Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Clinical observation of immune checkpoint inhibitors in the treatment of advanced pancreatic cancer: a real-world study in Chinese cohort
Authors Sun D, Ma J, Wang J, Zhang F, Wang L, Zhang S, Chen G, Li X, Du W, Cui P, Hu Y
Received 4 May 2018
Accepted for publication 4 July 2018
Published 11 September 2018 Volume 2018:14 Pages 1691—1700
DOI https://doi.org/10.2147/TCRM.S173041
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Danyang Sun,* Junxun Ma,* Jinliang Wang, Fan Zhang, Lijie Wang, Sujie Zhang, Guangying Chen, Xiaoyan Li, Wushuang Du, Pengfei Cui, Yi Hu
Department of Oncology, Chinese PLA General Hospital, Beijing, China
*These authors contributed equally to this work
Background: In recent years, immune checkpoint inhibitors have been used with great success in the treatment of various cancers. However, when used in monotherapy, immune checkpoint inhibitors have a poor effect on pancreatic cancer. This study assessed the efficacy and safety of the use of immune checkpoint inhibitors for the treatment of advanced pancreatic cancer.
Patients and methods: We evaluated patients with advanced pancreatic cancer who were treated with PD-1/PD-L1 inhibitors from 2015–2017. All the patients received PD-1/PD-L1 inhibitors as a monotherapy or in combination with other treatments, such as chemotherapy, targeted therapy, and CTLA-4 inhibitors at the recommended dosages.
Results: For the 43 patients enrolled, the objective response rate was 10.5%, the disease control rate was 50%, the median progression-free survival was 2.3 months, and the median overall survival (mOS) was 5.1 months. The mOS was longer for patients receiving combined therapy than for those receiving PD-1/PD-L1 inhibitor monotherapy (5.4 vs 2.0 months, P = 0.020). Patients receiving immune therapy as a first-line treatment had prolonged survival compared with those receiving it as a second-line or multiple-line treatment, but the difference was not statistically significant (mOS: 7.0 vs 5.1 vs 2.8 months, P = 0.161). There was a reduction in the serum level of CA19-9 associated with the response to treatment. Adverse events were tolerable and were mainly grade 1 and 2. The immune-related adverse events that occurred were hypothyroidism, diarrhea, and rash.
Conclusion: Immune checkpoint inhibitors showed a certain efficacy in the treatment of advanced pancreatic cancer and could confer long-term survival benefits. Combined therapy was more effective and may serve as an alternative option. Further studies should be performed.
Keywords: pancreatic cancer, immune therapy, checkpoint inhibitor
Introduction
Pancreatic cancer is a type of malignant tumor with a relatively high mortality rate. The 5-year survival rate of patients with pancreatic cancer is approximately 7%, and the incidence of pancreatic cancer is increasing annually. Radical operation is still the curative treatment for pancreatic cancer, while other options such as chemotherapy, radiotherapy, or targeted therapy have little curative effect and cannot confer obvious survival benefits. In recent years, immunotherapy, especially the use of immune checkpoint inhibitors, has been very successful in the treatment of various cancers. This class of drugs, through blocking the PD1/PD-L1 pathway and mobilizing the immune system to kill the tumor, has a broad spectrum of antitumor effects. Nonetheless, the current results of clinical trials in the treatment of pancreatic cancer show that monotherapy of immune checkpoint inhibitors does not yield satisfactory results, and further studies are underway. Our study observed 43 patients with advanced pancreatic cancer in our hospital who were treated with immune checkpoint inhibitors and analyzed the clinical efficacy of the treatment and the adverse events associated with it.
Patients and methods
Patients
From January 2015 to July 2017, patients with advanced pancreatic cancer receiving immune checkpoint inhibitors in our medical center were considered eligible for this retrospective analysis.
The recruitment criteria were as follows:
- Presence of clinicopathologically confirmed advanced pancreatic cancer that could not be surgically resected or that recurred after surgery.
- Absence of second primary malignancies.
- Administration of immune checkpoint inhibitors as the first-line therapy or after the failure of previous treatments.
- Administration of at least two cycles of immune checkpoint inhibitors.
