Back to Journals » Clinical Ophthalmology » Volume 15
Clinical Outcomes and Patient Satisfaction with a New Diffractive-Refractive Trifocal Intraocular Lens – A 12 Month Prospective Study
Authors Brar S , Ganesh S , RP N, CR R
Received 14 May 2021
Accepted for publication 5 July 2021
Published 3 August 2021 Volume 2021:15 Pages 3247—3257
DOI https://doi.org/10.2147/OPTH.S320202
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Scott Fraser
Supplementary video of "A new diffractive-refractive trifocal intraocular lens" [ID 320202].
Views: 184
Sheetal Brar, Sri Ganesh, Nikhil RP, Roopashree CR
Department of Phaco-Refractive Surgery, Nethradhama Super Speciality Eye Hospital, Jayanagar, Bengaluru, Karnataka, 560070, India
Correspondence: Sheetal Brar
Department of Phaco-Refractive Surgery, Nethradhama Super Speciality Eye Hospital, 256/14, Kanakapura Main Road, 7th Block, Jayanagar, Bengaluru, Karnataka, 560070, India
Tel +91 9591002092
Email [email protected]
Purpose: To evaluate the clinical outcomes and patient satisfaction after implantation of Optiflex Trio, a new trifocal intraocular lens (IOL) following cataract surgery.
Methods: Patients undergoing phacoemulsification for age-related cataracts and who satisfy the eligibility criteria underwent bilateral implantation with Optiflex Trio trifocal IOL. At follow -up visits of 1, 3, 6, and 12 months, binocular uncorrected and corrected distance, intermediate and near visual acuity, reading performance, contrast sensitivity (CS) and patient satisfaction for dysphotopsia and spectacle independence were evaluated using questionnaires.
Results: A total of 54 eyes from 27 patients with mean age of 66.30± 7.48 years were included in the study. At 12 months, 78% (n = 21) patients had binocular cumulative UDVA of 20/20 or better. Post-op SE refraction accuracy was within ± 0.50 D for 93% (n = 50) eyes, and refractive cylinder accuracy was within ≤ 0.50 D in 94% (n = 51) eyes. The mean binocular UNVA was 0.01± 0.05 LogMAR, and the mean UIVA at 60 and 80 cm was 0.07± 0.06 and 0.03± 0.05 LogMAR, respectively, at 12 months. Reading speeds at 40, 60 and 80 cm showed improvement overtime. No patient had complained of severe dysphotopsia, and none of the patients required glasses for any activity. No eye underwent YAG-laser capsulotomy for significant PCO at the end of mean follow-up.
Conclusion: After 12 months, Optiflex Trio trifocal IOL provided a complete visual restoration with good visual quality outcomes in terms of uncorrected distance, intermediate and near visual acuity. The incidence of dysphotopsia was low, and spectacle independence was high, resulting in good patient satisfaction.
Trial Registry: CTRI/2019/10/021647 (www.ctri.nic.in).
Keywords: Optiflex, trifocal, IOL, spectacle independence, intraocular lens
Introduction
Trifocal intraocular lenses (IOLs) were developed to bridge the gap between monofocal and bifocal multifocal IOLs in order to provide significant advantages in terms of post-operative unaided intermediate vision, due to the addition of a third foci in the diffractive platform of the optic of the lens.1–3
Ever since the introduction of the first trifocal IOL from Finevison,4 various trifocal IOLs evaluated in the past were shown to successfully reduce spectacle dependence without compromising the quality of vision (QOV) in patients desiring freedom from glasses following cataract surgery for age-related cataracts.5–7
Optiflex Trio trifocal IOL (Biotech Healthcare Holding GmbH, Obergrundstrasse 17, 6002 Luzern, Switzerland), is a relatively recent introduction in the field of trifocal intraocularIOL technology. The IOL is a single piece, hydrophobic acrylic, aspheric, IOLs containing natural chromophore, with a 360° square edge. According to the best of our knowledge, the clinical outcomes with this model of trifocal IOL have not been evaluated yet. In this study, we have reported one-year clinical outcomes related to safety, efficacy, predictability, contrast sensitivity (CS), reading performance, patient satisfaction, complications and overall results with this new trifocal IOL.
