Back to Journals » Cancer Management and Research » Volume 13
Clinical Outcome in Soft Tissue Sarcoma Patients with Lung Metastasis Who Received Metastasectomy and/or Radiofrequency Ablation: Tokai Musculoskeletal Oncology Consortium Study
Authors Nakamura T , Asanuma K , Takao M , Yamanaka T, Koike H, Chen-Yoshikawa TF, Tsukushi S, Kuroda H, Kozawa E, Sano M, Aiba H, Nakanishi R, Nagano A, Yamada K, Shido Y, Kawanami K, Izubuchi Y, Sudo A , Nishida Y
Received 13 August 2021
Accepted for publication 15 October 2021
Published 10 November 2021 Volume 2021:13 Pages 8473—8480
DOI https://doi.org/10.2147/CMAR.S333721
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Tomoki Nakamura,1 Kunihiro Asanuma,1 Motoshi Takao,2 Takashi Yamanaka,3 Hiroshi Koike,4 Toyofumi F Chen-Yoshikawa,5 Satoshi Tsukushi,6 Hiroaki Kuroda,7 Eiji Kozawa,8 Masaaki Sano,9 Hisaki Aiba,10 Ryoichi Nakanishi,11 Akihito Nagano,12 Kenji Yamada,13 Yoji Shido,14 Katsuhisa Kawanami,15 Yuya Izubuchi,16 Akihiro Sudo,1 Yoshihiro Nishida4,17
1Department of Orthopedic Surgery, Mie University Graduate School of Medicine, Tsu, Mie, Japan; 2Department of Thoracic Surgery, Mie University Graduate School of Medicine, Tsu, Mie, Japan; 3Department of Radiology, Mie University Graduate School of Medicine, Tsu, Mie, Japan; 4Department of Orthopedic Surgery, Nagoya University Graduate School of Medicine, Nagoya, Aichi, Japan; 5Department of Thoracic Surgery, Nagoya University Graduate School of Medicine, Nagoya, Aichi, Japan; 6Department of Orthopedic Surgery, Aichi Cancer Center Hospital, Nagoya, Aichi, Japan; 7Department of Thoracic Surgery, Aichi Cancer Center Hospital, Nagoya, Aichi, Japan; 8Department of Orthopedic Surgery, Nagoya Memorial Hospital, Nagoya, Aichi, Japan; 9Department of Thoracic Surgery, Nagoya Memorial Hospital, Nagoya, Aichi, Japan; 10Department of Orthopedic Surgery, Graduate School of Medical Sciences, Nagoya City University, Nagoya, Aichi, Japan; 11Department of Oncology, Immunology and Surgery, Graduate School of Medical Sciences, Nagoya City University, Nagoya, Aichi, Japan; 12Department of Orthopedic Surgery, Gifu University Graduate School of Medicine, Gifu, Gifu, Japan; 13Department of Musculoskeletal Oncology, Okazaki City Hospital, Okazaki, Aichi, Japan; 14Department of Orthopedic Surgery, Hamamatsu Medical University, Hamamatsu, Sizuoka, Japan; 15Department of Orthopedic Surgery, Aichi Medical University School of Medicine, Nagakute, Aichi, Japan; 16Department of Orthopedics and Rehabilitation Medicine, University of Fukui Faculty of Medical Sciences, Eiheiji, Fukui, Japan; 17Department of Rehabilitation, Nagoya University Hospital, Nagoya, Aichi, Japan
Correspondence: Tomoki Nakamura
Department of Orthopaedic Surgery, Mie University Graduate School of Medicine, 2-174 Edobashi, Tsu, Mie, 514-8507, Japan
Tel +81592315022
Fax +81592315211
Email [email protected]
Purpose: Here, we investigated the oncological outcomes of lung metastasectomy and/or radiofrequency ablation (RFA) of 92 patients with soft tissue sarcoma (STS) at nine institutions.
Methods: The study cohort included 65 men and 27 women with a mean age of 59 years at the time of metastasis. The mean follow-up duration was 51 months. All patients underwent metastasectomy and/or RFA for lung metastasis.
