Back to Journals » Orthopedic Research and Reviews » Volume 14
Clinical Outcomes of Delayed Osteoarticular Tuberculosis: A Review of 30 Cases
Authors Kamal AF , Oktari PR, Kurniawan A, Kodrat E, Mumpuni NA
Received 15 April 2022
Accepted for publication 26 July 2022
Published 20 October 2022 Volume 2022:14 Pages 351—363
DOI https://doi.org/10.2147/ORR.S366294
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Clark Hung
Achmad Fauzi Kamal,1 Prima Rizky Oktari,1 Aryadi Kurniawan,1 Evelina Kodrat,2 Nadia Asmirtania Mumpuni3
1Department of Orthopedic and Traumatology, Faculty of Medicine, Universitas Indonesia, Dr Cipto Mangunkusumo General Hospital, Jakarta, Indonesia; 2Department of Anatomical Pathology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia; 3Pelni General Hospital, Jakarta, Indonesia
Correspondence: Prima Rizky Oktari, Department of Orthopedic and Traumatology, Faculty of Medicine, Universitas Indonesia, Dr Cipto Mangunkusumo General Hospital, Jl Diponegoro No. 71, Central of Jakarta, Jakarta, Indonesia, Email [email protected]
Purpose: The lack of knowledge regarding osteoarticular tuberculosis (TB) cases in Indonesia leads to delayed and chronic conditions. This study aims to evaluate clinical outcomes of patients with osteoarticular TB.
Materials and Methods: Thirty osteoarticular cases were retrospectively analyzed, with a focus on non-immunocompromised patients without spine involvement. Chemotherapy length, operative treatment method, and infection recurrence were evaluated.
Results: The majority (60%) of patients were aged between 19 to 49 years. The most common complaint was painful swelling, particularly during physical activity. Weight-bearing joints, such as the hips, knees, and ankles, were the most affected. Laboratory results showed over half of the patients had anemia, 96% had elevated erythrocyte sedimentation rate (ESR), and 76% had elevated C-reactive protein (CRP) levels. Radiological findings varied, with lytic lesions, abscesses, and joint destruction observed. All patients presented with pathognomonic histological tubercle appearances, with caseous necrosis, lymphocytes, and Langhans giant cells present. Twenty-nine cases were treated with anti-TB drugs for 12 months, while one recurrent case received the drugs for 24 months. All patients underwent surgery to gain local infection control.
Conclusion: Osteoarticular TB is a common manifestation of extrapulmonary TB and must not be overlooked. Early detection of osteoarticular TB may prevent limb morbidity. Although anti-TB drugs are the primary treatment for osteoarticular TB, in some cases, surgery is required to establish a diagnosis and gain local infection control.
Keywords: osteoarticular TB, tuberculosis, extrapulmonary TB, caseous necrosis, Jakarta
Introduction
Indonesia has the third-highest prevalence of tuberculosis (TB) after India and China. In Indonesia, pulmonary TB is the second-highest cause of mortality among infectious diseases. Three-quarters of the TB prevalence in Indonesia is among those aged between 14 to 49 years old. Osteoarticular TB is an uncommon form of extrapulmonary TB (EPTB); it comprises 1–6% of all TB cases and 10–15% of all EPTB cases. The most frequent sites of osteoarticular TB infection are the spine, hip, and knee.1,2
Osteoarticular TB diagnosis is often delayed. Diagnostic delayed means that there is time interval between onset of symptoms and confirmation of TB to the patients. The cut-off point was 1 months (4 weeks).3,4 EPTB results from the hematogenous and lymphatic spread of M. tb bacilli. Tuberculous osteomyelitis and arthritis generally arise due to the reactivation of bacilli lodged in the bone during the original primary infection. The predilection of the bacillus for large joints is due to the rich vascular supply in the growth plates of the long bones. Tuberculous arthritis is believed to result from the extension of the initial infection of the bone to the joint. In the Netherlands and England, concomitant pulmonary TB has been reported in 29% and 15% of osteoarticular TB patients, respectively.5 In Karachi, Ali et al reported concurrent pulmonary TB in nearly 50% of osteoarticular TB cases.6 However, only 6.9% of 5337 osteoarticular TB cases had concurrent pulmonary TB. Similarly, a study performed in Turkey and Denmark also found low rates of concomitant pulmonary TB in osteoarticular TB cases.5 Despite its prevalence in other countries, osteoarticular TB is still underdiagnosed in Indonesia.