Materials and Methods
This prospective, single-centre single arm study was approved by the institutional ethics committee of Nethradhama Superspeciality Eye Hospital, Bangalore, and was conducted in accordance with the principles of the Declaration of Helsinki. All patients provided written informed consent.
Inclusion criteria were healthy eyes besides senile cataract, corneal astigmatism ≤0.75 dioptres (D), IOL power calculation resulting in dioptres between +7.0 D to +30.00 D, capsular bag IOL implantation, and ability to read English language fluently.
Exclusion criteria were patients with corneal astigmatism of >0.75 D, irregular astigmatism due to keratoconus, pellucid marginal degeneration, or corneal scars, corneal dystrophy, severe ocular surface disorders, pupillary abnormalities, history of glaucoma, intraocular inflammation, macular degenerations or retinopathies potentially affecting visual outcome, vulnerable subjects, neuro-ophthalmic diseases, intraoperative complications such as posterior capsule rupture, nucleus drop or capsular bag loss precluding the implantation of the planned IOL, and unassured follow-ups.
Pre-operatively, all patients underwent complete ophthalmologic examination including measurement of uncorrected and best distance corrected visual acuity (ETDRS charts, Precision Vision, LaSalle, IL, USA), manifest refraction, slit lamp biomicroscopy, non-contact tonometry (Tomey NCT, NishiKu, Nagoya, Japan), tomography using elevation based Scheimpflug imaging device Pentacam HR (Oculus Optikgeräte GmbH, Wetzlar, Germany) to rule out irregular astigmatism, HD Analyzer (Visiometrics, Spain) to assess ocular dryness, specular microscopy (Tomey, Japan), macular OCT (Optovue, Fremont, USA) and dilated fundus examination. Biometric assessments were performed using a swept source OCT based optical biometer, IOL Master-700 (Carl Zeiss Meditec, Jena, Germany) using Barrett TK Universal II formula. All eyes were targeted at emmetropia. An optimized A-constant of 118.5 was used for IOL power calculation.
Description of the Study IOL
The Optiflex Trio trifocal IOL is a single piece, hydrophobic acrylic, aspheric, diffractive-refractive trifocal IOL containing natural chromophore, with a 360° square edge for prevention of posterior capsular opacification (Table 1, Figure 1). Lens material with natural yellow chromophore prevents the risk of ARMD, does not disturb the circadian rhythm and does not attribute to altered color perception. The optic of the lens is aspheric with negative spherical aberrations (S.A.) of −0.2µ. The optic of the IOL is diffractive-refractive trifocal with a near add of +3.5 D, and an intermediate add of +1.85 D on the IOL plane. Optimum Asymmetric and balanced light distribution of 45% for far, 27% for intermediate and 28% for near, is designed to provide good unaided vision at all distances.
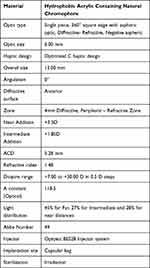 |
Table 1 Description of Optiflex Trio IOL Specifications |
 |
Figure 1 Representative image showing the design of the Optiflex Trio trifocal IOLs. Notes: Reproduced with permission from Biotech Healthcare Holding GmbH. |
Surgical Procedure
All surgeries were performed by a single experienced surgeon (S.G.), using a standard phacoemulsification technique under topical anaesthesia, using the Centurion Precision system (Alcon Laboratories, Fort Worth, TX, USA). Through a temporal clear corneal incision of 2.8 mm, a 5.0 −5.5 mm capsulorhexis was aimed and direct chop technique was used for nuclear deployment. After irrigation and aspiration of the cortex, the left side port was hydrated and BSS injected from the main wound to inflate the bag and form the anterior chamber. Followed by this, the Optiflex Trio trifocal IOL was injected into the capsular bag using its dedicated injector system (Optiject BES28 system). Supplementary Video S1 shows the surgical video of loading and implantation of the Optiflex Trio trifocal IOL, in the right eye of one of the study participants. Any device related to intra-operative complications such as haptic or optic breakage, or explantation of the IOL due to device damage or incorrect IOL power, were recorded.
Post-operative topical therapy included topical prednisolone (1%, Pred Forte, Allergan) 6 times for 6 weeks tapering weekly, moxifloxacin (0.5%, Vigamox, Alcon) 4 times for 2 weeks, nepafenac (0.1%, Nevanac, Alcon) 3 times for 4 weeks and lubricants 4 times or SOS for 4 weeks or more.