Treatments
All patients received the PD-1/PD-L1 inhibitors pembrolizumab, nivolumab, or atezolizumab until the cessation of treatment due to disease progression or drug toxicity. Pembrolizumab was administered 2 mg/kg once every three weeks, nivolumab was administered 3 mg/kg once every two weeks, and atezolizumab was administered 1,200 mg once every three weeks. The specific dosage was adjusted according to the condition of the patient. PD-1/PD-L1 inhibitors were administered as single agents or in combination with chemotherapy, targeted therapy or a CTLA-4 inhibitor (ipilimumab).
Before treatment, all patients who had at least one measurable lesion underwent routine blood tests including blood biochemistry and serum tumor marker levels and imaging examinations such as CT, MRI, and ultrasound to comprehensively assess the disease and obtain baseline information. Imaging examinations were used to evaluate the curative effect of immune checkpoint inhibitors after every two or three cycles. Adverse events were recorded.
Assessments
Based on Response Evaluation Criteria in Solid Tumors (RECIST) 1.1, tumor responses can be divided into complete remission (CR), partial remission (PR), stable disease (SD), and progression of the disease (PD). According to iRECIST for immunotherapy, an auxiliary evaluation, the curative effects can be categorized as immune complete response (iCR), immune partial response, immune unconfirmed progressive disease, and immune confirmed progressive disease. The objective response rate (ORR) was calculated based on the CR and PR; and the disease control rate (DCR) was calculated based on the CR, PR, and SD. At the same time, progression-free survival (PFS) and overall survival (OS) were evaluated. PFS was the duration from the time that the patient received treatment to the time of disease progression or to the patient’s death due to disease progression. OS was the duration from the time that the patient received treatment to the patient’s death time from any cause. Adverse events were classified and recorded based on National Cancer Institute-Common Terminology Criteria for Adverse Events 4.0 and related immunotherapeutic adverse events (such as dermal toxicity, intestinal toxicity, hepatotoxicity, immune-related pneumonia, and thyroid toxicity) were noted.
Statistical analysis
The primary end points of this study were the ORR, PFS, and OS. The secondary end point was safety. We also conducted some subgroup analyses to identify the population that might benefit the most from immune therapy. All the patient data were updated within one month of the follow-up visit on November 30, 2017. The statistical analyses were performed with SPSS version 22.0. All numerical data were analyzed with chi-square tests and Fisher’s exact tests, and all measurement data were analyzed with Independent-Samples t-tests. The survival analysis was conducted according to the Kaplan–Meier method and tested with log-rank tests. In this research, the result was considered statistically significantly different when P < 0.05. The original cutoff date for the analysis of PFS and OS was November 30, 2017.
Ethics approval
This retrospective study was approved by the ethics committee of Chinese PLA General Hospital. As this was a retrospective study based on real-word clinical data instead of clinical trial, patient consent to review their medical records was not required by ethics committee of Chinese PLA General Hospital. All the participants of this study guarantee the patient data confidentiality. All the patients were informed the possible adverse events and potential risk of treatment by doctors. All of them have signed informed consent before using PD-1/PD-L1 inhibitor.
Results
Patients
Patients with pancreatic cancer treated with immune checkpoint inhibitors from January 2015 to July 2017 in the Chinese People’s Liberation Army (PLA) General Hospital were enrolled; there were 51 patients pathologically and clinically diagnosed with advanced pancreatic cancer. Ultimately, 43 patients with advanced pancreatic cancer were enrolled, after excluding eight patients who were lost to follow-up after only one cycle of immunotherapy. The characteristics of the 43 enrolled patients are shown in Table 1.
  | Table 1 Characteristics of patients with advanced pancreatic cancer received |
Thirty-two patients received combined therapy, with therapeutic schedules including chemotherapy (29 patients), targeted drugs (nine patients), and the CTLA-4 inhibitor ipilimumab (three patients). Among the 29 patients treated with PD-1/PD-L1 inhibitor combined with chemotherapy, 12 patients (41.4%) were administered a single chemotherapy drug, and 17 patients (58.6%) were administered two chemotherapy drugs (Table 1).