In this study, 30 osteoarticular TB cases from two government hospitals in Jakarta, Indonesia, were retrospectively analyzed. This study aims to provide clinical and radiologic-histopathologic descriptions of delayed osteoarticular TB, its management, and outcomes.
Materials and Methods
This study was conducted at two government general hospitals in Jakarta, Indonesia, from July 2019 to December 2020. This study was approved by the ethical committee from Dr. Cipto Mangunkusumo Hospital (approval number 19121451). The ethical principles in this study are in accordance with the contents of the Declaration of Helsinki. The study was performed in accordance with relevant guidelines and regulations and in accordance with the ethical protocol of the hospital and institution. Patients with bone and/or joint TB were included, regardless of age or sex. Immunocompromised patients and those with spinal TB were excluded regarding its difference in prognosis. Delayed osteoarticular tuberculosis defined as the delay in diagnosis of patients for at least more than a month.4
Written informed consent for inclusion in this study was given by all patients or their care provider. A detailed history regarding general biodata, symptom presentations, prior treatments, concurrent illnesses, history of TB, and treatment history with anti-TB drugs, was taken. General and local examination of the involved joint or bone was carried out, with information regarding the site, swelling, tenderness, sinus discharge or ulcer, and joint mobility after treatment collected.
Information on hemoglobin levels, leucocyte counts, differential leukocyte counts, erythrocyte sedimentation rates (ESR), anteroposterior and lateral radiographs, and histopathological examination of the involved joint or bone was collected for each participant.
Magnetic resonance imaging (MRI) or computed tomography (CT) scans were performed when considered necessary. All patients underwent tissue debridement, and biopsy cultures were taken. A diagnosis of osteoarticular TB was established based on the pathological anatomical analysis of each tissue sample. Follow-up period was 2 years after patient intervention.
Results
This is the first comprehensive review of osteoarticular TB cases in Indonesia. There were 30 osteoarticular TB patients included in the study. Sixteen were male, and 14 were female. The patients’ ages ranged from 2 to 72 years old (Table 1). The majority of patients were aged between 19 to 49 years (63%), followed by less than 18 years old (27%), between 50 to 65 years old (7%), and more than 65 years old (3%). In our patients, all patients came months after their first symptoms appear due to financial problems. Also, majority of the patients came from places which were inaccessible to healthcare facilities and relatively low education level.
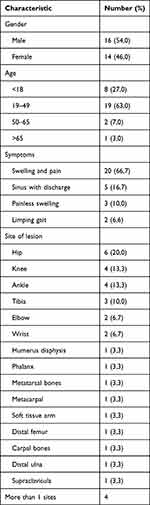 |
Table 1 Demographic and Clinical Characteristics of the Patients |
Functional outcomes were measured based on patient symptoms and signs. Patient complaints included painful swelling, swelling without pain, sinus with discharge, ulcers, and limping gaits (Table 1). Local pain worsened over time and was accompanied by swelling in 66.7% of the patients. Five patients complained about sinus and active discharge, and 2 patients complained about limping gaits due to extremity discrepancies related to hip region lesions. Symptom duration ranged from 2 to 6 months.
All patients were treated with four standard anti-TB drugs for two months: isoniazid (H), ethambutol (E), rifampicin (R), and pyrazinamide (Z). Following this, patients were treated with H and R only for at least a further 10 months. The involved bones or joints were debrided by 2 senior orthopedic surgeons. The post-debridement tissue samples were sent to histopathology for analysis.
Twenty-nine of the patients completely recovered within 4.23 months following treatment with the anti-TB drugs and surgical debridement. Surgical debridement was performed during a course of anti-TB drugs. One patient who had osteomyelitis TB in the proximal tibia had a more difficult infection to treat and experienced sinus with discharge that was aggravated by lymphadenopathy in the neck region. However, following 3 repeat debridements, treatment with anti-TB drugs for 24 months, and the addition of streptomycin injections for 2 months, the patient recovered from their osteomyelitis TB and lymphadenopathy.
