Back to Journals » International Journal of General Medicine » Volume 15
Clinical Outcomes of Left Bundle Branch Area Pacing in Comparison with Right Ventricular Septal Pacing in Patients with High Ventricular Pacing Ratio ≥40%
Authors Liu X , Li W , Zhou X, Huang H, Wang L , Wu M
Received 11 February 2022
Accepted for publication 1 April 2022
Published 19 April 2022 Volume 2022:15 Pages 4175—4185
DOI https://doi.org/10.2147/IJGM.S360522
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Xing Liu,* Wenbin Li,* Xiaolin Zhou, Haobo Huang, Lei Wang, Mingxing Wu
Department of Cardiology, Xiangtan Central Hospital, Xiangtan, Hunan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Mingxing Wu, Department of Cardiology, Xiangtan Central Hospital, Xiangtan, Hunan, People’s Republic of China, Email [email protected]
Background: The aim of the present study is to compare the clinical outcomes between left bundle branch area pacing (LBBaP) and right ventricular septal pacing (RVSP) in patients with percent ventricular pacing (VP%) ≥ 40%.
Methods: Fifty-four patients with VP% ≥ 40% were retrospectively studied, including 33 patients with LBBaP and 21 patients with RVSP. QRS duration (QRSd), interventricular mechanical delay (IVMD) and septal-posterior wall motion delay (SPWMD) were measured to evaluate ventricular synchrony. Heart failure hospitalization (HFH), pacing parameters, and complications were evaluated postoperatively and at follow-ups.
Results: The mean follow-up duration of the study participants was 13.80 ± 4.47 months. In the LBBaP group, no significant differences were noted in paced QRSd, IVMD and SPWMD of the LBBaP capture and intrinsic-conduction modes, but the paced QRSd was narrower (QRSd, 110.88 ± 7.37 vs 132.90 ± 14.78 ms, P< 0.0001) and the IVMD and SPWMD were lower when compared with the RVSP group (IVMD, 28.16 ± 4.76 vs 40.28 ± 6.97 ms, P < 0.0001; SPWMD, 43.68 ± 26.41 vs 97.94 ± 12.77 ms, P < 0.0001). LBBaP was associated with better left ventricular function in comparison with RVSP during follow-ups (LVEDD, 47.09 ± 4.47 vs 51.28 ± 7.58, P = 0.017; LVEF, 64.81± 5.49 vs 60.44 ± 9.28, P = 0.041). Patients with LBBaP had lower occurrences of HFH than patients with RVSP (3.13% vs 27.78%, P = 0.034). Pacing parameters showed no differences between the two groups and remained stable throughout the study period.
Conclusion: The results of this study suggest that LBBaP may be more suitable for patients requiring long-term high ventricular pacing ratio.
Keywords: left bundle branch area pacing, right ventricular septal pacing, high ventricular pacing ratio
Introduction
Right ventricular pacing (RVP) has been served as a treatment option for bradyarrhythmias and heart conduction abnormalities for more than half a century. However, RVP can cause artificial left bundle branch block, and long-term high ratio pacing may lead to uneven ventricular hypertrophy, myocardial disorder and fibrosis, ventricular dyssynchrony and structural changes of the left ventricle, which result in cardiac dyssynchrony, heart failure, mitral regurgitation, atrial fibrillation and so on.1–3 Moreover, a meta-analysis showed that right ventricular septal pacing (RVSP) or right ventricular outflow tract pacing (RVOTP) were not found to be superior to right ventricular apical pacing (RVAP).4 Most studies have shown that high-burden right ventricular (RV) pacing (threshold of ≥40%) is related to the development of heart failure (HF) symptoms and pacing-induced cardiomyopathy (PICM).5–7 Recently, a study with a large real-world cohort of patients (n = 21,202) confirmed that a higher burden RV pacing was associated with an obviously heightened risk of developing HF after short- and long-term follow-ups and it may occur more rapidly than previously anticipated.8 And in a large (n = 823) retrospective study by Kiehl et al.,7 101 patients (12.3%) developed PICM by the end of follow-up (mean 4.3 ± 3.9 years), with left ventricular ejection fraction (LVEF) being 33.7% ± 7.4% in patients with PICM vs 57.6% ± 6.1% in patients without PICM (P< 0.001), and the observed incidence of PICM was consistent with similar previous studies.9,10 Subsequent upgrade to cardiac resynchronization therapy (CRT) was effective clinically,7 but it was expensive and had no tools to recognize patients at high risk of developing HF in the setting of RVP and to judge whether these patients can benefit from CRT based on biventricular pacing (BVP).8