Follow-up examinations were performed at 1 day, 1 week, 1 month, 3 months, 6 months, and 12 months after surgery. Dilated slit-lamp examination was performed on post-op day 1 to assess the corneal clarity, anterior chamber inflammation and IOL position. From one month onwards, in addition to the above, assessment of manifest refraction, uniocular and binocular uncorrected and corrected distance visual acuity (UDVA, CDVA), uniocular and binocular uncorrected and corrected near visual acuity (UNVA, CNVA), photopic CS using CSV-1000 (Vector Vision, Greenville, Ohio), reading performance using Salzburg Reading Desk (SRD, University Eye Clinic, Paracelsus Medical University of Salzburg, Austria), defocus curve charting from +2 to −4 D. From 3 months onwards, and a QOV and patient satisfaction questionnaire regarding dysphotopsia symptoms and spectacle independence was also obtained.
Statistical Analysis
SPSS software for Windows version 17.0.0 (IBM Corp., Armonk, NY) was used for statistical analysis. All values were expressed as mean ± standard deviation (SD). Data was checked for normality before subjecting to analysis. A p-value of 0.05 or less was considered statistically significant. Outcome analysis was performed according to the Standard Graphs for Reporting Refractive Outcomes following Intraocular Lens-Based Refractive Surgery.
Results
Fifty-four eyes of 27 patients were evaluated in the study. Table 2 shows the demographic profile and baseline pre-operative parameters of the eyes included in the study. Table 3 shows the visual and refractive outcomes evaluated for distance and near vision at all post-operative visits of 1 week, 1, 3, 6 and 12 months.
 |
Table 2 Demographics and Baseline Pre-Operative Parameters of All Eyes (n = 54) Included in the Study |
 |
Table 3 Visual and Refractive Outcomes at 1 Week, 1 Month, 3 Months, 6 Months, and 12 Months Post-Op |
Visual Outcomes
At 12 months, the mean binocular UDVA and CDVA were −0.00±0.07 and −0.06±0.04 LogMAR respectively. There was no significant difference in the binocular UDVA and CDVA at 12 months compared to post-op visits of 1 week, 1 month, 3 months, and 6 months (p-values for change in UDVA and CDVA >0.05), Table 3.
Uni-ocularly, 59% (32) eyes had UDVA of 20/20 or better, while all eyes (54) had a minimum UDVA of 20/32 (Supplementary file 1). Binocularly, 78% (21) patients had UDVA of 20/20 or better, while all patients (27), had a minimum UDVA of 20/25 (Figure 2). Fifty-two percent (28) of eyes had post-op UDVA same as post-op CDVA, whereas 35% (19) of eyes had post-op UDVA better than post-op CDVA by 1 line and 13% (7) eyes had post-op UDVA better than post-op CDVA by 2 lines (Supplementary file 2).
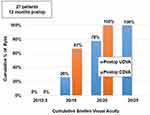 |
Figure 2 Histogram showing binocular results for UDVA and CDVA obtained following implantation of Optiflex Trio IOL at 12 months post-operatively. |
The mean binocular UNVA at 12 months was 0.01±0.05 LogMAR and mean binocular DCNVA was −0.01±0.03 LogMAR, both the parameters were not statistically significantly different from the post-op values at 1 week, 1 month, 3 months, and 6 months (Table 3). Ninety-seven percent (n = 26) of patients had binocular uncorrected near vision of N6 or better, while all patients had a minimum UNVA of N8 (Figure 3).
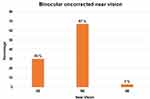 |
Figure 3 Binocular uncorrected near vision results at 12 months post-op. |
Refractive Outcome
At 12 months, the mean sphere, cylinder and SE was −0.07 D±0.19, −0.18 D±0.23, and −0.16 D±0.21, which was not significantly different from their respective values at post-op 1 week, 1 month, 3 months and 6 months (p-values> 0.05 for all parameters), Table 3. Ninety-three percent (50) of eyes were within ±0.50 D of SE correction, while 94% (51) eyes were within ±0.50 D of cylinder correction (Figure 4).