Results: The mean maximum size of the initial lung metastasis was 14.6 mm. At the initial evaluation, 41 patients had a single metastasis, whereas 51 patients had multiple metastases. The mean number of metastasectomies and/or RFA was 2 per patient. A total of 70 patients underwent lung metastasectomy, whereas the other 13 underwent lung RFA. The remaining nine patients underwent both RFA and metastasectomy. The 5-year post-metastatic survival rate was 52%. The patients who underwent complete treatment for the initial metastasis had better post-metastatic survival rates than those who underwent incomplete treatment. A univariate analysis of all possible prognostic factors for complete treatment confirmed the predictive value of disease-free interval, metastasis at initial presentation, distribution, tumor size, and number of lung metastases. Of the 92 patients, 74 underwent complete treatment for initial metastasis; in these patients, univariate and multivariate analyses showed that a smaller tumor size and single-lung metastasis were prognostic factors for superior post-metastatic survival. The patients with a smaller (< 11.5 mm) single metastasis had better post-metastasis survival. The 5-year post-metastatic survival rates were 89.9% for patients with a smaller (< 11.5 mm) single metastasis versus 22.7% for patients with larger (> 11.5 mm) and multiple metastases.
Discussion: We propose that complete treatment for lung metastasis in patients with STS may improve post-metastatic survival rates. Furthermore, tumor number and size are important variables for clinical decision-making.
Keywords: lung metastasis, metastasectomy, radiofrequency ablation, soft tissue sarcoma
Introduction
Soft-tissue sarcoma (STS) is a rare and heterogeneous group of cancers.1 The incidence of STS is less than six cases per 100,000 individuals, representing 1–2% of all cancer cases in adults.1 Lung metastasis from STS occurs in 20–50% of these patients. Several relevant prognostic factors have been defined for these cases. Metastasectomy is the standard treatment for improving the survival rates of patients with lung metastasis from STS.2–4 Radiofrequency ablation (RFA) of the lung was recently proven a useful option, as it promises outcomes similar to those of metastasectomy.3,5,6 Age, tumor size, disease-free interval (DFI), number of metastases, and tumor volume doubling time are predictive factors of survival.3,4,7–9 However, previous reports included a relatively small number of patients with STS or patients with STS and bone sarcoma due to the rarity of the disease itself. Here we investigated the oncological outcomes of lung metastasectomy and/or RFA in 92 patients with STS at nine institutions under the Tokai Musculoskeletal Oncology Consortium.
Materials and Methods
This study was approved by the Clinical Research Ethics Review Committee of Mie University Hospital (H2020-208), and it was conducted in accordance with the Declaration of Helsinki. The need for informed consent was waived due to the retrospective study design. Instead, patients were allowed to opt-out. Data from 2008–2018 were obtained from nine hospitals. The study cohort included 65 men and 27 women with a mean age of 59 years (range, 16–87 years) at the time of the metastasis. The mean follow-up duration was 51 months (range, 3.8–159 months). All patients underwent metastasectomy and/or RFA for lung metastasis treatment.
Metastasis was diagnosed on the presence of at least one of the following criteria: 1) histological examinations confirmed a metastatic lesion in specimens obtained by surgical resection; and 2) the lesions obviously progressed in number and/or size.
The primary aim of the study was to examine the factors associated with curability of the initial lung metastasis. We defined curability as complete or incomplete treatment of all initial lung metastases with metastasectomy or RFA.3 The secondary objective was to examine the prognostic factors associated with oncological outcomes in patients with STS who underwent complete resection or ablation.
Statistical Analyses
Statistical associations between clinicopathological variables were evaluated using the chi-square test for qualitative data. Post-metastatic disease-specific survival (DSS) was defined as the time from the diagnosis of lung metastasis to the date of death due to sarcoma or the last follow-up examination. Survival curves were constructed using the Kaplan–Meier method. The univariate and multivariate analyses were conducted using a log rank test and a Cox proportional hazards model, respectively, to compare overall survival rates. Values of P <0.05 were included as variables in the multivariate analysis. All statistical analyses were performed with the EZR graphical user interface (Saitama Medical Center, Jichi Medical University, Saitama, Japan) for R (R Foundation for Statistical Computing, Vienna, Austria), a modified version of R Commander designed to add statistical functions frequently used in biostatistics. Probability P values <0.05 were considered significant in all statistical analyses.