In the patients receiving PD-1/PD-L1 inhibitor combined with chemotherapy, 15 patients were administered gemcitabine-based chemotherapy, while 10 were administered nab-paclitaxel and gemcitabine. Seventeen patients received nab-paclitaxel, seven received nimotuzumab, and three patients received ipilimumab (Figure 1).
  | Figure 1 PD-1/PD-L1 inhibitor combined with double chemotherapy drugs. |
Efficacy analysis
Among the 43 patients, 38 were evaluated for the efficacy of the treatment. No patients achieved CR, four patients achieved PR, 15 patients had SD, and 19 patients had PD. The ORR was 10.5% (95% confidence interval [CI] 2.6–21.1), and the DCR was 50.0% (95% CI 34.2–65.8) (Table 2).
Because this study was a retrospective analysis, among the 38 patients who were evaluated for the efficacy of treatment, imaging studies were not identified for 12 patients. The imaging data of the other 26 patients were used to evaluate their targeted lesion sizes. Among these 26 patients, 10 patients experienced shrinkage of their target lesions, and four of those whose lesions shrank significantly and achieved PR with rates of 86%, 72%, 36%, and 25% (Figure 2A).
All patients received follow-up, ranging from 0.7 to 80.0 weeks, with a median follow-up period of 15.0 weeks. The duration of the administration of medication ranged from 2.8 to 59 weeks, with a median of 8.2 weeks. Eleven patients were still receiving immune checkpoint inhibitors at the end of follow-up. The drug onset times for the four patients who achieved PR were 5.9, 7.6, 7.6, and 8.0 weeks. Furthermore, two of these four patients experienced disease progression, with PFS times of 30.4 and 31.7 weeks, while the other two patients had maintained PR at the end of follow-up (Figure 2B).
Survival analysis
Among the 43 patients, four lost PFS, and 28 of the remaining 39 had progressive disease, with a median PFS of 2.3 months (95% CI 1.971–2.659) and a 6-month PFS rate of 34.2%; four lost OS, and 20 of the other 39 died, with a median OS of 5.1 months (95% CI 3.776–6.409). The 1-year OS rate was 32.6% (Table 3; Figure 3).
Subgroup analysis
Comparison of the line of treatment
Among the 39 patients who were followed up to the median OS, 18 patients received the immune checkpoint inhibitor as a first-line treatment, 13 patients received it as a second-line treatment, and eight patients received it as a third-line or multiple-line treatment. Patients who received the checkpoint inhibitor as a second-line treatment or earlier all received chemotherapy regimens related to gemcitabine and 5-Fu. The results showed that using the immune checkpoint inhibitor as a first-line treatment resulted in a longer median overall survival (mOS) than using it as a second-line or multiple-line treatment, but the difference was not statistically significant (7.0 vs 5.1 vs 2.8 months, P = 0.161) (Table 4; Figure 4).
Monotherapy and combined therapy
Ten patients received monotherapy with the immune checkpoint inhibitor, and 29 patients received combined therapy. Among them, 26 patients used chemotherapy combined with immune check point inhibitor. The comparisons between monotherapy and combined therapy were as follows: DCR 50.0% versus 44.4% (P = 0.538), median progression-free survival (mPFS) 2.9 months versus 2.0 months (P = 0.227), and mOS 5.4 months versus 2.0 months (P = 0.020). In terms of DCR and mPFS, monotherapy and combined therapy had similar results, while in regard to OS, combined therapy was significantly better than monotherapy (P < 0.05) (Table 5; Figure 5).
Analysis of the continuation of PD-1 therapy after disease progression
Seven patients continued to be treated with the immune checkpoint inhibitor after immune checkpoint inhibitor therapy had failed. Among them, five had PD, and two had SD by the time the follow-up period ended. When patients continued to be treated with the immune checkpoint inhibitor after disease progression, the median PFS was 2.2 months (95% CI 1.780–2.623) (Figure 6).