 |
Figure 4 Histogram showing the accuracy to the intended spherical equivalent refraction (A) and refractive astigmatism (B) at 12 months post-op. |
Intermediate Visual Outcomes at 60, 80 Cm Using ETDRS Charts
At 12 months, the mean uncorrected visual acuity at 60 cm was 0.07±0.06 LogMAR and at 80 cm was 0.03±0.05 LogMAR. No significant differences were found between uncorrected and corrected intermediate values at 60 and 80 cm, and also when these were compared to the previous follow-ups, i.e., 1 week, 1 month, 3 months, and 6 months (Table 4).
 |
Table 4 Binocular Near and Intermediate Visual Acuity with ETDRS Charts and Reading Speeds at 40, 60, and 80 Cm and CS with CSV-1000 at Post-Operative Visits from 1-Week Through 12 Months |
Reading Speeds
The binocular uncorrected reading speeds (words per minute) assessed with Salzburg Reading Desk (SRD) at 40, 60 and 80 cm showed improvement from 1 week to 12 months, however, it was not statistically significant. A similar trend was observed for corrected reading speeds for all distances (Table 4).
Contrast Sensitivity
Photopic CS evaluated binocularly at 1 week, 1 month, 3 months, 6 months, and 12 months showed improvement in the log values of all spatial frequencies over time; however, this was not statistically significant (Table 4, Figure 5).
 |
Figure 5 Photopic CS evaluated binocularly (with correction) at 12 months. |
Defocus Curve
Binocular defocus curves were charted with correction at 1 week, 1 month, 3 months, 6 months, and 12 months using defocusing lenses from +2.00 to −4.00. At 12 months post-op, the defocus curve showed two peaks corresponding to 0.00 D and −2.5D, with a gradual decline of the slope in the intermediate range of vision (−1.50 D) (Figure 6). Overall, a full range of functional vision (20/32 or better), was maintained through a defocus of 4.5D.
 |
Figure 6 Binocular distance corrected defocus curve evaluated from +2 to −4 D defocus at 12 months. |
Endothelial Cell Count
Endothelial cell count (ECC) was calculated using specular microscopy. Mean ECC at pre-op, 6 months and 1 year were 2618±186, 2591±362, 2576±96 cells/mm2, respectively, and were found to be non-significant (p value: 0.20).
Patient Satisfaction & Spectacle Independence
A QOV questionnaire was obtained for all patients from 3 months onwards. The mean score of dysphotopsia symptoms (graded from 0–10, 0 being minimal and 10 being severe) reduced significantly at 12 months (0.61±0.49) when compared to 3 months (3.11±0.43), p = 0.00. Spectacle independence scores (graded from 0–10, 0 being dependent on glasses and 10 being completely glass free) for distance, intermediate and near vision were 9.56±0.09, 9.55±0.09 and 9.25±0.36 respectively at 12 months. Overall patient satisfaction score (graded in percentages from 0–100%, 0 being not satisfied at all and 100 being fully satisfied) was 97.07±2.23 at the end of 12 months (Supplementary file 3).
Adverse Effects and Complications
Dilated clinical examination was performed at 3, 6, and 12 months to assess optical clarity of the IOL in terms of glistenings, opacification, calcification, or posterior capsule opacification (PCO), and IOL centration. All IOLs were found to be well-centered in the bag with 360° overlap of capsulorhexis and without any significant tilt or decentration. None of the eyes IOL glistening, calcification, or PCO affecting the visual outcomes or patient satisfaction at the last follow-up. No other vision threatening complications such as cystoid macular oedema, post-operative uveitis, secondary glaucoma or endophthalmitis occurred in any of the eyes included in the study.
Discussion
Several studies have evaluated the clinical outcomes of various trifocal IOLs.4,5,7,8 In the present study, we evaluated the results of Optiflex Trio, which is a recently introduced trifocal IOLs in the market. Table 5 shows the comparative analysis of the design and clinical outcomes between Optiflex Trio and published results of currently available trifocal IOLs.