Results
Clinicopathological Characteristics (Table 1)
A total of 92 patients were included in this study. The site of the primary tumor included the thigh (n=31), leg (n=9), chest wall (n=8), knee (n=7), buttock (n=6), upper arm (n=5), foot (n=4), retroperitoneum (n=3), abdominal wall (n=3), and other (n=16). The primary STS was classified histologically as follows: 23 undifferentiated pleomorphic sarcomas, 22 leiomyosarcomas, 12 malignant peripheral nerve sheath tumors, 8 synovial sarcoma, 7 myxofibrosarcomas, 4 dedifferentiated liposarcoma, 4 extra-skeletal chondrosarcoma, and 12 tumors of other histological types. Primary tumor resection was performed in 89 patients. The remaining 3 patients underwent radical radiotherapy (n=2) or carbon ion radiotherapy (n=1) due to wide resection difficulty. All local tumors were controlled when an initial lung metastasis was found.
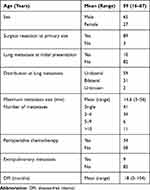 |
Table 1 Patients’ Characteristics |
A total of 10 patients developed lung metastasis at initial presentation, and all 10 underwent surgical resection at the primary site. The mean maximum tumor size of the initial lung metastasis was 14.6 mm (range, 3–56 mm; unknown size in five patients). At the initial evaluation, the lung metastasis was a single one in 41 patients and multiple in 51 patients. Moreover, the distribution was unilateral in 59 patients and bilateral in 31 patients (unknown in 2 patients). The mean DFI between primary tumor treatment and initial metastasis was 18 months (range, 0–104 months). The mean number of metastasectomies and/or RFA was 2 (range, 1 to >10). A total of 70 patients underwent lung metastasectomy. The other 13 patients underwent lung RFA. The remaining nine patients underwent both RFA and metastasectomy. Perioperative chemotherapy for lung metastasectomy or RFA was administered in 34 patients (Table 2).
 |
Table 2 Patient Backgrounds According to Treatment |
Clinical Outcome in 92 Patients
At the final follow-up, 48 patients were alive, 43 patients had died of the disease, and 1 patient had died of other causes. The 3- and 5-year post-metastatic DSS were 62% (95% confidence interval [CI], 54.3–74.1%] and 52% [95% CI, 40.3–62.4%]), respectively (Figure 1). The patients who underwent complete treatment of the initial metastasis had better post-metastatic DSS than those with incomplete treatment (Figure 2). The 3- and 5-year post-metastatic survival rates of patients with complete treatment were 69% (95% CI, 56.7–78.4%) and 58.4% (95% CI, 45.4–69.3%), respectively, whereas the results for the foregoing criteria were 50% (95% CI, 25.9–70.1%) and 27.8% (95% CI, 8.39–51.6%), respectively (p=0.02) (Figure 2). RFA was more likely to be administered to patients with multiple and/or bilateral metastases (Table 2). Univariate analysis of all possible prognostic factors for complete treatment confirmed the predictive value of DFI (p<0.0001), metastasis at initial presentation (p=0.0003), distribution (p=0.001), tumor size (p=0.03), and number of lung metastases (p<0.0001) (Table 3).
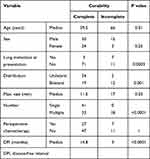 |
Table 3 Possible Variables for Curability |
 |
Figure 1 Kaplan–Meier curves showing the post-metastatic survival of 92 patients with soft tissue sarcoma after initial lung metastasis. |
 |
Figure 2 Kaplan–Meier curves showing post-metastatic survival according to the curability of the initial lung metastasis (A: complete treatment, B: incomplete treatment). |
Predictors for Survival in 74 Patients with Complete Treatment
Of the 92 patients, 74 received complete treatment for initial metastasis. The mean maximum tumor size of the initial lung metastasis was 11.5 mm (range, 3–45 mm; unknown in two patients). At the initial evaluation, 41 patients had a single metastasis and 33 patients had multiple metastases. The distribution was unilateral in 55 patients and bilateral in 19 patients. The mean DFI between primary tumor treatment and initial metastasis was 20 months (range, 0–104 months).
At the final follow-up, 43 patients were alive, 30 patients had died of the disease, and one patient had died of other causes. The univariate and multivariate analysis showed that a smaller tumor size and single-lung metastasis were prognostic factors for superior post-metastatic survival (Tables 4 and 5). Finally, the patients were divided into four groups according to metastasis size (11.5 mm) and number (single vs multiple). Two patients were excluded because of unknown metastasis size. The patients with a smaller (<11.5 mm) single metastasis had better post-metastasis survival (Table 6). The 3- and 5-year post-metastatic DSS rates were 89.9% (95% CI, 65.3–97.4%) and 89.9% (95% CI, 65.3–97.4%) for the patients with a smaller (<11.5mm) and single metastasis, respectively, whereas the same were 44.1% (95% CI, 20.7–65.9%) and 22.7% (95% CI, 5.96–45.8%), respectively, for patients with larger (>11.5 mm) or multiple metastases (Figure 3).
