: a systematic review and bayesian network meta-analysis. JAMA Ophthalmol. 2022;22:e223667. doi:10.1001/jamaophthalmol.2022.3667
17. Alfonso JF, Fernández-Vega Cueto L, Belda-Salmerón L, Montés-Micó R, Fernández-Vega L. Visual function after implantation of a diffractive aspheric trifocal intraocular lens. Eur J Ophthalmol. 2016;26(5):405–411. doi:10.5301/ejo.5000741
18. Alfonso JF, Fernández-Vega-Cueto L, Fernández-Vega L, Montés-Micó R. Visual function after implantation of a Presbyopia-Correcting trifocal intraocular lens. Ophthalmic Res. 2020;63(2):152–164. doi:10.1159/000500834
19. Lapid-Gortzak R, Bhatt U, Sanchez JG, et al. Multicenter visual outcomes comparison of 2 trifocal presbyopia-correcting IOLs: 6-month postoperative results. J Cataract Refract Surg. 2020;46(11):1534–1542. doi:10.1097/j.jcrs.0000000000000274
20. Torky MA, Nokrashy AE, Metwally H, Abdelhameed AG. Visual performance following implantation of presbyopia correcting intraocular lenses. Eye (Lond). 2022. doi:10.1038/s41433-022-02188-y
21. Ferreira TB, Ribeiro FJ, Silva D, Matos AC, Gaspar S, Almeida S. Comparison of refractive and visual outcomes of 3 presbyopia-correcting intraocular lenses. J Cataract Refract Surg. 2022;48(3):280–287. doi:10.1097/j.jcrs.0000000000000743
22. Schrecker J, Seitz B, Langenbucher A. Performance einer neuen 7-mm-Intraokularlinse mit Nachbeobachtung über 1,5 Jahre [Performance of a new 7 mm intraocular lens with follow-up over 1.5 years]. Ophthalmologe. 2022;119(4):367–373. doi:10.1007/s00347-021-01504-3
23. Borkenstein AF, Borkenstein EM. Efficacy of large optic intraocular lenses in myopic eyes with posterior segment pathology. Ophthalmol Ther. 2022;11(1):443–452. doi:10.1007/s40123-021-00433-3
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