 |
Table 5 Comparison of the Design and Clinical Outcomes Between Optiflex Trio and Published Results of Currently Available Trifocal Intraocular Lenses |
Ribeiro et al compared the clinical outcomes of 3 different models of diffractive trifocal IOLs: FineVision POD F (PhysIOL), RayOne Trifocal (Rayner IOL, Ltd.), or AcrySof IQ PanOptix (Alcon Laboratories, Inc.) IOL in terms of visual acuity, refraction, CS, and visual quality.8 The study did not find any statistically significant differences between groups in distance, intermediate, and near visual acuity, and postoperative refraction. Postoperative binocular uncorrected intermediate VA of 0.10 logMAR or better was found in 14 (93.33%) patients in the 3 groups. In the present study, however, the postoperative binocular uncorrected intermediate VA of 0.10 logMAR or better was found in 24 (88.8%) patients. The mean UIVA in our study was 0.07±0.06 logMAR, which was slightly lower than the 3 trifocal IOLs evaluated in the study by Ribeiro et al, wherein the RayOne trifocal showed maximum mean UIVA of 0.00 logMAR.
However, this may be explained by the fact that in our study intermediate visual acuity was evaluated at 60 cm versus 66 cm in their study. Despite this, none of the patients in our study complained of dissatisfaction with intermediate vision or required glasses for the same. However, when compared to studies by Kim9 and Asena7 et al, which evaluated outcomes with AT LISA 839 MP IOL, the intermediate VA results appear to be far superior with Optiflex Trio at a 12 months post-op.
In terms of uncorrected near vision results, Ribeiro8 et al found that a postoperative binocular uncorrected near VA of 0.10 logMAR or better was found in 13 (86.67%), 14 (93.33%), and 13 (86.67%) patients in the POD F, RayOne, and PanOptix IOLs groups, respectively. In contrast, a binocular uncorrected near VA of 0.10 logMAR or better was found in all (100%) patients in the present study. Since the near vision was evaluated at same distance in both our study and their study (40 cm), this suggests that Optiflex Trio trifocal IOL may provide uncorrected near vision as good as or better than the currently available trifocal IOLs. Although, the near addition for Optiflex Trio IOL (+3.5 D at 38 cm), which appears to be almost similar to that of the POD F and RayOne trifocal IOLs (Table 5), its high percentage of overall light utilization (88.8%), combined with high light distribution for near (28%), may theoretically result in effective near visual acuity. However, future prospective, comparative studies between Optiflex Trio and different trifocal IOLs are needed to establish this preliminary observation.
Various studies have reported reduction in CS following implantation of multifocal IOLs.10–12 However, modern multifocal IOLs based upon trifocal and EDOF technologies did not show a significant reduction in CS and visual quality.13–15 The CS with trifocal IOLs has been shown to provide conflicting results when compared to found to extended depth of focus lenses. Gunderson16 et al suggested comparable results between the two technologies, while Mencucci17 et al showed that Symfony ERV IOL resulted in significantly better CS than AT LISA Tri 839 MP trifocal IOL. However, while comparing 3 different trifocal IOLs, Ribiero8 et al did not find any statistically significant differences between groups for any of the spatial frequencies evaluated for photopic CS without glare (P > 0.05)8. In the present study, Optiflex Trio achieved CS results within the physiologic CS range set for the measuring device for normal subjects of similar age (Figure 5).
Few studies have evaluated reading performance following implantation of trifocal IOLs. Compared to the study by Mencucci17 et al, the reading speed with Optiflex Trio was slightly less than that with AT LISA Tri and AcrySof IQ Panoptix trifocal IOL. This may be due to the difference on the tools used for evaluating reading performance in both studies, which was SRD in the present and MNREAD charts in Mencucci17 et al study. However, using the same tool, i.e., SRD, the mean reading speeds were found to be higher with Optiflex Trio versus Zeiss Trifocal IOL at the end of 12 months, Table 5. The relatively higher amount of light distribution dedicated for intermediate in Optiflex Trio (27% vs 20% in Zeiss Trifocal), and nil PCO incidence at 12 months may possibly explain relatively better reading speeds with Optiflex Trio trifocal IOL.
When compared to our published results of AT LISA TRI trifocal IOL, wherein, 5 eyes underwent YAG laser capsulotomy for early PCO at a mean follow-up of 7.2±2.9 months, no eye developed PCO following Optiflex Trio IOL implantation in the present study.18 This may be explained by the fact that Optiflex Trio is a hydrophobic acrylic foldable IOL, whereas the material of AT LISA Tri is hydrophilic acrylic, and it is well-known that hydrophilic IOLs have been shown to have significantly higher rates of PCO formation.19–21
In conclusion, Optiflex Trio trifocal IOLs provided good visual outcomes at all distances at 12 months, which were comparable with the published results of currently available trifocal IOLs. Patients achieved high level of satisfaction in terms of spectacle independence as well as comfort with their vision due to minimal dysphotopsia symptoms and nil incidence of posterior capsular opacification at the end of 12 months. To our knowledge, this is the first study reporting the outcomes of Optiflex Trio trifocal IOL. Further prospective, randomised comparative studies with larger sample sizes are suggested to compare the long-term results of this lens with other available trifocal IOLs.