 |
Table 4 Univariate Analysis for Predicting Post-Metastatic Survival |
 |
Table 5 Multivariate Analysis for Predicting Post-Metastatic Survival |
 |
Table 6 Post-Metastatic Survival According to Lung Condition |
Discussion
In the present study, we analyzed 92 patients with STS who underwent lung metastasectomy and/or RFA. The 3- and 5-year post-metastatic DSS rates were 62% and 52%, respectively. The 5-year survival rate after lung metastasectomy was reportedly 15–50% in patients with bone sarcoma and STS, which is consistent with the present study’s results.4,7,10 The patients who received complete treatment for initial metastasis had better post-metastatic survival than those who received incomplete treatment. DFI, metastasis at initial presentation, tumor distribution, tumor size, and number of lung metastases were associated with complete treatment of lung metastasis.
The survival of patients who received incomplete treatment was generally poor. Nakamura et al reported that the 1- and 3-year survival rates after incomplete RFA treatment in nine patients were 29.6% and 0%, respectively.5 Suzuki et al reported that the 5-year survival rate of patients who received incomplete resection was 8.3%.11 However, in the present study, the 3- and 5-year post-metastatic survival rates of patients who received incomplete treatment were 50% and 27.8%, respectively. Therefore, there could have been some bias in the current study, as some patients may have had low-grade STS and slow-growing metastases. In these cases, metastasectomy and/or RFA may be considered if one of the metastases gradually grows.
The chemosensitivity may be better in other patients with advanced STS. If the patients received chemotherapy and maintained disease control, with the exception of those with a single metastasis, metastasectomy and/or RFA may be considered. Therefore, we excluded patients who received incomplete treatment from the analysis of further survival. For the 74 patients who received complete treatment, univariate and multivariate analyses showed that a smaller tumor size and a single lung metastasis were prognostic factors for better post-metastatic survival. However, there is no agreement about the maximum number and size of metastases indicated for metastasectomy and/or RFA. A tumor size of 1–2 cm reportedly influenced survival.8,12 Similarly, a single to three metastases affected survival.7,8,12,13 In contrast, Rehders et al suggested that aggressive treatment be considered despite differences in lung metastasis number and size whenever complete treatment was likely to be achieved.14 In this study, however, patients with multiple or larger metastases had relatively poor survival. Therefore, we strongly suggest that metastasectomy and/or RFA be considered for patents with single and/or smaller (around 10 mm) metastases. A 5-mm lung nodule with a short volume doubling time (within 30 days) would grow to approximately 10 mm within 3 months. Therefore, a repeat chest computed tomography scan should be obtained within 3 months in patients if small lung nodules are found on the follow-up computed tomography.15
In this study, age was not related to prognosis. In general, age is considered only a relative contraindication to metastasectomy. When complete treatment is indicated in older and generally healthy patients, metastasectomy should be considered. If patients have comorbidities, RFA may be considered since it is a less invasive local treatment with reported good local control.3,5,6
This study has some limitations, including that adjuvant chemotherapy and surgical technique were highly individualized to patients and institutions. In this study, we did not know the type of surgery and adjuvant chemotherapy administered to each patient, although they have minimal to no impact on the survival rate of patients undergoing metastasectomy.16,17 Moreover, we did not examine the treatment at an advanced stage after relapse. New drugs, such as pazopanib, eribulin, and trabectedin, could be administered in cases of advanced STS and may contribute to survival.18 The retrospective nature of the study was another limitation. However, this was a large multicenter study that enrolled a large number of Japanese patients with STS who underwent lung metastasectomy and/or RFA.
In conclusion, here we proposed that complete treatment for lung metastasis in patients with STS may improve post-metastatic survival. Furthermore, tumor number and size are also important variables in clinical decision-making.
Funding
This study received no financial support.
Disclosure
The authors declare no conflicts of interest for this study.