CA19-9 level change
The levels of the serum tumor marker CA19-9 were tested during the treatment of 34 patients, and treatment efficacy was evaluated in 31 of those patients. After immune checkpoint inhibitor therapy had been used for two or three cycles, the results of the reexamination showed that the CA19-9 levels of nine patients (30%) decreased compared to the levels before treatment, with two patients achieving PR, six with SD, and one with PD. Compared with the levels before the treatment, the CA19-9 levels of 22 patients (70%) increased. Among them, the levels increased less than 100% in 11 patients and more than 100% in 11 patients. Among these 22 patients, two achieved PR, seven had SD and 13 had PD. There was an obvious correlation between changes in the level of CA 19–9 and curative effect (P = 0.028, 95% CI 0.266–0.761) (Table 6; Figure 7).
  | Table 6 The efficacy of patients with different CA19-9 level changes |
  | Figure 7 The association between the response status and the percentage change in CA19-9 level. |
PD-L1 expression and genetic mutation
Fifteen of the 43 patients were tested for the protein expression level of PD-L1/PD-1, with seven testing negative and eight testing positive for PD-L1 protein expression. Thirteen patients were evaluated for treatment efficacy. Among the seven negative patients, three had SD, and four had PD. Among the eight positive patients, one achieved PR (PD-L1+ 50%–75%), three had SD, and two had PD. The median PFS and mOS for the negative patients were 2.9 months (95% CI 0–6.095) and 7.0 months (95% CI 3.570–10.426), respectively. The median OS for the positive patients was 5.1 months (95% CI not reach [NR]), and the median PFS had not been reached by the end of the follow-up period. There were no significant differences between these two groups (P > 0.05) (Figure 8).
  | Figure 8 The efficacy of treatment in patients with different levels of PD-L1 expression. |
Six patients underwent second-generation gene sequencing, and genetic mutations were found. One patient had an MET genetic mutation, and five patients had KRAS genetic mutations. In addition to the KRAS mutation, one patient also had TP53, MYC, CNKN2A, and NF2 genetic mutations (Table 7).
Safety
The adverse events reported in the 43 patients were mainly neutropenia (33%), nausea and vomiting (16%), alopecia (16%), fatigue (12%), and thrombocytopenia (12%). These events were mainly grade 1 or grade 2. Grade 3 or grade 4 adverse events were mainly neutropenia (two cases of grade 3) and thrombocytopenia (two cases of grade 3 and two cases of grade 4). In terms of immune-related adverse events, two patients (5%) had hypothyroidism, and one of the three patients with diarrhea or rash might have been experiencing symptoms related to the immunotherapy (Table 8).
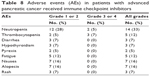  | Table 8 Adverse events (AEs) in patients with advanced pancreatic cancer received immune checkpoint inhibitors |
Discussion
Pancreatic cancer, which is notorious for its severity and poor curative effects and prognosis, ranks as the sixth most common cause of cancer-related deaths in China and has a 5-year survival rate of 7%.1,2 Radical operation may be the only method of curing the disease; however, due to the difficulty of early diagnosis, approximately 80%–85% of the patients who are diagnosed have already entered the disease progression stage or the advanced stage and have lost the chance to undergo the operation.3 At present, these patients mainly depend on systemic chemotherapy, targeted therapy, local radiotherapy, and symptomatic and supportive treatments.
In recent years, immune checkpoint inhibitors have been successfully used to treat various solid tumors, such as malignant melanoma, lung carcinoma, head and neck neoplasms, bladder carcinoma, renal carcinoma, and Hodgkin’s lymphoma.4 PD-1, a type of immunosuppressive signaling molecule, can be expressed on the surfaces of various cells, including activated T lymphocytes, B lymphocytes, Treg cells, and natural killer (NK) cells. Its ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC), are mainly expressed on the surfaces of antigen-presenting cells and tumor cells. The activated PD-1/PD-L1 pathway can suppress the function of T cells, promote T cell apoptosis, downregulate the secretion of inflammatory cytokines, and reduce the toxicity of cells, which can eventually lead to tumor immune escape.5,6 Immune checkpoint inhibitors, through blocking the PD1/PD-L1 signaling pathway and mobilizing the immune system to kill the tumor, have a broad spectrum of antitumor effects.7