Operative wounds healed 3–4 weeks following debridement. In 2 patients, discharge persisted and required a repeat debridement.
The clinical complaint, wound, functional status, and patient activity related to the affected limb were evaluated in all patients.
The histopathological results of all patients were consistent with TB. Pathological findings revealed tubercles, which are characteristic of TB lesions. The tubercle consist of central casseous necrosis surrounded by epitheloid cells, Langhans giant cells and lymphocytes.
Discussion
According to the World Health Organization (WHO), EPTB is defined as an infection by Mycobacterium tuberculosis (M. tb) that affects tissues and organs outside the pulmonary parenchyma. Extrapulmonary TB represents 20–25% of all TB cases. Osteoarticular TB in Indonesia is not well understood, and consequently, cases of osteoarticular TB are often delayed in diagnosis. Pulmonary TB is the third most common cause of mortality due to infection in Indonesia.2 The hospital used in this study reported numerous delayed diagnoses of osteoarticular TB in patients. Several factors are thought to contribute to this, both from practitioners and patients. For practitioners, non-specific presenting symptoms and a lack of familiarity with the disease may contribute. For patients, a lack of knowledge, ignorance, and socioeconomic conditions may contribute.
In this study, most of the patients were aged between 18–49 years old, followed by those younger than 18 and then those older than 49. This is in line with the ages reported by Ali et al, who reported that most osteoarticular TB patients are 21 to 40 years old, while older individuals are less likely to develop it, showing a bimodal pattern of TB dispersion.6 In developing countries, such as Indonesia, these lesions are more common in children and young adults, while in developed countries, those over 55 years old are most commonly affected.7
Just over half of the patients in the current study were male. Other studies showed no significant difference in gender in osteoarticular TB cases.6,7 In the study by Ali et al, 66.6% of osteoarticular TB cases were female.6 Procopie showed that there is no relationship between gender and TB.
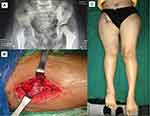
In the current study, most of the patients complained of chronic, dull pain and swelling that was aggravated by movement. Others complained of painless chronic swelling, while two patients complained of chronic wounds. Two cases were children with TB coxitis who complained of a limping gait. In these cases, chronic joint destruction and epiphyseal plate destruction resulted in leg length discrepancies (Figure 1).
Twenty-one cases in the current study involved the joint (Table 1), with 14 involving a weight-bearing joint (hip, knee, ankle). Those that did not involve a weight-bearing joint involved the elbow, wrist, and carpal joints. The remaining nine cases involved bones, including the diaphyseal tibia, humerus, phalanges, metatarsals, and metacarpals. Two cases involved the metaphyseal ulna and femur. Similar to the results of the current study, Tuli reported that the hips, knees, feet, elbows, hands, and shoulders were the most commonly affected sites,8 while Ali et al found that the knees and hips were most affected (in 15.5% and 13.63% of 66 cases, respectively), followed by the ankles and elbows.6
An osteoarticular lesion involves hematogenous spread from the primary infected organ, such as the lungs, lymph glands, or other viscera, where the infection may be active or quiescent. As a result of bacteremia, the infection enters the musculoskeletal system through vascular pathways, most often via the arteries. Tubercular bacilli enter the joint space directly through the subsynovial arteries or indirectly through eroded epiphyseal or metaphyseal lesions. Eroded epiphyseal lesions are more frequently seen in adults, while metaphyseal lesions are commonly seen in younger individuals. As the metaphyseal portion of the hip is enclosed by a capsule, it is the most common route of infection. In cases where the hip is involved, the weight-bearing areas of articular cartilage are maintained for a few months while the peripheral cartilage is destroyed.8
The infection can begin in the bone or synovial membrane. In childhood cases, the initial lesion is commonly in the metaphyseal part of the long bone, while in adult cases, it is typically at the end. In the hip, osseous tuberculous foci may start in the acetabular, epiphyseal, trochanteric, or metaphyseal regions.8 The infection moves to the joint following capsule or epiphyseal plate destruction. When the infection reaches the subchondral region, articular cartilage loses its source of nutrition and, as a result, detaches from the bone. This detached cartilage becomes loose bodies in the joint cavity. Damage to the epiphyseal plate results in leg length discrepancy and a limping gait.9
