Back to Journals » Clinical Ophthalmology » Volume 16
Clinical Performance and Surgeon Acceptability of a New Dual Mode Phacoemulsification System
Authors Quesada G, Chang DH , Waltz KL, Kao AA, Quesada R, Wang Y, Ji L, Parizadeh D, Atiles L
Received 18 February 2022
Accepted for publication 25 July 2022
Published 6 August 2022 Volume 2022:16 Pages 2441—2451
DOI https://doi.org/10.2147/OPTH.S363061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Gabriel Quesada,1 Daniel H Chang,2 Kevin L Waltz,3 Andrew A Kao,2 Rodrigo Quesada,1 Ying Wang,4 Leilei Ji,4 Dari Parizadeh,4 Luis Atiles4
1Grupo Oftalmo & Plastico, San Salvador, El Salvador; 2Empire Eye and Laser Center, Bakersfield, CA, USA; 3Central American Ophthalmic Research Consultants, Indianapolis, IN, USA; 4Johnson & Johnson Surgical Vision, Inc, Santa Ana, CA, USA
Correspondence: Gabriel Quesada, Grupo Oftalmo & Plastico, 9 C Pte, 4625, Col Escalon, San Salvador, El Salvador, Tel +1 (503) 22579003, Email [email protected]
Purpose: The purpose of this first in-human study was to evaluate the overall clinical performance of the VERITAS™ Vision System in patients scheduled to undergo cataract extraction and to confirm overall surgeon acceptability.
Patients and methods: This prospective, open-label multinational study included adults with cataracts scheduled for planned cataract extraction and posterior chamber IOL implantation. Standard small-incision phacoemulsification cataract surgery with the VERITAS Vision System was conducted. Surgeons completed a questionnaire regarding their clinical experience with the VERITAS Vision System for each patient following surgery and 1-day postoperative. Corneal clarity and adverse events (AEs) were assessed. Surgeon acceptability was scored on a 5-point scale, with acceptability considered favorable for scores of 4 and 5.
Results: A total of 115 eyes (79 patients) were treated. The El Salvador site treated 41 patients (58 eyes), and the US site treated 38 patients (57 eyes). Overall, surgeons were satisfied with the clinical performance regardless of the cataract grade. The satisfaction with anterior chamber stability, post-occlusion surge, followability, holdability, cutting efficiency, usability, and overall satisfaction with the VERITAS Vision System was clinically favorable in ≥ 99% of cases. Overall satisfaction with the swivel handpiece, foot pedal, and enhanced ergonomics were clinically favorable in ≥ 97% of cases regardless of the cataract grade. Satisfaction with corneal clarity at same-day postoperative and 1-day postoperative, and 1-day overall clinical results of surgery with the VERITAS Vision System were clinically favorable in ≥ 94% of cases regardless of cataract grade.
Conclusion: The new dual-mode phacoemulsification system with dual-durometer tubing, gas forced infusion, new swivel handpiece, and ergonomics improvements resulted in a high rate of user satisfaction with clinical performance and ergonomics. The VERITAS Vision System is safe and effective when used as indicated.
Keywords: VERITAS Vision System, cataract, ergonomics, fluidics, satisfaction
Introduction
Advancements in technology and ultrasound for cataract removal surgery have three goals: to be less traumatic, more precise, and more efficient. Phacoemulsification (phaco) systems that facilitate improved fluidics, lower ultrasound energy, and have more efficient cataract removal may reduce the risk of surgical complications. Patient safety is affected by fluidics (the way fluid is moved through the eye during surgery1). Fluidics impact how easily nuclear fragments are removed from the eye and how much surge is created by occlusion-break at the aspiration tip.1 Fluid dynamics can have a negative effect on the corneal endothelium,2 and enhancement in fluidics can improve anterior chamber stability. For example, post-occlusion vacuum surge, caused by the release of the occluded aspiration system, can cause an abrupt decrease in intraocular pressure (IOP), shallowing of the anterior chamber, iris trauma, and posterior capsule rupture.3 Surge is less dependent on surgical skills than on machine fluidics.4 Therefore, less surge and better chamber stability can improve patient safety.