Studies showed that PD-L1 expression was upregulated in pancreatic cancer cells.8 In a preclinical study, PD-1 inhibitors were shown to effectively suppress tumors through upregulating the secretion of IFN-γ and downregulating the secretion of IL-10.9 In a Phase I clinical trial with 207 patients with solid tumors (including 14 patients with pancreatic cancer), the condition of 166 patients improved, but no patients with pancreatic cancer achieved remission.10 In another Phase I clinical trial related to the PD-1 inhibitor pembrolizumab, among the 32 patients enrolled, there was one patient with pancreatic cancer, whose best curative effect was evaluated as SD and whose PFS was 20 weeks.11 In a Phase II clinical trial of the CTLA-4 inhibitor ipilimumab, 27 patients with advanced pancreatic cancer were enrolled. After two periods of treatment, reexamination showed that no patients were in remission. Only one achieved PR after continuing to be treated with the original therapeutic strategy for another cycle.12 Although the cases enrolled in these clinical trials were limited, based on the current results, monotherapy with immune checkpoint inhibitors does not yield satisfactory effects in the treatment of pancreatic cancer. On the one hand, this might be because patients with pancreatic cancer have comparatively high tumor loads; on the other hand, it might be because pancreatic cancer has comparatively low immunogenicity.13 Studies showed that chemotherapy could increase the release of tumor antigens and reactivate the antitumor immune response. Chemotherapy can also influence tumor microenvironment to help in promoting antigen expression and antitumor immune response.14 The combination of immunotherapy and chemotherapy can achieve a synergistic effect.15 The synergistic effect of chemotherapy combined with immune checkpoint inhibitor depends both on the dosage of chemotherapy and the stimulation of tumor antigen presenting capacity. Clinical trials combining PD-1, PD-L1, and CTLA-4 inhibitors with other therapeutic methods (immune vaccine, chemotherapy, and multitarget inhibitors) are in progress.
This study retrospectively analyzed 43 patients with advanced pancreatic cancer who were treated with a PD-1/PD-L1 inhibitor. Twenty received first-line treatment, 14 received second-line treatment, and nine received multiple-line treatment. Eleven received monotherapy of the PD-1/PD-L1 inhibitor, and the others received combined therapy of the PD-1/PD-L1 inhibitor and another treatment (including chemotherapy, targeted drugs, and CTLA-4 inhibitors). The results of the analysis showed that the ORR was 10.5%, the DCR was 50.5%, the mPFS was 2.3 months, and the mOS was 5.1 months. The PD-1/PD-L1 inhibitor showed a certain degree of effectiveness in the treatment of advanced pancreatic cancer because four patients who were treated with the PD-1 inhibitor and dual-drug chemotherapy achieved PR. The subgroup analysis revealed that the use of combination therapy significantly improved the OS compared with the use of a single drug, but there were no significant differences in DCR and PFS, which might be attributed to the limited number of cases. The OS for patients receiving the PD-1/PD-L1 inhibitor as a first-line treatment was clearly longer than that of those receiving it as a second-line and multiple-line treatment. This might be because most patients who received it as a multiple-line treatment were in poor physical condition, with poor chemotherapy tolerance, poor immunity, and high tumor load; they were in the end-stage phase, when using immune checkpoint inhibitors could not achieve a desirable antitumor effect. This study also revealed that the level of CA19-9, a well-recognized sensitive tumor marker for pancreatic cancer, had an obvious correlation with the curative effect. Therefore, CA19-9 could work as a biological marker indicating the efficacy of the treatment. At present, the expression of PD-L1 is the most widely recognized marker used to predict the efficacy of the PD-1/PD-L1 inhibitor. In this research, the expression of PD-L1 was tested in 15 patients; one patient had a high level of PD-L1 expression (>50%). The curative effect for this patient with a high expression level was PR, and the best curative effect for PD-L1-negative patients was SD. However, the expression of PD-L1 had no obvious correlation with the curative effect (P > 0.05), which could be explained by the limited number of cases and different PD-L1 testing methods.