References
1. Bourcier K, Le Cense A, Tselikas L, et al. Basic knowledge in soft tissue sarcoma. Cardiovasc Intervent Radiol. 2019;42(9):1255–1261. doi:10.1007/s00270-019-02259-w
2. Stamenovic D, Hohenberger P, Roessner E. Pulmonary metastasectomy in soft tissue sarcomas: a systematic review. J Thorac Dis. 2021;13(4):2649–2660. doi:10.21037/jtd-2019-pm-13
3. Nakamura T, Matsumine A, Takao M, et al. Impact of tumor volume doubling time on post-metastatic survival in bone and soft- tissue sarcoma patients treated with metastasectomy and/or radiofrequency ablation of the lung. Onco Targets Ther. 2017;10:559–564. doi:10.2147/OTT.S121562
4. Marulli G, Mammana M, Comacchio G, Rea F. Survival and prognostic factors following pulmonary metastasectomy for sarcoma. J Thorac Dis. 2017;9(Suppl 12):S1305–S1315. doi:10.21037/jtd.2017.03.177
5. Nakamura T, Matsumine A, Yamakado K, et al. Lung radiofrequency ablation in patients with pulmonary metastases from musculoskeletal sarcomas. Cancer. 2009;115(16):3774–3781. doi:10.1002/cncr.24420
6. Koelblinger C, Strauss S, Gillams A. Outcome after radiofrequency ablation of sarcoma lung metastases. Cardiovasc Intervent Radiol. 2014;37(1):147–153. doi:10.1007/s00270-013-0644-9
7. Nevala R, Jaamaa S, Tukuainen E, et al. Long-term results of surgical resection of lung metastases from soft tissue sarcoma: a single center experience. J Surg Oncol. 2019;120(2):168–175.
8. Chudgar NP, Brennan MF, Munhoz RR, et al. Pulmonary metastasectomy with therapeutic intent for soft-tissue sarcoma. J Thorac Cardiovasc Surg. 2017;154(1):319–330.e1. doi:10.1016/j.jtcvs.2017.02.061
9. Van Geel AN, Pastorino U, Jauch KW, et al. Surgical treatment of lung metastases: the European Organization for Research and Treatment of Cancer-Soft Tissue and Bone Sarcoma Group study of 255 patients. Cancer. 1996;77(4):675–682. doi:10.1002/(SICI)1097-0142(19960215)77:4<675::AID-CNCR13>3.0.CO;2-Y
10. Yamamoto Y, Kanzaki R, Kanou T, et al. Long-term outcomes and prognostic factors of pulmonary metastasectomy for osteosarcoma and soft tissue sarcoma. Int J Clin Oncol. 2019;24(7):863–870. doi:10.1007/s10147-019-01422-0
11. Suzuki M, Iwata T, Ando S, et al. Predictors of long-term survival with pulmonary metastasectomy for osteosarcoma and soft tissue sarcomas. J Cardiovasc Surg (Torino). 2006;47(5):603–608.
12. Predina JD, Puc MM, Bergey MR, et al. Improved survival after pulmonary metastasectomy for soft tissue sarcoma. J Thorac Oncol. 2011;6(5):913–919. doi:10.1097/JTO.0b013e3182106f5c
13. Sanderberg RA, Figueiredo LP, Haddad FJ, Gross JL, Younes RN. Pulmonary metastasectomy from soft tissue sarcomas. Clinics. 2010;65(9):871–876. doi:10.1590/S1807-59322010000900010
14. Rhhders A, Hosch SB, Scheunemann P, Stoeklein NH, Knoefel WT, Peiper M. Benefit of surgical treatment of lung metastasis in soft tissue sarcoma. Arch Surg. 2007;142(1):70–75. doi:10.1001/archsurg.142.1.70
15. Nakamura T, Matsumine A, Matsubara T, Asanuma K, Uchida A, Sudo A. Clinical impact of the tumor volume doubling time on sarcoma patients with lung metastases. Clin Exp Metastasis. 2011;28(8):819–825. doi:10.1007/s10585-011-9413-9
16. Carter RJ, Qin LX, Downey RJ, Brennan MF, Singer S, Maki RG. Perioperative chemotherapy in patients undergoing pulmonary resection for metastatic soft-tissue sarcoma of the extremity. Cancer. 2007;110(9):2050–2060. doi:10.1002/cncr.23023
17. Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Interact Cardiovasc Thorac Surg. 2013;17(4):720–724. doi:10.1093/icvts/ivt300
18. Nakamura T, Asanuma K, Hagi T, Sudo A. Clinical outcome of systemic treatment for advanced soft tissue sarcoma: real-life perspective in Japan. Drug Des Devel Ther. 2020;14:4215–4220. doi:10.2147/DDDT.S275526
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