Technological advances in surgical efficiency reduce the total energy in the eye.5 Pulses of energy with brief cooling periods improve the cutting efficiency. Followability, the ability to attract cataract pieces to the phaco tip, is important for efficient and safe surgery. Improvements in fluidics (attractive forces) and more efficient energy delivery (repulsive force) have led to improvements in followability. Traditionally, surgeons prefer to use a peristaltic pump for holdability (the ability to hold the tissue to the phaco tip) and intraoperative control and to utilize a Venturi pump for followability and improved efficiency (phaco time).6 Advances in tubing and Venturi pump dynamics can potentially improve holdability and control while maintaining followability and efficiency.
Another consideration is the value of a comfortable, ergonomic phacoemulsification experience for surgeons due to the volume of cataract surgeries performed daily. The delicate nature of the procedure requires significant effort and control, which can be taxing on the surgeon. Surgeon-centered equipment design would help alleviate musculoskeletal disorders, which are common in ophthalmologists.7,8
The VERITAS Vision System (Johnson & Johnson Vision, Santa Ana, CA) is a next-generation phacoemulsification system (Figure 1). The VERITAS Vision System was designed to address three critical areas: patient safety, surgeon efficiency, and surgeon comfort. The overall design of the system console chassis allows for a similar workflow to the Whitestar Signature™ Pro System (Johnson & Johnson Vision, Santa Ana, CA) but with modifications featuring improvements in fluidics that include new packs with small bore and dual-durometer aspiration tubing, a new gas-forced infusion (GFI) functionality called Advanced Infusion, and an updated venting feature. The dual-durometer tubing comprises a multi-layer tubing with a stiff inner wall and a softer outer wall, providing improved surge protection and chamber stability without excessively reducing the tube flexibility. The Advanced Infusion feature works with the intravenous pole and adds 22 mmHg (30 cm H2O) pressure to the balanced salt solution bottle. The new fluidics feature includes a continuous irrigation auto-off feature where continuous irrigation will automatically turn on when the foot pedal is pressed, and automatically turn off when the handpiece is removed from the eye. Variable venting relieves vacuum when the foot pedal travels rapidly from the aspiration position to irrigation position. Ergonomic improvements include a new VERITAS Swivel handpiece, new foot pedal, smaller remote control, and larger touch monitor with 40° of side-to-side rotation and 15° of up-and-down tilt (Figure 1).
The VERITAS Swivel handpiece has a lighter and smaller configuration, which includes a rotational aspect of the distal tip that swivels to provide more maneuverability with respect to the proximal handle (Figure 2). The ergonomic design allows surgeon’s fingers to independently control the distal tip without having to move the hand, forearm, elbow, arm, and even shoulder. This could potentially improve surgeon dexterity and reduce surgeon arm-hand fatigue. The VERITAS Swivel handpiece provides both longitudinal and transversal movement.
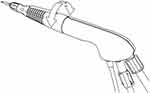 |
Figure 2 The VERITAS Swivel Handpiece. The VERITAS Swivel Handpiece has up to 220° of rotation for ease of maneuvering and surgeon comfort for less fatigue. It is light weight and shorter. |
The foot pedal controls the various operating modes of the instrument. The foot pedal design offers control through the use of increased linearity with the foot pedal movement (Figure 3). The design provides uniform pressure throughout the foot pedal movement, easing foot and leg fatigue. The degrees of movement for each foot pedal position can be selected by the surgeon.
The purpose of this first in-human study was to evaluate the overall clinical performance of the VERITAS Vision System in patients scheduled to undergo cataract extraction and to confirm the overall surgeon acceptability.
Materials and Methods
Study Design and Patients
This was a prospective, open-label study conducted at 2 study sites with 2 investigators in El Salvador and 4 investigators in the United States. The study protocol was reviewed and approved by the Institutional Review Board (Salus IRB; Austin, Texas) and the Government of El Salvador National Office of Medicines Ethical Review Board (Santa Tecla, La Libertad) in accordance with the Declaration of Helsinki and the International Council for Harmonisation Guideline for Good Clinical Practice. All patients provided written informed consent and HIPAA regulations were followed. The study was registered on www.clinicaltrial.gov (NCT04332640).