Disclosure
Dr Sri Ganesh and Dr Sheetal Brar are consultants to Biotech Vision Care, Ahmedabad, India. The study was supported by a limited grant from Biotech Vision Care Pvt Ltd. The authors reported no other potential conflicts of interest for this work.
References
1. Zamora-de La Cruz D, Zúñiga-Posselt K, Bartlett J, Gutierrez M, Abariga SA. Trifocal intraocular lenses versus bifocal intraocular lenses after cataract extraction among participants with presbyopia. Cochrane Database Syst Rev. 2020;6(6):CD012648. doi:10.1002/14651858.CD012648.pub2. PMID: 32584432; PMCID: PMC7388867.
2. Xu Z, Cao D, Chen X, Wu S, Wang X, Wu Q. Comparison of clinical performance between trifocal and bifocal intraocular lenses: a meta-analysis. PLoS One. 2017;12(10):e0186522. doi:10.1371/journal.pone.0186522. PMID: 29073156; PMCID: PMC5657996.
3. Jin S, Friedman DS, Cao K, et al. Comparison of postoperative visual performance between bifocal and trifocal intraocular Lens based on randomized controlled trails: a meta-analysis. BMC Ophthalmol. 2019;19(1):78. doi:10.1186/s12886-019-1078-1. PMID: 30871503; PMCID: PMC6419463.
4. Bilbao-Calabuig R, Llovet-Rausell A, Ortega-Usobiaga J, et al. Visual outcomes following bilateral lmplantation of two diffractive trifocal intraocular lenses in 10 084 eyes. Am J Ophthalmol. 2017;179:55–66. doi:10.1016/j.ajo.2017.04.013. PMID: 28456547.
5. Akman A, Asena L, Ozturk C, Gür Güngör S. Evaluation of quality of life after implantation of a new trifocal intraocular lens. J Cataract Refract Surg. 2019;45(2):130–134. doi:10.1016/j.jcrs.2018.12.003. PMID: 30612749.
6. Klyushnikova EV, Hurtzilava OG, Latariya EL, Dautova ZA. Kachestvo zhizni patsientov posle fakoemul’sifikatsii katarakty s implantatsiei trifokal’noi intraokulyarnoi linzy [Quality of life of patients after phacoemulsification with implantation of trifocal intraocular lens]. Vestn Oftalmol. 2020. 136(6):195–201. doi:10.17116/oftalma2020136062195. Russian. PMID: 33371649.
7. Sezgin asena B. Visual and refractive outcomes, spectacle independence, and visual disturbances after cataract or refractive lens exchange surgery: comparison of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2019;45(11):1539–1546. doi:10.1016/j.jcrs.2019.06.005. PMID: 31587938.
8. Ribeiro F, Ferreira TB. Comparison of clinical outcomes of 3 trifocal IOLs. J Cataract Refract Surg. 2020;46(9):1247–1252. doi:10.1097/j.jcrs.0000000000000212. PMID: 32898095.
9. Kim M, Kim JH, Lim TH, Cho BJ. Comparison of reading speed after bilateral bifocal and trifocal intraocular lens implantation. Korean J Ophthalmol. 2018;32(2):77–82. doi:10.3341/kjo.2017.0057. PMID: 29560618; PMCID: PMC5906405.
10. Lord SR, Dayhew J, Howland A. Multifocal glasses impair edge-contrast sensitivity and depth perception and increase the risk of falls in older people. J Am Geriatr Soc. 2002;50(11):1760–1766. doi:10.1046/j.1532-5415.2002.50502.x. PMID: 12410892.
11. Kenneth MP, Nguyen ZJ, Nguyen-Cuu J, Sebag J. Impact of multifocal intraocular lenses on contrast sensitivity function in vision degrading vitreopathy. Invest Ophthalmol Vis Sci. 2018;59(9):2199.