In addition to clinical and radiological examinations, evaluation of hematological parameters is important for establishing TB diagnosis. Anemia is commonly found in cases of TB infection.10–12 The characteristics of anemia in cases of TB are the same as in chronic anemia, with both being normocytic normochromic anemias.13 Several studies have shown that TB-associated anemia involves the suppression of erythropoiesis by inflammatory mediators. Furthermore, the absence of bone marrow iron has also been found with TB-associated anemia due to M. Tb utilizing iron for survival.11
Of the patients in the current study, 59% had TB-associated anemia (Table 2), with most having moderate anemia when Hb levels were adjusted for age and gender. Several studies have also reported high rates of anemia in TB patients. Abay et al found that 46% of TB patients had anemia, with the majority being mild to moderately anemic. Abay et al also found that the anemia rate was higher, and more severe cases of HIV concomitant with TB (60%).14 Similarly, Atomsa reported that 37% of patients in their study were moderately anemic.15
 |
Table 2 Laboratory Profile of the Patients |
However, other studies have found low rates of TB-associated anemia. Lee et al found that only 31.9% of TB cases had mild anemia.16 Similarly, Kahase et al reported that only 25% of patients had mild anemia, with the majority of patients having normal hemoglobin values.11 Cereijo et al found no anemia in 32 cases of osteoarticular TB.17
In the current study, the majority of patients had normal leucocyte values. This is in agreement with the findings of previous studies.10,15,16 Kahase et al reported that 72.5% of TB patients had normal total white blood cell (WBC) counts; however, the mean WBC count among TB patients was significantly higher than in normal patients (p = 0.04).10 Hadadi et al found that 74.8% of osteoarticular TB patients had normal leukocyte counts.16 Similarly, Cereijo et al showed that only 16.6% of patients had leucocytosis.15 Atomsa found that the mean absolute WBC counts in TB patients were in the normal range, albeit statistically higher (8.35 ± 3.3×103 cells/µL) than those of healthy controls (6.83 ± 2.17 cells/µL).13
Rohini et al showed that the total WBC counts in pulmonary TB patients were 1.1 times higher than those of healthy controls. WBC counts increase during infection due to the actions of polymorphonuclear leucocytes and macrophages as part of the body’s mechanism to combat the bacterial population.11
WBC counts are sensitive to inflammatory reactions but have low sensitivity. For instance, normal leukocyte counts may be due to chronic infection or immunosuppression. Therefore, differential white blood cell component counts must be conducted. In the current study, lymphopenia was found in 5 patients, probably due to the chronic nature of their disease. Similar to these findings, Kahase found that 60% of patients had lymphopenia, with the mean lymphocyte values of osteoarticular TB patients lower than those of healthy controls.10 Similarly, Abay found that 8% of TB patients had lymphopenia, while 12% of TB patients who also had HIV had lymphopenia. In the current study, all patients had normal neutrophil values.
Thrombocytosis was found in 77.2% of patients in the current study. The cause of thrombocytosis is typically attributed to an immune phenomenon, namely the production of platelet antibodies and reactivation of myeloid hyperplasia.12
In the current study, increased ESR was observed in 95% of patients as a sign of the ongoing inflammation process, while CRP was increased in 76% of patients. Increased ESR and CRP are often found in active forms of the disease, although increased ESR is not proof that the infection is in its active form. However, CRP is an acute phase reactant, and levels of CRIP rise in response to IL-6 mediated pyogenic infections, such as TB.19 These findings are in line with the findings of previous studies.10,11,19 Hadadi found that the mean ESR among osteoarticular TB patients was 57.0 ± 35.7 mm/h. Similarly, Cereijo found that the mean ESR value of osteoarticular TB patients at the time of diagnosis was 55.7 ± 29 mm/h, with only 4 patients having an ESR below 10 mm/h. ESR values tend to decrease following anti-TB drug administration.17,18 While ESR and CRP levels were equal during screening tests, ESR is more sensitive to age changes and is slower to respond to inflammatory changes.20
