Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Clinical Importance of FNDC-5 and Selectin-E mRNA Expression Among Type 2 Diabetics with and without Obesity
Authors Verma AK, Aladel A , Dabeer S , Ahmad I , Khan MI, Almutairi MG , Al-Harbi AI , Beg MMA
Received 4 December 2021
Accepted for publication 10 March 2022
Published 2 April 2022 Volume 2022:15 Pages 1011—1021
DOI https://doi.org/10.2147/DMSO.S352483
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Antonio Brunetti
Amit K Verma,1,2 Alanoud Aladel,3 Sadaf Dabeer,4 Irfan Ahmad,5 Mohammad Idreesh Khan,6 Malak Ghazi Almutairi,7 Alhanouf I Al-Harbi,8 Mirza Masroor Ali Beg9,10
1Department of Biotechnology, Jamia Millia Islamia University, New Delhi, India; 2Department of Zoology and Environmental Sciences, Gurukul Kangri Vishwavidyalaya, Haridwar, India; 3Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia; 4Department of Exercise Science and Sport Management, Kennesaw State University, Kennesaw, GA, USA; 5Department of Clinical Laboratory Science, College of Applied Medical Sciences, King Khalid University, Abha, Saudi Arabia; 6Department of Clinical Nutrition, College of Applied Health Sciences in Arras, Qassim University, Buraidah, Saudi Arabia; 7Department of nutrition, Almethnab General Hospital, Qassim Health Cluster, Ministry of Health, Al Mithnab, Saudi Arabia; 8Department of Medical Laboratory, College of Applied Medical Sciences, Taibah University, Yanbu, Saudi Arabia; 9Faculty of Medicine, Ala-Too International University, Bishkek, Kyrgyzstan; 10Centre for Promotion of Medical Research, Ala-Too International University, Bishkek, Kyrgyzstan
Correspondence: Mirza Masroor Ali Beg, Email [email protected]
Background: Type 2 diabetes mellitus (T2DM) is growing illnesses associated with metabolic dysregulation such as obesity affecting a large population become leading causes of death worldwide. Fibronectin type III domain-containing protein 5 (FNDC-5) and selectin-E were suggested to have effects on metabolism and diabetes, therefore present study aimed to evaluate the clinical importance of FNDC-5 and selectin-E among the T2DM patients with and without obesity.
Methods: Study included cohort of 200 T2DM patients with and without obesity. We evaluated FNDC-5, selectin-E mRNA expression as well as vitamin-D, and vitamin-B12 levels in among the T2DM patients with and without obesity.
Results: Study observed significant difference in biochemical parameters included in study. T2DM patients with obesity had significantly higher fasting blood glucose levels (p< 0.0001) and HbA1c (glycated hemoglobin) (p< 0.0001) compared to those T2DM patients without obesity. T2DM patients with obesity also had higher systolic blood pressure (p=0.001), LDL (low density lipoprotein) (p=0.02), TG (triglycerides) (p=0.02) and cholesterol (p=0.01) compared to T2DM patients without obesity. The mRNA expression of FNDC-5 (p< 0.0001) was lower in T2DM patients with obesity compared to T2DM patients without obesity. It was observed that the T2DM patients with vitamin-D deficiency had significantly lower FNDC-5 mRNA expression (p=0.03) when compared with those with sufficient vitamin-D level. T2DM patients with clinically normal vitamin-B12 level expressed 0.60 fold FNDC-5 mRNA expression while B12 deficient T2DM patients had 0.28 fold FNDC-5 mRNA expression (p=0.005). No as such significant association was was observed with selectin-E. A negative correlation of FNDC-5 mRNA expression with Post prandial glucose (mg/dl) (p=0.04) and TG (mg/dl) (p=0.02) was observed.
Conclusion: FNDC-5 down regulation was observed with T2DM with obesity, vitamin-D and vitamin-B12 deficiency suggesting obesity, vitamin-D and vitamin-B12 deficiency could be the factor for FNDC-5 down-regulation leading to worseness or progression of disease. We suggest that FNDC-5 down-regulation could be used as an indicator for T2DM worseness and development of other associated complications.
Keywords: type 2 diabetes mellitus, obesity, FNDC-5, selectin-E, mRNA, expression
Introduction
T2DM and associated comorbidity like obesity is an epidemic health problem that has raised concern worldwide. The multifactorial causes of T2DM and obesity lead to health burden among all age groups. Prevention can be achieved by lifestyle and behavioral interventions. However, Because of complex and long-lasting hormonal, metabolic changes, the effectiveness is limited and these adaptations protect against weight loss and promote weight gain.