His-bundle pacing (HBP) is a pacing method that can rapidly activate in both ventricles and synchronizes contraction by facilitating conduction through the native His Purkinje system.11 However, there are still some challenges to HBP, including the difficulty in implantation, high capture thresholds, electrode dislocation and lower success rates particularly in patients with infranodal block.3,12 Left bundle branch area pacing (LBBaP) is a novel physiological pacing pattern and overcomes the clinical limitations of traditional HBP.13 Recent studies have demonstrated that LBBaP can generate narrow QRS duration (QRSd) and possesses a low and stable threshold.14,15 Some studies16,17 even have suggested that LBBaP may be superior to BVP-based CRT in improving left ventricular (LV) function. However, the effect of LBBaP and RVP on cardiac function is not clear, especially in patients with high demand RV pacing (threshold of ≥40%). Therefore, the aim of the present study is to assess the clinical effect of LBBaP in patients with percent ventricular pacing (VP%) ≥40%.
Methods
Study Population
This was an observational retrospective single-center study. Pacemaker-indicated patients from February 1, 2019 to May 31, 2020 at Xiangtan Central Hospital were consecutively enrolled, according to 2013 ESC/EHRA Guidelines on cardiac pacing. Only patients with VP% ≥40% confirmed by programming examination about 1 month after pacemaker surgery were included in this study. Patients in this study were divided into two groups based on the pacing site, one group underwent traditional RVSP and the other group underwent LBBaP. The exclusion criteria were as follows: (1) previously implanted with cardiac devices; (2) underwent CRT or implantable cardioverter defibrillator (ICD); (3) myocardial infarction; (4) valvular heart disease or hypertrophic cardiomyopathy; (5) congestive heart failure. The study protocol was approved by the Ethics Committee of Xiangtan Central Hospital. All patients signed written informed consent. All data from registered research were registered in Chinese Clinical Trial Registry (http://www.chictr.org.cn, No. ChiCTR2100046901, registered on 30/05/2021, retrospectively registered). This study was conducted in accordance with the Declaration of Helsinki.
Procedure
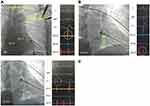
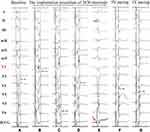
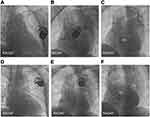
LBBaP was performed following the description in the literature.18–20 One 4-pole electrophysiology catheter (Synaptic Medical) was sent into the RV through the femoral vein, and right bundle branch (RBB) potential or far-field His bundle (HB) potential was recorded as a marker to find the HB region21 (Figure 1A). At the same time, we performed axillary vein angiography via cubital vein in all patients with a venous approach (Figure 1A). Utilizing the RBB potential and 4-pole electrophsiology catheter image location as markers, a fixed-curve sheath (C315 His, Medtronic Inc.) with the Select Secure pacing lead (model 3830, Medtronic Inc., Minneapolis, MN, USA) was introduced into the HB region via the left axillary vein to record HB potential (Figure 1B). Then, the delivery sheath with the 3830 lead was moved approximately 1–2 cm toward the RV septum along the line between the HB site and RV apex in right anterior oblique (RAO) 30° fluoroscopic view to reach the initial site for LBBaP (Figure 1B). At this site, intrinsic rhythm in LBBaP was shown in Figure 2A and unipolar paced QRS morphology usually appeared as a “W” pattern with a notch at the nadir of the QRS in lead V1 (Figure 2B). Next, the 3830 lead tip was adjusted in the vertical direction of the interventricular septum (IVS) by rotating the sheath counterclockwise appropriately and was screwed in with 8–10 clockwise rotation. As the lead tip was gradually screwed into the IVS, notch in the nadir in lead V1 moved back gradually until the form of a QR or rSR morphology appeared (Figure 2C and D). Moreover, premature