Mantoux tests or tuberculin skin tests (TSTs) use a purified protein derivative of M. tb. In this study, 6 pediatric patients (< 17 years old) underwent a TST; three returned a positive result. As TSTs have low sensitivity and specificity, they have higher rates of false-positive and false-negative results and cannot differentiate between latent and active TB. Therefore, a positive TST result could suggest active TB, a past infection, BCG vaccination, or sensitization by environmental mycobacteria. Additionally, a negative result may not necessarily exclude a TB diagnosis, as a false negative result could be seen in immunocompromised patients. In regions with endemic TB, EPTB in adults cannot be diagnosed solely using TSTs.21 Previous studies have shown that patients with negative TST results were significantly more likely to have miliary TB or combined pulmonary and EPBT, while those with positive TST results were more likely to have cavitary pulmonary disease. Based on these limitations, TSTs are rarely done as the possible interpretations of test results are wide.22
In the current study, radiological examinations uncovered sclerotic lesions (35.7%), joint destruction (17.9%), cortical destruction, and osteopenia (7.1%). Although not all patients had MRI scans, the most frequent findings were effusion, bone marrow edema, synovitis, and joint space narrowing. In addition, four patients had abscesses. In articular TB cases, subluxation, dislocation, and femoral head destruction were also observed. In children, osteoarticular TB begins in the metaphyseal bone region, while in adults, it begins in the epiphyseal region. As the disease progresses, it erodes the joint space, which is called transphyseal spread (Figure 1). Reactive hyperemia results in juxta-articular bone demineralization and local bone destruction. Periosteal reaction also occurs as the disease progresses; when the subchondral region is reached, articular cartilage nutrition becomes blocked, causing the cartilage to become detached from the bone.23 This can result in loose cartilage bodies within the joint that cause pain during motion. Damage to the physis during childhood will result in deformities, such as bowing or leg length discrepancy.
While this study included one case where a 6-year-old had osteoarticular TB in the diaphyseal part of the tibia that had not yet eroded the joint, the majority of childhood cases included had resulted in joint destruction (Figure 2). In these cases, the disease onset occurred more than 6 months before the study began, which may explain why sclerotic lesions were commonly found. In the early stages of the disease, soft tissue swelling is often found; however, in later stages, osteopenia, periosteal thickening, and periarticular bone destruction are observed. Furthermore, cold abscesses and fistulae may develop in late-stage cases.5
Four of the cases in the current study involved the knee; these cases showed synovitis, joint effusion, soft tissue swelling, and bone marrow edema during MRI examination. Osteoarticular TB can also begin with synovial inflammation. In these cases, the synovial fluid becomes congested and induces heightened inflammatory reactions, thereby causing joint effusion. In the later stages of the disease, granulomatous synovial lesions expand over the bone at the synovial reflections, causing cartilage destruction. When the disease becomes chronic, loose sheets of necrotic articular cartilage and an accumulation of fibrinous material in the synovial fluid may produce rice bodies, which are typically found in synovial joints, tendon sheaths, and bursae. If untreated, this production of rice bodies will cause the disease to progress to tuberculous arthritis and may result in the development of para-articular soft tissue masses, cold abscesses, and sinus tracts.
The current study included 1 rare case of tuberculous tenosynovitis with sinus of the right-hand middle finger (Figure 3). Tuberculous tenosynovitis may result from direct hematogenous spread or periarticular extension of tuberculous arthritis. In these cases, the tendon will be infiltrated and thickened. Tubercle formation may result in caseation, necrosis, and secondary effusion within the tendon sheath. Disease progression may lead to the thinning of the tendon and, ultimately, tendon rupture. Sinus formation is a rare finding in such cases. Four other patients in the current study also had tuberculous tenosynovitis, albeit without sinus. Each of these 4 patients had more than 1 site involved; 2 patients also had spondylitis TB, 1 patient had lesions in the carpal and ankle, and 1 patient had lesions in the carpal and wrist.
 |
Figure 3 (A) sinus on lateral part of middle phalanx; (B) Tenosynovitys appearance from MRI; (C) Granulation tissue was found below extensor tendon. |
Based on chest radiography, pulmonary involvement was present in 50% of the cases examined in this study. Early-stage osteoarticular TB is often misdiagnosed, with the joint disease attributed to traumatism, degenerative disease, gout, pseudogout, or rheumatic disease.