Included patients were adults ≥22 years of age with cataracts for which cataract extraction and posterior chamber IOL implantation had been planned. Key exclusion criteria were as follows: expected surgical difficulties that may increase the potential for complications, monocular status, pupil or zonular abnormalities, history or current use of alpha-1 antagonists, any condition which could make it unsafe to treat the patient, and pregnant or breast-feeding.
Surgical Procedure and Follow-Up
Each surgeon used his own standard small-incision cataract surgery technique with phacoemulsification and the VERITAS Vision System. Surgeons completed a questionnaire regarding the clinical use of the VERITAS Vision System for each patient immediately following surgery and 1-day postoperative. Immediately after surgery at the operative visit, a biomicroscopic slit-lamp exam was performed to assess corneal clarity and determine the presence or absence of any medical or lens findings, complications, or adverse events (AEs). One-day postoperatively, the treated eye was also examined for ocular symptoms, visual acuity, IOP, corneal clarity, and other slit-lamp findings.
Outcome Measures
The primary endpoint was a questionnaire assessment of overall clinical performance based on surgeon ratings of anterior chamber stability, followability, holdability, phaco (cutting) efficiency, satisfaction with usability of the VERITAS Vision System, and overall satisfaction with the VERITAS Vision System. Other endpoints assessed via questionnaire were satisfaction with the VERITAS Swivel handpiece, satisfaction with the VERITAS Foot Pedal, rating of corneal clarity at 1-day postoperative, and satisfaction with 1-day postoperative clinical results of surgery with the VERITAS Vision System. Surgeon rating was on a scale from 1 to 5, defined as 1 = unsatisfied, 2 = somewhat unsatisfied, 3 = neither satisfied nor unsatisfied, 4 = satisfied, and 5 = very satisfied. The system log files automatically generated by the VERITAS Vision system after completion of each surgery were collected to assess surgical parameters.
Data Analysis
Surgeon acceptability was considered favorable for scores of 4 and 5. The proportion and associated 95% confidence interval for each question rated ≥4 were computed.
The sample size was determined to be minimum 55 eyes and up to 150 eyes to gauge surgeon experience with the new system. A two-sided 95% confidence interval for an expected proportion of 0.95 using the large sample normal approximation extends to 0.058 (ie, a precision of 5.8%) with a sample size of 55 eyes and extends to 0.035 (ie, a precision of 3.5%) with a sample size of 150 eyes.
Results
A total of 115 eyes of 79 patients were enrolled and treated. Of these 115 eyes, 36 patients had bilateral surgeries and 43 patients had unilateral surgeries. The El Salvador site treated 41 patients (58 eyes), and the US site treated 38 patients (57 eyes). A total of 107 of 115 eyes were treated with the VERITAS Swivel handpiece (some surgeries were back to back and the VERITAS Swivel handpiece was being sterilized and not available).
The population consisted of 36 (45.6%) Caucasians and 41 (51.9%) Central/South Americans. The mean age was 65.9 ± 8.9 years, and 72.2% (57/79) of patients were women (Table 1). The cataract was classified as nuclear for 98.3% (113/115) of eyes and cortical for 80.0% (92/115) of eyes, and density was mild (2+) for 45.2% (52/115), moderate (3+) for 37.4% (43/115) and severe (4+) for 7.8% (9/115) of the cataracts.
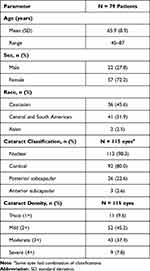 |
Table 1 Demographics and Baseline Characteristics |
Surgical parameters were within the expected ranges for a phaco procedure (Table 2). Namely, the mean effective phaco time was 4.9 ± 7.0 seconds, mean ultrasonic time was 74.2 ± 71.6 seconds, mean average phaco power was 10.0 ± 7.0%, and mean volume of the balanced salt solution was 170.0 ± 86.0 mL.
 |
Table 2 Surgical Parameters |
Primary Outcome
Overall, surgeons were satisfied with the clinical performance regardless of the cataract grade (Figure 4). There were no ratings of 1 or 2 for the primary outcome measures. In all cataract grades, satisfaction with usability and phaco (cutting) efficiency were considered clinically favorable (score 4 or 5) in 100% (95% CI: 0.97, 1.00) of cases. In all cataract grades, satisfaction with followability, holdabillity, and overall satisfaction with the VERITAS Vision System were considered clinically favorable in 99.1% (95% CI: 0.95, 1.00) of cases. Satisfaction with anterior chamber stability was assessed via three similar questions, and 99.1% (95% CI: 0.95, 1.00) of cases had clinically favorable satisfaction with overall anterior chamber stability, responsive fluidics to quickly achieve and maintain chamber stability, and satisfaction with post-occlusion surge.