ventricular contraction (PVC) emerging from endometrial surface of the left ventricular septum (Figure 1C) may occasionally be recorded during the lead rotation or LBB potential (Figure 2E) may be recorded at the final lead location. It is important to pay close attention to the variation of output threshold and pacing impedance during lead implantation in order to avoid ventricular septum perforation caused by the lead. Finally, according to previously published criteria,18–20 successful LBBaP was characterized by the paced QRS morphology in V1 lead showing right bundle branch block (RBBB) or near-normal QRS complex (less than 130 ms). The stimulus to peak left ventricular activation time (S-PLVAT) is defined as the duration between the ventricular stimulation signal and R spike in lead V5. The S-PLVAT remained shortest and constant at different testing outputs (Figure 2F and G). Postoperative images of LBBaP are shown in Figure 3A and B and the penetration depth in the IVS could be estimated by fluoroscopic imaging with contrast injection (Figure 3C).
RVSP: Under the support of the C315 S10 sheath (Medtronic Inc., Minneapolis, MN), the Select Secure pacing lead (model 3830, Medtronic Inc., Minneapolis, MN, USA) was screwed towards the IVS vertically with 4–6 clockwise rotation. Postoperative images of RVSP and fluoroscopic imaging with contrast injection are shown in Figure 3.
Data Collecting and Follow-Up
Demographic data and medical history were documented. S-PLVAT was measured from pacing stimulus to the peak of R wave in lead V5 at low and high outputs. LBB potential was recorded during the implantation procedure. QRSd was documented in all patients before and after implantation and at last follow-up. We recorded total procedure duration (defined as the time from sterilization to the end of the operation), fluoroscopy dose and imaging data. All patients’ follow-up started one month after surgery in the device clinic, then continued at a 6–8 months interval, and ended with the last follow-up on February 5, 2021.
Echocardiographic Parameters
Transthoracic echocardiography was performed at baseline and several months (6–24) follow-up. LV end-diastolic dimension (LVEDD) and left ventricular ejection fraction (LVEF) (by Simpson’s method) were measured. Cardiac synchrony was evaluated by measurements of the interventricular mechanical delay (IVMD) and the septal-posterior wall motion delay (SPWMD) by one experienced specialist. IVMD was measured by echocardiography as the interval between the start of the QRS and the start of the aortic and pulmonary ejection. If the IVMD was greater than 40 ms, the dyssynchrony between the left and right ventricle was indicated. SPWMD was measured by M-mode as the distance between the first maximum systolic inward motion of the septum and the maximum inward motion of the posterior wall. If the SPWMD was greater than 130 ms, intraventricular dyssynchrony was indicated.
Programming
The pacing thresholds, R-wave amplitudes and impedances were collected at implantation and during follow-ups. ECG-gated AV optimization was done in all patients before discharge, which corrected BBB and yielded narrower QRSd. Pacing parameters were measured 1 month and several months (6–24) postoperatively.
Safety Endpoints
Procedure-related complications including hematoma, pericardial effusion, pocket or lead infection, and lead dislodgment were recorded at any time during follow-up. Heart failure hospitalization (HFH) was tracked at follow-ups. HFH was defined as an outpatient or inpatient hospitalization in which the patient showed signs and symptoms of heart failure.
Statistical Analysis
Data were expressed as means ± standard deviations for continuous variables and percentages for categorical variables. A chi-square test or Fisher’s exact probability test was used for comparison of categorical variables as appropriate. Differences among groups were analyzed by Student’s t-tests or one-way ANOVA, and LSD method was performed for pairwise comparison. All statistical analyses were performed using commercially available software (IBM SPSS Statistics 26), and the P value reported was two-sided and a value of less than 0.05 was considered statistically significant.