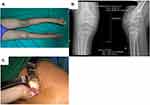
Each patient in the current study underwent debridement, synovectomy, and biopsy that was either preceded or followed by anti-TB drug treatment. The indication for surgical involvement in early-stage disease is primarily to obtain tissue for diagnosis. Deep-seated lesions in the hip, ankle, or knee require open biopsies. In the healed TB phase, surgery is required for deformity correction and joint reconstruction. There are several important principles to follow when surgery is required for osteoarticular TB cases.24 For example, if there is a sinus tract, it has to be excised; therefore, a standard incision is not always suitable in the extremities. Thorough excision of the infected and dead tissue has to be completed, including the bone. Infected granulated tissue must be taken from the deepest points of the infected structures for histopathological analysis and culture. Primary soft tissue closure should not be forced; if there is a soft tissue defect, a secondary closure is preferred. In 1 case in the current study, an external fixator joint that spanned the entire wrist joint was used to maintain stability as the bone destruction was too extensive (Figures 4 and 5). When there is less extensive bone destruction, post-debridement external support, such as a back slab or cast, should be used.
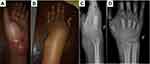 |
Figure 4 (A) Left wrist swelling with discharge on volar area; (B) Dorsal area of the left wrist; (C and D) X ray of the left wrist shows juxta articular osteopenia and destruction of carpal bones. |
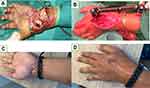 |
Figure 5 (A) Casseous necrosis was found in the volar side of patient in Figure 3; (B) External fixator span the wrist joint; (C and D) 1 year post operative. |
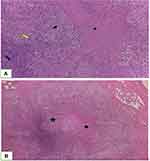
Pathological examination is mandatory to establish a diagnosis of osteoarticular TB. Due to the unavailability of high-profile diagnostic tests like PCR in poor socioeconomic and, most importantly, high TB prevalence areas, histopathological features are better able to diagnose osteoarticular TB. In the current study, Intraoperative procedures revealed found cortex thinning and whitish caseous-like materials. The histopathological analysis of the samples taken during these procedures demonstrated that the samples contained tubercle that has area of caseous necrosis in the central, surrounded by epitheloid cells, multinucleated Langhans giant cells and lymphocyte infiltration (Figure 6A and 6B). A positive histopathological diagnosis of osteoarticular TB is seen in 72–97% of cases, indicating high accuracy.25 In a study by Jain et al, the histopathological diagnosis was positive in 100% of cases that had been clinocoradiologically diagnosed as osteoarticular TB.26 Moreover, Jain et al stated that the accuracy of the histopathological diagnoses was higher than that of acid-fast bacilli staining, which only returned positive results for 12% of the cases.
The use of invasive procedures to establish TB diagnoses is not always possible; WHO recommends that a clinician should use their clinical judgment in determining whether to start anti-TB drug treatment without a histopathological or microbial diagnosis. In patients with strong clinical and radiological evidence of osteoarticular TB, starting anti-TB drug treatment is recommended, although their progress should be monitored. If the diagnosis is uncertain, tissue specimens should be taken and analyzed prior to administering anti-TB drugs.
WHO recommends osteoarticular TB patients should be treated with the anti-TB drugs R, H, Z, and E for 2 months, followed by a 10-month regimen of R, H, and E.
A patient is considered to have reached healed status following the completion of the anti-TB drug regimen, if they have no relapses for 2 years, and if their fever, night sweats, weight loss (if any), sinus, and/or ulcer are resolved. In the current study, laboratory tests following debridement and 6 months of anti-TB drug treatment showed increased hemoglobin levels, decreased thrombocytes, leucocytes, and infection markers (ESR and CRP; Table 2). Radiological indications of bone healing were also observed, including remineralization of affected bone and sharpening of the joint or vertebral margin. MRI scans during the resolution phase demonstrated marrow edema, fatty replacement in the marrow, and no contrast enhancement.27
Unhealed Osteoarticular TB
In the current study, one patient complained of a lump on her right cruris and an abscess above her left clavicle (Figures 7 and 8). They also complained of weight loss and intermittent fever for the 6 months prior to the study. During a physical examination, a tender lump was found on the patient’s right tibia, and an abscess was found above the left clavicle. Based on the examination of a chest x-ray, infiltrates were seen in the lungs. An open biopsy procedure was used, and pathological examination of the biopsy sample showed tubercles, granulomas, and epithelioid cells. The patient was subsequently given anti-TB drugs. After 3 months of anti-TB drug treatment, the patient underwent debridement and another open biopsy. These procedures led to the discovery of caseous tissue inside the bone, and pathological tissue examination revealed the presence of tubercles, epithelioid cells, and caseous necrosis. During follow-up, wound dehiscence, sinus in the central operative scar, and internal serous fluid were observed (Figure 7). As the wound had not healed following 12 months of anti-TB drug treatment, 2 more operations were performed. After the last operation, the sinus was still present; however, it was treated with tulle and gauze that were changed daily. The wound on the patient’s left clavicle was also treated with tulle and gauze (Figure 8).