There were a number of factors affecting the efficacy of the immunotherapy. A previous study found that genetic mutation was an influencing factor when immune checkpoint inhibitors were used to treat lung cancer. According to the recommendation of the National Comprehensive Cancer Network Guidelines 2017 for the Treatment of Lung Cancer, immunotherapy is suitable for EGFR-, ALK-, and ROS1-negative patients.16 The results of a meta-analysis by Jiang et al showed that immunotherapy was inferior to chemotherapy in the treatment of patients with lung cancer with EGFR mutations.17 EGFR mutations were negatively correlated with PD-L1 expression, and mutated tissues without T cell infiltration reflected no immunogenic tolerance. In addition, they also found that patients with lung cancer with KRAS mutations had high levels of expression of PD-L1 and increased infiltration of T cells; the OS of patients receiving immunotherapy was clearly better than that of those receiving chemotherapy. Double mutation of KRAS and TP53 significantly increased the expression of PD-L1 in lung adenocarcinoma and the double positive proportion of PD-L1+/TIL+. In addition, the double mutation of KRAS and TP53, which was significantly correlated with increased tumor mutation load, might be a potential marker of tumor response to immunotherapy.18 In this study, six patients received second-generation genetic testing, with two achieving PR, two with SD, and two with PD as their best tumor response. For the two patients who achieved PR, one had a KRAS genetic mutation and one had multigene mutations including double mutation of KRAS and TP53; their tumor sizes shrank by 72% and 86%, respectively. The correlation between genetic mutations in patients with pancreatic cancer and the curative effect of the PD-1/PD-L1 inhibitor and the internal mechanism driving that correlation deserve further study.
Adverse events experienced by patients in this study mainly belonged to grades 1 or 2, with a few experiencing grades 3 or 4 neutropenia and thrombocytopenia, which might have been related to chemotherapy and could be improved after treatment. Immune-related adverse events were mainly mild thrombocytopenia, diarrhea, and rash, and the overall adverse events were tolerable.
Conclusion
The tumor microenvironment inhibited by hyperimmunity causes pancreatic cancer to be less sensitive to such treatments as chemotherapy and radiotherapy. Generally, monotherapy or combined therapy with an immune checkpoint inhibitor showed certain efficacy in the treatment of advanced pancreatic cancer and provided some survival benefit to patients. Studies should be conducted to explore how to expose more tumor-specific antigens, how to foster the ability of T cells to recognize antigens and kill cells, how to select the most suitable patients for immunotherapy, and how to reduce related adverse events.
Acknowledgment
We thank all the coworkers in our medical department who contributed to providing information for all enrolled patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. | ||
Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66(2):115–132. | ||
Bond-Smith G, Banga N, Hammond TM, Imber CJ. Pancreatic adenocarcinoma. Br Med J. 2012;344:e2476. | ||
Ma W, Gilligan BM, Yuan J, Li T. Current status and perspectives in translational biomarker research for PD-1/PD-L1 immune checkpoint blockade therapy. J Hematol Oncol. 2016;9(1):47. | ||
Harvey RD. Immunologic and clinical effects of targeting PD-1 in lung cancer. Clin Pharmacol Ther. 2014;96(2):214–223. | ||
Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16(5):275–287. | ||
Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207–212. | ||
Geng L, Huang D, Liu J, et al. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol. 2008;134(9):1021–1027. | ||
Okudaira K, Hokari R, Tsuzuki Y, et al. Blockade of B7-H1 or B7-DC induces an anti-tumor effect in a mouse pancreatic cancer model. Int J Oncol. 2009;35(4):741–749. | ||
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–2465. | ||
Patnaik A, Kang SP, Rasco D, et al. Phase I Study of Pembrolizumab (MK-3475; Anti-PD-1 Monoclonal Antibody) in Patients with Advanced Solid Tumors. Clin Cancer Res. 2015;21(19):4286–4293. | ||
Royal RE, Levy C, Turner K, et al. Phase 2 trial of single agent Ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J Immunother. 2010;33(8):828–833. | ||
Pico de Coaña Y, Choudhury A, Kiessling R. Checkpoint blockade for cancer therapy: revitalizing a suppressed immune system. Trends Mol Med. 2015;21(8):482–491. | ||
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity. 2013;39(1):74–88. | ||
Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–158. | ||
Ettinger DS, Wood DE, Aisner DL, et al. Non-Small Cell Lung Cancer, Version 5.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. | ||
Jiang Q, Xie M, He M, et al. Anti-PD-1/PD-L1 antibodies versus docetaxel in patients with previously treated non-small-cell lung cancer. Oncotarget. 2017;9(7):7672–7683. | ||
Dong ZY, Zhong WZ, Zhang XC, et al. Potential Predictive Value of TP53 and KRAS Mutation Status for Response to PD-1 Blockade Immunotherapy in Lung Adenocarcinoma. Clin Cancer Res. 2017; 23(12):3012–3024. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.