Results
Baseline Outcomes
A total of 91 patients received LBBaP or RVSP in Xiangtan Central Hospital from February 2019 to May 2020. Based on inclusion and exclusion criteria, 54 patients with VP% ≥40% were included in the study, of whom 33 patients (age 73.67 ± 11.87 years; 63.6% male) underwent LBBaP, of whom 21 patients (age 68.14 ± 11.66 years; 52.4% male) received RVSP. The mean follow-up time was similar in the LBBaP group and the RVSP group (13.94 ± 4.44 months vs 13.56 ± 4.64 months, P = 0.775). During follow-up, one patient in the LBBaP group who suffered acute myocardial infarction was excluded from the study and three patients in the RVSP group were excluded due to death (one patient suffered from a traffic accident, one from a cerebral hemorrhage, and another died from an unknown cause). The proportion of patients who had AF with slow ventricular rate was lower in the LBBP group than in the RVSP group (0% vs 19.1%, P = 0.019). Other baseline clinical characteristics amongst the two groups showed no statistical difference (Table 1).
 |
Table 1 Baseline Patient Characteristics and Procedure Outcomes |
Implant Outcomes
LBB potential was recorded in 23 of 33 (69.7%) patients who underwent LBBaP. The mean S-PLVAT was 71.18 ± 5.23 ms and that was similar in patients in whom LBB potential could be recorded and patients in whom it could not be recorded in the LBBaP group (72.13 ± 4.56 vs 75.60 ± 6.10 ms, P = 0.079). Compared with the RVSP group, total procedure duration was longer (120.82 ± 11.46 vs 93.19 ± 8.7 minutes, P<0.0001) and fluoroscopic dose was higher (79.36 ± 8.79 vs 53.38 ± 9.02 mGy, P< 0.0001) in the LBBaP group (Table 1).
During the follow-up period, there were 6 HFH events, there was a downward trend toward HFH in patients who underwent LBBaP (1/32, 3.13%) compared with patients who underwent RVSP (5/18, 27.78%; P = 0.034).
Electrocardiogram and Echocardiography Outcomes
Table 2 showed electrocardiogram and echocardiogram outcomes of the patients in both groups at baseline and last follow-up. The QRSd was similar in the LBBaP group and the RVSP group at baseline (113.58 ± 21.22 ms vs 113.48 ± 21.80 ms, P = 0.987). In the LBBaP group, no significant differences were noted in paced QRSd of the LBBaP capture and intrinsic-conduction modes (P = 0.493), but the paced QRSd was shorter (QRSd, 110.88 ± 7.37 vs 132.90 ± 14.78 ms, P <0.0001) when compared with the RVSP group, and the difference persisted during follow-ups. The paced QRSd was 109.39 ± 6.61 ms during LBBaP in patients with observed LBB potential and 114.30 ± 8.23 ms during LBBaP in patients without LBB potential observed (P = 0.174). In addition, we observed LBBB patients in the LBBaP group (n = 5) and discovered that the paced QRSd was obviously shorter than their baseline level (120.00 ± 1.58 vs 152.40 ± 6.34 ms, P = 0.001), suggesting that the LBBB was corrected.
 |
Table 2 Comparison of QRSd and Echocardiographic Parameters Between the LBBaP and RVSP Groups Before and After Pacemaker Implantation |
IVMD pre-procedure was similar in the LBBaP group and the RVSP group (30.82 ± 10.77 ms vs 30.05 ± 12.82 ms, P = 0.813). IVMD was less in LBBaP group than RVSP group (28.16 ± 4.76 vs 40.28 ± 6.97 ms, P <0.0001) at last follow-up, which showed a good interventricular synchrony caused by LBBaP. SPWMD pre-procedure had no difference in both two groups (44.27 ± 33.96 vs 46.95 ± 36.36, P = 0.784), but it was less in LBBaP group at last follow-up (43.68 ± 26.41 vs 97.94 ± 12.77 ms, P < 0.0001), which suggested a good intraventricular synchrony resulting from LBBaP.