 |
Figure 7 (A) Before Operation; (B) After 1st operation, sinus and discharging; (C) Third operation; (D) Wound healed with secondary intention. |
 |
Figure 8 Scrofluroderma; (A) before anti TB drugs; (B) after 18 months of anti TB drugs; (C) After 24 months of anti TB drugs. |
After the patient had been receiving anti-TB drugs for 24 months, the complaints about pain and fever ceased. Furthermore, the wound on the tibia healed, and there was no sinus or discharge. The scrofuloderma in the left clavicle had already healed, leaving a hypertrophic scar.
However, our study has its limitation. Firstly, the sample size is relatively small due to osteoarticular TB being one of the most common underdiagnosed extrapulmonary TB. Therefore, we provide wider range of age for inclusion criteria which includes pediatric patients. In further studies, multicenter research in more remote settings may be required to include patients that were previously not diagnosed. Also, longer period of follow-up may be necessary to address the risk of recurrence in osteoarticular TB.
Conclusion
Osteoarticular TB is not always in concomitant with active pulmonary TB. In endemic areas like Indonesia, musculoskeletal complaints must not be overlooked. Due to its chronic and progressive nature, many cases of osteoarticular TB are not diagnosed, leading to morbidity. Anti-TB drugs remains the main treatment for osteoarticular TB. However, surgery may be needed in particular cases such as early-stage disease or deep-seated lesions to establish a diagnosis and subsequent debridement for local control of infection.
Acknowledgments
The authors would like to give thanks to the Department of Orthopedics and Traumatology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hu S, Guo JIA, Ji T, Shen G, Kuang A. Multifocal osteoarticular tuberculosis of the extremities in an immunocompetent young man without pulmonary disease: a case report. Exp Ther Med. 2015;9(6):2299–2302. doi:10.3892/etm.2015.2425
2. Kementrian Kesehatan RI. Infodatin: Tuberkulosis. [Internet]. Jakarta. Kementrian Kesehatan RI; [2018; cited 2021 October 10th]. Available from: https://pusdatin.kemkes.go.id/resources/download/pusdatin/infodatin/infodatin-tuberkulosis-2018.pdf.
3. Erdem H, Baylan O, Simsek I, Dinc A, Pay S, Kocaoglu M. Delayed diagnosis of tuberculous arthritis. Jpn J Infect Dis. 2005;58(6):373–375.
4. Al-Sayyad MJ, Abumunaser LA. Tuberculous arthritis revisited as a forgotten cause of monoarticular arthritis. Ann Saudi Med. 2011;31(4):398–401. doi:10.4103/0256-4947.83210
5. Pigrau-serrallach C, Rodríguez-Pardo D. Bone and joint tuberculosis. Eur Spine J. 2013;22(Suppl 4):556–566. doi:10.1007/s00586-012-2331-y
6. Ali R, Jalil A, Qureshi A. Case Series Extra spinal osteoarticular tuberculosis: a case series of 66 patients from a tertiary care hospital in Karachi. JPMA. 2012;62(12):1344–1348.
7. Procopie IP, Opescu ELLEP, Uplea VEH, et al. Review osteoraticular tuberculosis-brief review of clinical morphological and therapeutic profiles. Curr Health Sci J. 2017;43(3):171–190.
8. Tuli SM. General principles of osteoarticular tuberculosis. Clin Orthop Relat Res. 2002;398:11–19.
9. Saraf SK, Tuli SM. Tuberculosis of Hip. Indian J Orthop. 2015;49(1):1–10. doi:10.4103/0019-5413.143903
10. Kahase D. Evaluation of peripheral blood parameters of pulmonary tuberculosis patients at St. Paul ’ s hospital millennium medical college, Addis Ababa, Ethiopia: comparative Study. J Blood Med. 2020;11:115.