12. Schmitz S, Dick HB, Krummenauer F, Schwenn O, Krist R. Contrast sensitivity and glare disability by halogen light after monofocal and multifocal lens implantation. Br J Ophthalmol. 2000;84(10):1109–1112. doi:10.1136/bjo.84.10.1109. PMID: 11004093; PMCID: PMC1723267.
13. Alfonso JF, Fernández-Vega Cueto L, Belda-Salmerón L, Montés-Micó R, Fernández-Vega L. Visual function after implantation of a diffractive aspheric trifocal intraocular lens. Eur J Ophthalmol. 2016;26(5):405–411. doi:10.5301/ejo.5000741. PMID: 26797852.
14. Law EM, Aggarwal RK, Kasaby H. Clinical outcomes with a new trifocal intraocular lens. Eur J Ophthalmol. 2014;24(4):501–508. doi:10.5301/ejo.5000407. PMID: 24366771.
15. Kim TI, Chung TY, Kim MJ, Lee K, Hyon JY. Visual outcomes and safety after bilateral implantation of a trifocal presbyopia correcting intraocular lens in a Korean population: a prospective single-arm study. BMC Ophthalmol. 2020;20(1):288. doi:10.1186/s12886-020-01549-z. PMID: 32669090; PMCID: PMC7362483.
16. Gundersen KG, Potvin R. Comparing visual acuity, low contrast acuity and contrast sensitivity after trifocal toric and extended depth of focus toric intraocular lens implantation. Clin Ophthalmol. 2020;14:1071–1078. doi:10.2147/OPTH.S253250. PMID: 32368005; PMCID: PMC7183779.
17. Mencucci R, Favuzza E, Caporossi O, Savastano A, Rizzo S. Comparative analysis of visual outcomes, reading skills, contrast sensitivity, and patient satisfaction with two models of trifocal diffractive intraocular lenses and an extended range of vision intraocular lens. Graefes Arch Clin Exp Ophthalmol. 2018;256(10):1913–1922. doi:10.1007/s00417-018-4052-3. PMID: 29980919.
18. Ganesh S, Brar S, Pawar A. Long-term visual outcomes and patient satisfaction following bilateral implantation of trifocal intraocular lenses. Clin Ophthalmol. 2017;11:1453–1459. doi:10.2147/OPTH.S125921. PMID: 28860693; PMCID: PMC5558567.
19. Vasavada AR, Raj SM, Shah A, Shah G, Vasavada V, Vasavada V. Comparison of posterior capsule opacification with hydrophobic acrylic and hydrophilic acrylic intraocular lenses. J Cataract Refract Surg. 2011;37(6):1050–1059. doi:10.1016/j.jcrs.2010.12.060. PMID: 21596247.
20. Iwase T, Nishi Y, Oveson BC, Jo YJ. Hydrophobic versus double-square-edged hydrophilic foldable acrylic intraocular lens: effect on posterior capsule opacification. J Cataract Refract Surg. 2011;37(6):1060–1068. doi:10.1016/j.jcrs.2010.12.059. PMID: 21596248.
21. Nanu RV, Ungureanu E, Instrate SL, et al. An overview of the influence and design of biomaterial of the intraocular implant of the posterior capsule opacification. Rom J Ophthalmol. 2018;62(3):188–193. [PMID: 30505987; PMCID: PMC6256071]. doi:10.22336/rjo.2018.29
22. Doroodgar F, Niazi F, Sanginabadi A, et al. Visual performance of four types of diffractive multifocal intraocular lenses and a review of articles. Int J Ophthalmol. 2021;14(3):356–365. doi:10.18240/ijo.2021.03.04. PMID: 33747809; PMCID: PMC7930542.
23. Ferreira TB, Ribeiro FJ. Prospective Comparison of clinical performance and subjective outcomes between two diffractive trifocal intraocular lenses in bilateral cataract surgery. J Refract Surg. 2019;35(7):418–425. doi:10.3928/1081597X-20190528-02. PMID: 31298721.
24. Marques EF, Ferreira TB. Comparison of visual outcomes of 2 diffractive trifocal intraocular lenses. J Cataract Refract Surg. 2015;41(2):354–363. doi:10.1016/j.jcrs.2014.05.048. PMID: 25661129.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