LVEDD (47.48 ± 4.69 vs 50.62 ± 7.10, P = 0.056) and LVEF (64.97 ± 6.15 vs 63.57 ± 9.98, P = 0.526) preoperatively had no difference in both two groups. However, LVEDD was shorter (47.09 ± 4.47 vs 51.28 ± 7.58, P = 0.017) and the LVEF was higher (64.81 ± 5.49 vs 60.44 ± 9.28, P = 0.041) in the LBBaP group at last follow-up when compared with RVSP group.
Pacing Parameters and Complications
Table 3 and Figure 4 respectively show the results and variation trends of pacing parameters in the two groups at implantation, 1 month, and last follow-up. Pacing capture threshold, R-wave amplitude and impedance were similar in both the groups at implantation, 1 month and last follow-up, and remained stable. Interestingly, we found that the R-wave amplitude increased and impedance dropped in both groups with the delay of follow-up time.
 |
Table 3 Changes in Pacing Parameters at Follow-Ups |
One patient in LBBaP group suffered a complicated acute perforation of the LV septum during the lead implantation, and finally the reimplantation was successful. Other procedure-related complications including hematoma, pericardial effusion, pocket or lead infection, and lead removal in either groups were not observed at any time during follow-up.
Discussion
This study revealed that the paced QRSd was clearly shorter in the LBBaP group in comparison with the RVSP group and it was similar to intrinsic conduction QRSd, which was consistent with other studies.22,23 Meanwhile, some studies24,25 have shown that QRSd is a representation of ventricular activation time, and it has been recognized as an alternative indicator for assessing ventricular electrical synchrony. Therefore, the ventricular electrical synchronization induced by LBBaP was superior to that induced by RVSP. The possible reason is that the left ventricular His-Purkinje system was paced directly during LBBaP while RVSP caused an artificial left bundle branch block. Meanwhile, some studies have shown that a longer QRS duration is associated with a higher risk of developing HF or PICM.10,26
Secondly, studies24,27 have shown that good ventricular mechanical synchronization is associated with good ventricular electrical synchronization caused by a narrow QRSd. Indeed, recent studies have demonstrated that LBPaP produces better LV mechanical synchrony than RVSP,14,23,28 which is consistent with the results of our recently published meta-analysis.29 In our study, IVMD and SPWMD were lower in the LBBaP group in comparison with the RVSP group, further suggesting that LBBaP could generate better ventricular mechanical synchrony than RVSP. Therefore, as a novel physiological pacing mode, LBBaP can produce good ventricular electrical-mechanical synchronization that is similar to the result of the preoperative native conduction system and better than that of RVSP.
Additionally, our study found that the incidence of pacing-induced LV dysfunction was lower in the LBBaP group compared with the RVSP group at the middle-term follow-up because LBBaP could generate better LV electrical-mechanical synchrony than RVSP. The result was consistent with those of Das et al.’s study21 and Zhang et al.’s study.30 However, LVEDD and LVEF were similar in both groups at follow-up in another two studies,23,28 indicating that LBBaP and RVSP show no differences in postoperative LV function. This difference may be explained by patients with a higher percentage of bundle branch block (BBB) and RV pacing being enrolled into Das et al.’s study21 and Zhang et al.’s study,30 respectively, and longer follow-up in the two studies. In Das et al.’s study,21 31 (62%) patients with BBB were enrolled. BBB occurred in 13 of 22 (59.1%) patients in the LBBaP group, and was corrected in 11 of 13 (84.6%) patients, suggesting a significant improvement in cardiac electrical synchronization. In Zhang et al.’s study,30 only AVB patients were included, resulting in a high rate of RV pacing in the RVSP and LBBaP groups (94.86 ± 1.56 vs 95.47 ± 1.22, P = 0.768). In the present study, Cum% ventricular pacing (VP) was lower in the RVSP group compared with the LBBaP group (84.25 ± 21.3 vs 98.52 ± 2.59, P < 0.001), which was the main reason for the high proportion of AVB (90.9%) in the LBBaP group. When only complete AVB patients (13 in the RVSP group and 30 in the LBBaP group) were enrolled