11. Surekha KRM, Srikumar BPS, Kumar AM, Mahesh Kumar A. Assessment of hematological parameters in pulmonary tuberculosis patients. Indian J Clin Biochem. 2016;31(3):332–335. doi:10.1007/s12291-015-0535-8
12. Shah I-A-A-OM-A-AH. Hematological abnormalities in Saudis suffering from pulmonary tuberculosis and their response to the treatment. Res J Pharmacol. 2009;3:78–85.
13. Nandennavar M, Cyriac S, Sagar T. Immune hemolytic anemia in a patient with tuberculous lymphadenitis. J Glob Infect Dis. 2011;3(1):89–92. doi:10.4103/0974-777X.77303
14. Abay F, Yalew A, Shibabaw A, Enawgaw B. Hematological abnormalities of pulmonary tuberculosis patients with and without HIV at the University of Gondar Hospital, Northwest Ethiopia: a comparative cross-sectional study. Tuberc Res Treat. 2018;2018:1–7.
15. Atomsa D, Abebe G, Sewunet T. Original article immunological markers and hematological parameters among newly diagnosed tuberculosis patients at Jimma university specialized hospital. Ethiop J Health Sci. 2011;2.
16. Lee SW, Kang YA, Yoon YS, et al. The Prevalence and Evolution of Anemia Associated with Tuberculosis. J Korean Med Sci. 2006;21(12):1028–1032. doi:10.3346/jkms.2006.21.6.1028
17. Cereijo MJ, Rivas MJ, Ibañez D, Mayo J, Ibañez D, Mayo J. The clinical spectrum of osteoarticular tuberculosis in non-human immunodeficiency virus patients in a defined area of northwestern Spain (1988–1997). Clin Exp Rheumatol. 1999;17(6):663–669.
18. Hadadi A, Rasoulinejad M, Khashayar P, Mosavi M, Morad MM. Osteoarticular tuberculosis in Tehran, Iran: a 2-year study. Eur Soc Clin Infect Dis. 2010;16(8):1270–1273. doi:10.1111/j.1469-0691.2009.03082.x
19. Yoon C, Chaisson LH, Patel SM, et al. Diagnostic accuracy for C reactive protein for active pulmonary tuberculosis: a Systematic review and meta analysis. Int J Tuberc Lung Dis. 2018;21(9):1013–1019.
20. Meek JH, Lewis SM, Meek JH, Lewis SM. ESR or CRP? A comparison of their clinical utility. Hematology. 2007;2013:8454.
21. Article R, Purohit M. Laboratory diagnosis of extra-pulmonary tuberculosis (EPTB) in resource- constrained setting: state of the art, challenges and the need. JCDR. 2015;9(4):EE01.
22. Auld SC, Click ES, Heilig CM, et al. Association between tuberculin skin test result and clinical presentation of tuberculosis disease. BMC Infect Dis. 2013;13(1). doi:10.1186/1471-2334-13-460
23. Vanhoenacker FM, Sanghvi DA, Backer AID. Imaging features of extraaxial musculoskeletal tuberculosis. Indian J Radiol Imaging. 2009;19(3):176–186. doi:10.4103/0971-3026.54873
24. Dhillon MS, Agashe V, Patil SD. Role of surgery in management of osteo-articular tuberculosis of the foot and ankle abstract. Open Orthop J. 2017;11:633–650. doi:10.2174/1874325001711010633
25. Vardhan V, Yanamandra U. Diagnosis of osteoarticular tuberculosis. Indian J Rheumatol. 2011;6(1):87–94. doi:10.1016/S0973-3698(11)60038-1
26. Jain AK, Jena SK, Singh MP, Dhammi IK, Ramachandran VG, Dev G. Evaluation of clinico-radiological, bacteriological, serological, molecular and histological diagnosis of osteoarticular TB New Delhi. Indian J Orthop. 2008;42(2):173–177. doi:10.4103/0019-5413.40253
27. World Health Organization. Index TB guidelines, guidelines for extrapulmonary TB in India; 2016.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.