One case with a mild (2+) cataract was rated 3 (neither satisfied or unsatisfied) for all primary endpoint scores except rating of phaco efficiency, satisfaction with usability, and satisfaction with post-occlusion surge (all rated 4).
Satisfaction with Other Questionnaire Items
Overall, surgeons were satisfied with the ergonomics of the VERITAS Vision System regardless of the cataract grade (Figure 5). In all cataract grades, 97.2% (95% CI: 0.92, 0.99) of cases had clinically favorable satisfaction with the overall VERITAS Swivel handpiece, the weight and size of the VERITAS Swivel handpiece, and the surgeon control of VERITAS Swivel handpiece. In all cataract grades, 100% of cases had clinically favorable satisfaction with the VERITAS Foot Pedal (95% CI: 0.97, 1.00) and the enhanced ergonomics of the VERITAS Foot Pedal (95% CI: 0.95, 1.00).
Surgeons were satisfied with the one-day postoperative clinical results of the VERITAS Vision System regardless of the cataract grade (Figure 6). In all cataract grades, 97.4% (95% CI: 0.83, 0.99) of cases had clinically favorable satisfaction with 1-day postoperative clinical results of surgery (based on the results of uncorrected distance visual acuity [UCDVA], corneal clarity, AE rate, and other relevant clinical assessments). Also, in all cataract grades, 94.8% (95% CI: 0.89, 0.98) of cases had clinically favorable satisfaction with corneal clarity 1-day postoperative. There were 2 eyes with moderate/severe cataract with corneal clarity rated 1 at 1-day postoperative. In both cases, the surgeons reported that the surgical technique, not the device, contributed to the unsatisfied rating of corneal clarity. Clinically favorable satisfaction with corneal clarity at same-day postoperative was reported for 100% (95% CI: 0.97, 1.00) of cases in all cataract grades.
Safety
Operative complications and/or additional surgical procedures were reported for five (4.3%) eyes. Namely, 2 eyes had posterior capsule rupture (one required vitrectomy and was recorded as an AE), and 1 eye had a corneal abrasion. In addition, 1 eye had placement of an iStent device, and 1 eye had a nylon conjunctival suture placed due to excessive balanced salt solution underneath the conjunctiva.
Medical findings, including elevated IOP, were typical of cataract surgery 1-day postoperative. Presence of cells was the most reported postoperative medical finding, which occurred in 52.2% (60/115) of eyes (Table 3). Corneal edema was present in 21.7% (25/115) of eyes (trace corneal edema: 12.2% [14/115], mild corneal edema: 8.7% [10/115], and moderate corneal edema: 0.9% [1/115]). The majority of eyes with edema had moderate-to-severe cataracts. Folds in Descemet’s membrane were reported in 7.8% (9/115) of eyes (3 eyes had mild cataract, 3 eyes had moderate cataract, and 3 eyes had unreported cataract grade).
 |
Table 3 Safety Findings |
All procedures were completed successfully for each case, and there were no patient complications or injuries attributed to the VERITAS System.
Discussion
The VERITAS Vision System features technological advancements in fluidics management and ergonomic improvements for the console, handpiece, foot pedal, and remote control. In this first in-human use study, the satisfaction with anterior chamber stability, post-occlusion surge, followability, holdability, cutting efficiency, usability, and overall satisfaction with the VERITAS Vision System was clinically favorable in ≥99% of cases. Other endpoints such as overall satisfaction with the swivel handpiece, foot pedal, and enhanced ergonomics were clinically favorable in ≥97% of cases. Satisfaction with corneal clarity at same-day postoperative, corneal clarity at 1-day postoperative, and 1-day overall clinical results of surgery with the VERITAS Vision System was clinically favorable in ≥94% of cases. It is well known that phacoemulsification cataract surgery is a very safe surgical procedure, with few patients experiencing serious (sight-threatening) AEs. Accordingly, in this study, medical findings were typical of cataract surgery, and there were 2 cases of posterior capsule rupture.