in the present study, Cum% VP was similar between the two groups (97.08 ± 3.59 vs 98.57 ± 2.62, P = 0.135). Likewise, LVEDD was shorter (47.07 ± 4.69 mm vs 54.64 ± 6.41 mm, P <0.001) and the LVEF was higher (65.55 ± 4.82 vs 57.45 ± 10.62, P = 0.002) in the LBBaP group compared with the RVSP group at last follow-up, which was consistent with the result of Zhang et al.’s study.30 Finally, our study also found that LBBaP had lower occurrences of HFH than with RVSP in patients with high ventricular pacing ratio ≥40%. Recently, two studies30,31 showed that LBBaP could reduce the incidence of HFH in comparison with RVP in patients with atrioventricular block, and one study31 also indicated that a high burden of VP >40% was a risk factor for HF events. The mechanism for the reduction of pacing-induced cardiac dysfunction and prevention of HFH may be related to the maintenance of electrical-mechanical synchrony during LBBaP. It should be noted that recent non-randomized clinical trials have indicated that LBBaP may be superior to BVP-based CRT in improving LV function for the LBBB patients with heart failure.16,17,32 Therefore, we believe that LBBaP may be more suitable for patients with indications for pacemaker implantation and requiring long-term high ventricular pacing ratio, or CRT patients.
This study showed that pacing parameters were not significantly different between the two groups at implantation, 1 month after implantation and at last follow-up, and remained stable throughout the study period. The R-wave amplitude and pacing impedance of the two groups were gradually increasing and decreasing respectively with the prolongation of follow-up time. This phenomenon may be related to myocardial edema caused by lead implantation. As the myocardial edema decreased after implantation, the electrical conduction between the lead and the myocardium might have increased, leading to this trend. Other studies have also demonstrated that LBBaP has good and stable pacing parameters.14,15,22,30
Procedure-related complications including pocket hematoma, pericardial effusion, pocket or lead infection, and lead removal in both the groups were not observed at any time during the follow-up. But one patient in the LBBaP group suffered acute perforation of LV septum accompanied with a sudden loss of capture and a reduction in lead impedance from 780Ω to 350Ω, and the reimplantation was successful in the end. One of the main challenges for LBBaP is how to insert the lead deep enough into the IVS without causing septal perforation. There are several ways to monitor lead depth and avoid perforation, such as fulcrum sign, changes in the QRS notch in V1 lead, sheath angiography, impedance monitoring, observing fixation beats33 and pacing from the ring electrode. Recent study found that cTnT elevation was more significant in LBBaP within 2 days post-procedure compared with RVSP,15 and another study showed that the number of attempts was an independent risk factor correlated with the myocardial damage.34 So, during LBBaP surgery, special attention should be paid to injury to ventricular septal coronary artery and excessive attempts avoided.
Nevertheless, this research study also has limitations. First, the sample size is relatively small as a single-center, retrospective and observational study. Second, although RV pacing ratio of the two groups was different in this study, which may have contributed to the biased results, RV pacing percentages of the two groups were similar and the same results could be obtained when only complete AVB patients were enrolled. Third, the clinical homogeneity of patients could not be guaranteed between LBBaP and RVSP, and there is selection bias in choosing two surgical procedures. Finally, considering the complexity of HF etiology, the strict balance of etiologies of HF other than pacing ratio ≥40% for the included patients, coupled with sustained and longer follow-up, make the clinical outcomes of HFH more reliable and may provide more solid evidence for the superiority of LBBaP. Thus, randomized multicenter prospective studies with larger sample sizes and longer follow-up periods will be beneficial for evaluating the cardiac function and HFH of patients with high-burden ventricular pacing receiving LBBaP and further confirming our research results.
Conclusions
LBBaP is a new physiologic ventricular pacing pattern, which leads to ventricular electrical-mechanical synchronization. LBBaP is associated with reduction in the occurrence of pacing-induced LV dysfunction and HFH in patients requiring a high burden of VP compared with RVSP. The results of this study suggest that LBBaP may be more suitable for patients requiring long-term high ventricular pacing ratio.
Abbreviations
HB, His bundle; IEGM, intracardiac electrogram; IVS, interventricular septum; LBB, left bundle branch; LBBaP, left bundle branch area pacing; PVC, premature ventricular contraction; RBB, right bundle branch; RV, right ventricular; RVSP, right ventricular septal pacing; S-PLVAT, stimulus to peak left ventricular activation time.
Data Sharing Statement
The data in this study are available from the corresponding author (Mingxing Wu) on a reasonable request.
Funding
This study was supported by Hunan Province Science and Technology Department in China (2018SK52104).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Nahlawi M, Waligora M, Spies SM, Bonow RO, Kadish AH, Goldberger JJ. Left ventricular function during and after right ventricular pacing. J Am Coll Cardiol. 2004;44(9):1883–1888.
2. Thambo JB, Bordachar P, Garrigue S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation. 2004;110(25):3766–3772.
3. Abdelrahman M, Subzposh FA, Beer D, et al. Clinical Outcomes of His Bundle Pacing Compared to Right Ventricular Pacing. J Am Coll Cardiol. 2018;71(20):2319–2330.
4. Zografos TA, Siontis KC, Jastrzebski M, et al. Apical vs. non-apical right ventricular pacing in cardiac resynchronization therapy: a meta-analysis. Europace. 2015;17(8):1259–1266.
5. Sweeney MO, Hellkamp AS, Ellenbogen KA, et al. Adverse effect of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duration in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation. 2003;107(23):2932–2937.
6. Sharma AD, Rizo-Patron C, Hallstrom AP, et al. Percent right ventricular pacing predicts outcomes in the DAVID trial. Heart Rhythm. 2005;2(8):830–834.
7. Kiehl EL, Makki T, Kumar R, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm. 2016;13(12):2272–2278.
8. Merchant FM, Hoskins MH, Musat DL, et al. Incidence and Time Course for Developing Heart Failure With High-Burden Right Ventricular Pacing. Circ Cardiovasc Qual Outcomes. 2017;10(6):65.
9. Dreger H, Maethner K, Bondke H, Baumann G, Melzer C. Pacing-induced cardiomyopathy in patients with right ventricular stimulation for >15 years. Europace. 2012;14(2):238–242.
10. Khurshid S, Epstein AE, Verdino RJ, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy. Heart Rhythm. 2014;11(9):1619–1625.
11. Arnold AD, Shun-Shin MJ, Keene D, et al. His Resynchronization Versus Biventricular Pacing in Patients With Heart Failure and Left Bundle Branch Block. J Am Coll Cardiol. 2018;72(24):3112–3122.
12. Upadhyay GA, Cherian T, Shatz DY, et al. Intracardiac Delineation of Septal Conduction in Left Bundle-Branch Block Patterns. Circulation. 2019;139(16):1876–1888.
13. Huang W, Su L, Wu S, et al. A Novel Pacing Strategy With Low and Stable Output: pacing the Left Bundle Branch Immediately Beyond the Conduction Block. Can J Cardiol. 2017;33(12):
14. Hou X, Qian Z, Wang Y, et al. Feasibility and cardiac synchrony of permanent left bundle branch pacing through the interventricular septum. Europace. 2019;21(11):1694–1702.
15. Chen X, Jin Q, Bai J, et al. The feasibility and safety of left bundle branch pacing vs. right ventricular pacing after mid-long-term follow-up: a single-centre experience. Europace. 2020;22(Suppl_2):ii36–ii44.
16. Li X, Qiu C, Xie R, et al. Left bundle branch area pacing delivery of cardiac resynchronization therapy and comparison with biventricular pacing. ESC Heart Fail. 2020;7(4):1711–1722.