It would be expected that cases of moderate/severe cataracts would yield less satisfaction with the VERITAS Vision System clinical performance (primary endpoint). However, the results demonstrate that satisfaction with clinical performance was not altered by the cataract density. There was 1 case with a mild (2+) cataract that was rated 3 for most primary endpoint scores. This rating of 3 was reported for the surgeon’s first case. Thus, the rating was likely due to a learning curve, as subsequent cases by this surgeon were rated 4 or 5. This indicates that the rating of 3 in overall satisfaction is not attributed to a severe cataract and demonstrates that the equipment performed well even with hard cataracts as indicated by high satisfaction scores with dense cataracts.
The VERITAS Vision System has a dual pump with access to both peristaltic and Venturi fluidics. Studies suggest that peristaltic fluidics have better control and holdability.6 Also, studies show that Venturi pumps are more efficient, use less total ultrasound, decrease case time, and decrease fluid usage.6 The fluidic properties of the dual-durometer tubing were designed to facilitate improved chamber stability and to reduce surge, which allows surgeons to have improved holdability and followability with both pump systems. In this study, the surgeons frequently switched between modes during various stages of the surgery, and the ability to do so may account for the high satisfaction scores with the VERITAS Vision System. By improving chamber stability in the Venturi mode, the dual-durometer tubing could potentially improve usability of the Venturi setting, which may allow more surgeons to feel confident to use the Venturi mode for certain stages of the procedure, such as fragment and epinucleus removal.
Musculoskeletal disorders afflict ophthalmologists; uncomfortable postures lead to disabling back and neck pain, numbness in the hands and legs, and carpal tunnel syndrome.8,9 Ophthalmologists report that performing the same task repeatedly, working in awkward/cramped positions, working in the same position for prolonged periods, and bending/twisting the back combined with the stressful demands of ocular surgery contribute to musculoskeletal pain as a direct result of their work.7,10 There has been a call for manufacturers to consider ergonomic factors when designing surgical equipment,10,11 The VERITAS Vision System features several innovations to provide a better surgical experience, including ergonomic improvements to the handpiece, foot pedal, remote control, and monitor. The VERITAS Swivel Handpiece has up to 220° of rotation for ease of maneuvering and surgeon comfort for less fatigue. The soft, flexible, light-weight advanced tubing system creates ease of handling and maneuverability. The foot pedal has 11° of total treadle travel and reduced switch actuation force for comfortable procedures. The foot pedal has a metal foot loop for ease of equipment repositioning. The 19-inch monitor has 15° of tilt and 80° of side-to-side rotation for ease of viewing. The user interface has been enhanced for ease of access to menus and case information. These surgeon-centered equipment designs produced clinically favorable satisfaction scores in ≥97% of cases.
A limitation of this study is that there is no comparative group. As Mamalis (2015) points out, the superiority of a particular technology cannot be assessed unless models are used that optimize comparison of all ultrasound modalities. For this reason, we are unable to compare our results with those of other phaco systems.12 However, the patients were selected from multinational, normal cataract populations—a real-world setting, which is a benefit of the study design. However, potentially more complex cases (ie, eyes with significant iris issues, uncontrolled IOP, significant vitreous prolapse, capsular or zonular abnormalities, and risk of persistent bleeding) were excluded. Including complex cases would provide additional data regarding the surgical efficiency of this system and could be the subject of further study. Other future studies focusing on outcome metrics, such as postoperative changes in central corneal thickness, endothelial cell density, and refractive outcomes, are needed to confirm that the excellent surgeon satisfaction seen in this study is associated with positive long-term clinical outcomes.
Conclusion
In conclusion, the new dual-mode phacoemulsification system with dual-durometer tubing, GFI, new swivel handpiece, and ergonomics improvements resulted in a high rate of user satisfaction with clinical performance and ergonomics. The VERITAS Vision System is safe and effective when used according to the indications for use.