17. Wu S, Su L, Vijayaraman P, et al. Left Bundle Branch Pacing for Cardiac Resynchronization Therapy: nonrandomized On-Treatment Comparison With His Bundle Pacing and Biventricular Pacing. Can J Cardiol. 2021;37(2):319–328.
18. Huang W, Chen X, Su L, Wu S, Xia X, Vijayaraman P. A beginner’s guide to permanent left bundle branch pacing. Heart Rhythm. 2019;16(12):1791–1796.
19. Li X, Li H, Ma W, et al. Permanent left bundle branch area pacing for atrioventricular block: feasibility, safety, and acute effect. Heart Rhythm. 2019;16(12):1766–1773.
20. Vijayaraman P, Subzposh FA, Naperkowski A, et al. Prospective evaluation of feasibility and electrophysiologic and echocardiographic characteristics of left bundle branch area pacing. Heart Rhythm. 2019;16(12):1774–1782.
21. Das A, Islam SS, Pathak SK, et al. Left bundle branch area. A new site for physiological pacing: a pilot study. Heart Vessels. 2020;35(11):1563–1572.
22. Chen K, Li Y, Dai Y, et al. Comparison of electrocardiogram characteristics and pacing parameters between left bundle branch pacing and right ventricular pacing in patients receiving pacemaker therapy. Europace. 2019;21(4):673–680.
23. Sun Z, Di B, Gao H, Lan D, Peng H. Assessment of ventricular mechanical synchronization after left bundle branch pacing using 2-D speckle tracking echocardiography. Clin Cardiol. 2020;43(12):1562–1572.
24. Sandhu R, Bahler RC. Prevalence of QRS prolongation in a community hospital cohort of patients with heart failure and its relation to left ventricular systolic dysfunction. Am J Cardiol. 2004;93(2):244–246.
25. Pang BJ, Kumar S, Tacey MA, Mond HG. Capturing the His-Purkinje system is not possible from conventional right ventricular apical and nonapical pacing sites. Pacing Clin Electrophysiol. 2014;37(6):724–730.
26. Lee WC, Fang HY, Chen HC, et al. Post-pacemaker implant QRS duration and heart failure admission in patients with sick sinus syndrome and complete atrioventricular block. ESC Heart Fail. 2019;6(4):686–693.
27. Kirk JA, Kass DA. Electromechanical dyssynchrony and resynchronization of the failing heart. Circ Res. 2013;113(6):765–776.
28. Cai B, Huang X, Li L, et al. Evaluation of cardiac synchrony in left bundle branch pacing: insights from echocardiographic research. J Cardiovasc Electrophysiol. 2020;31(2):560–569.
29. Liu X, Li W, Wang L, Tian S, Zhou X, Wu M. Safety and efficacy of left bundle branch pacing in comparison with conventional right ventricular pacing: a systematic review and meta-analysis. Medicine. 2021;100(27):e26560.
30. Zhang S, Guo J, Tao A, Zhang B, Bao Z, Zhang G. Clinical outcomes of left bundle branch pacing compared to right ventricular apical pacing in patients with atrioventricular block. Clin Cardiol. 2021;44(4):481–487.
31. Li X, Zhang J, Qiu C, et al. Clinical Outcomes in Patients With Left Bundle Branch Area Pacing vs. Right Ventricular Pacing for Atrioventricular Block. Front Cardiovasc Med. 2021;8:685253.
32. Wang Y, Gu K, Qian Z, et al. The efficacy of left bundle branch area pacing compared with biventricular pacing in patients with heart failure: a matched case-control study. J Cardiovasc Electrophysiol. 2020;31(8):2068–2077.
33. Jastrzębski M, Kiełbasa G, Moskal P, et al. Fixation beats: a novel marker for reaching the left bundle branch area during deep septal lead implantation. Heart Rhythm. 2021;18(4):562–569.
34. Wu Y, Chen Y, Chen M, et al. Quantification of Acute Myocardial Damage Secondary to Implantation of Electrodes for the Left Bundle Branch Area Pacing. Rev Invest Clin. 2020;1:34.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.