Data Sharing Statement
No further data will be shared.
Acknowledgments
Brian Schwam (Johnson & Johnson Surgical Vision) was the medical monitor for the study. Heather S Oliff (Science Consulting Group, LLC) provided writing support.
Funding
The study was funded by Johnson & Johnson Surgical Vision, Inc., Santa Ana, CA, USA.
Disclosure
GQ, DHC, KLW, AK, and RQ are consultants to Johnson & Johnson Surgical Vision, Inc. YW, LJ, DP, and LA are employees of Johnson & Johnson Surgical Vision, Inc. DHC reports grants, personal fees, and non-financial support from Johnson & Johnson Vision during the conduct of the study; grants, personal fees, and non-financial support from Johnson & Johnson Vision; and personal fees from Carl Zeiss Meditec outside the submitted work. AK reports grants from Johnson and Johnson during the conduct of the study; consulting fees and travel arrangements for Veritas Surgical System meeting at their headquarters July 2021 from Johnson and Johnson; grants; and lecture fees for dinner meeting January 2020; and research and grant support for clinical trial ongoing from Glaukos outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Kent C. Phaco: know your fluidics options. Rev Opthalmol. 2017;5:e34.
2. Gonzalez-Salinas R, Garza-Leon M, Saenz-de-Viteri M, Solis SJ, Gulias-Canizo R, Quiroz-Mercado H. Comparison of cumulative dissipated energy delivered by active-fluidic pressure control phacoemulsification system versus gravity-fluidics. Int Ophthalmol. 2018;38(5):1907–1913. doi:10.1007/s10792-017-0674-4
3. Sharif-Kashani P, Fanney D, Injev V. Comparison of occlusion break responses and vacuum rise times of phacoemulsification systems. BMC Ophthalmol. 2014;14:96. doi:10.1186/1471-2415-14-96
4. Georgescu D, Payne M, Olson RJ. Objective measurement of postocclusion surge during phacoemulsification in human eye-bank eyes. Am J Ophthalmol. 2007;143(3):437–440. doi:10.1016/j.ajo.2006.11.017
5. Shah PA, Yoo S. Innovations in phacoemulsification technology. Curr Opin Ophthalmol. 2007;18(1):23–26. doi:10.1097/ICU.0b013e328011f9d0
6. Hida WT, de Medeiros AL, de Araujo Rolim AG, et al. Prospective randomized comparative study between venturi and peristaltic pumps in WhiteStar Signature((R)) phacoemulsification machine. Clin Ophthalmol. 2019;13:49–52. doi:10.2147/OPTH.S177978
7. Kitzmann AS, Fethke NB, Baratz KH, Zimmerman MB, Hackbarth DJ, Gehrs KM. A survey study of musculoskeletal disorders among eye care physicians compared with family medicine physicians. Ophthalmology. 2012;119(2):213–220. doi:10.1016/j.ophtha.2011.06.034
8. Honavar SG. Head up, heels down, posture perfect: ergonomics for an ophthalmologist. Indian J Ophthalmol. 2017;65(8):647–650. doi:10.4103/ijo.IJO_711_17
9. Betsch D, Gjerde H, Lewis D, Tresidder R, Gupta RR. Ergonomics in the operating room: it doesn’t hurt to think about it, but it may hurt not to! Can J Ophthalmol. 2020;55(3 Suppl 1):17–21. doi:10.1016/j.jcjo.2020.04.004
10. Venkatesh R, Kumar S. Back pain in ophthalmology: national survey of Indian ophthalmologists. Indian J Ophthalmol. 2017;65(8):678–682. doi:10.4103/ijo.IJO_344_17
11. Soueid A, Oudit D, Thiagarajah S, Laitung G. The pain of surgery: pain experienced by surgeons while operating. Int j Surg. 2010;8(2):118–120. doi:10.1016/j.ijsu.2009.11.008
12. Mamalis N. Which phacoemulsification modalities are the best? Comparing apples to apples. J Cataract Refract Surg. 2015;41(2):255–256. doi:10.1016/j.jcrs.2015.01.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