Obesity, excess body weight for height, but this simple definition belies an etiologically complex phenotype primarily associated with excess adiposity, or body fatness.1 Obesity significantly surges risk of chronic disease morbidity such as disability, depression, type 2 diabetes, cardiovascular disease.1
FNDC5 encodes irisin hormone has the capacity to trigger beneficial alterations in adipose tissue to improve muscle activity, it’s a thermogenic adipomyokine made when the precursor plasma membrane protein FNDC5 is cleaved.2 FNDC5 contributes to muscle-adipose tissue crosstalk by converting white to brown fat (browning of adipose tissue) in the metabolic energy process.3 FNDC5 regulates skeletal muscle, hepatic glucose uptake, and lipid metabolism, and has a protective effect against hyperlipidemia, hyperglycemia, and metabolic syndrome in obese people,4 and has lower expression in WAT than in muscle in humans.5 It has been linked to changes in glucose and lipid levels in obese and metabolic syndrome patients6 and weight loss happens due to decreased FNDC5 mRNA expression in skeletal muscle.7 Selectins are calcium-dependent transmembrane lectins that include three functionally distinct adhesion molecules expressed as E-selectin, P-selectin, and L-selectin where selectin-E plays an essential role in cell injury, vascular occlusion, and angiogenesis.8 TNF and IL-1 are induced by the expression of Selectin-E on endothelial cells,9 In both human and mouse endothelial cells, these cytokines induce transient transcription after exposure to E-selectin mRNA.10 Selectin-E ligands that are activated during rolling transduce signals that affect the function of the b2 integrin.11 Role of FNDC5 and selectin-E is novel and not well explored in T2DM patients with and without obesity. Therefore, Present study aimed to explore the role of FNDC-5, selectin-E mRNA expression in T2DM cases with and without obesity as well as their association with vitamin-D and B12 level.
Materials and Methods
Study Population and Sample Collection
Present research work included 200 T2DM patients with and without obesity (BMI) and 200 healthy controls. From the entire study participants 2 mL of peripheral blood samples were withdrawn in plain vial, 2mL in EDTA vials plain vials and 1 mL collected in fluoride vial.
Blood sample collected in fluoride vials (before and after meals) and EDTA vials were used for diagnosis of T2DM based on blood glucose and HbA1c level. Diagnostic criteria included, fasting blood glucose (> 126 mg/dL) and postprandial glucose (2hrs > 200 mg/dL) as well as glycated haemoglobin (HbA1C) were taken in consideration. Blood samples withdrawn in EDTA vials from all the participants were stored in −80°C for further processing such as total RNA extraction and blood sample of plain vials were centrifuged to separate the serum for biochemical investigations. This research study was ethically approved (12/9/176/JMI/IEC/2018) by the ethical committee board, Jamia Millia Islamia, New Delhi, India and all participants gave their written informed consent before the study began. Present study was conducted at Department of Biotechnology, Jamia Millia Islamia, New Delhi, India.
Total RNA Extraction, Complementary DNA Synthesis and Quantitative PCR for Expression Study
Total RNA was extracted using RNA extraction kit (GeneAid, Taiwan) and further extracted total RNA stored at −70°C. The quality and concentration of total RNA was checked by taking absorbance on 260/280 ratio using nanophotometer (NanoPhotometer p330, IMPLEN), further 100ng of total extracted RNA from each study participant was used to generate the cDNA library using kit (Verso, Thermo Scientific, USA) following manufacturer’s instructions. The expressions of FNDC-5 and Selectin-E mRNA were analyzed by quantitative real-time PCR using SYBR green dye using specific primer sequences. The program followed as: qRT-PCR for FNDC-5 (F; 5-CCAGTATGACATCATTGAAGCGT-3 and R; 5-TGCAGTTGTCCCTCTCCCT-3, 108bp) and Selectin-E (F; 5-AAGGCTTCATGTTGCAGGGA-3 and R; 5-ATTCATGTAGCCTCGCTCGG-3, 128bp), and β-actin (F; 5- AGCACAGAGCCTCGCCTTT −3 and R; 5- GAGCGCGGCGATATCATCA-3, 82bp) was performed for 40 cycles, initial denaturation was at 94°C for 35 second, annealing temperature for FNDC-5, Selectin-E, and β-actin was at 60°C for 35 seconds, extension at 72°C for 35 seconds and 20µL reaction volume was used. To confirm the target amplification, an additional step at 72°C for 10 minutes was performed to finish the reaction and a melting curve examination was performed between 35°C and 90°C. A control without cDNA was included in each experiment, and every reaction was done in duplicate. The FNDC-5 and selectin-E mRNA expression levels were calculated using the relative quantification by 2−(ΔΔCT) method.
Vitamin-D and B12 Level Assessment
Sored serum of T2DM cases and healthy controls in −80°C were thawed further vitamin-D and B12 levels were assessed by the electrochemiluminescence immunoassay (Cobas e411, Roche, Basel, Switzerland). Serum 25 (OH) D levels ≤20 ng/mL was considered as Vitamin-D deficiency, <30 ng/mL considered as insufficiency and 30ng/mL or above considered as sufficiency.12 Vitamin B12 deficiency was defined as <148 pmol/L in serum and above was considered as normal range.13
Statistical Analysis
Data analysis was performed using Graph Pad Prism software version 6.05 and SPSS 21.0. The Mann–Whitney U-test (2 groups), Kruskal–Wallis post hoc Dunn test (>2 groups) was used to analyze the significant differences among the groups. The relative cycle threshold (Ct) method was used to analyze the QRT-PCR results and the relative quantification method was used to calculate the FNDC-5 and Selectin-E mRNA expression levels using the 2–(ΔΔCt) method. Expression was defined as up- or down-regulated as a result of more than or less than one and all values were normalized relative to the control values, which were depicted as a value of 1. p< 0.05 was considered significant.
Population Demographics
Present study included 200 T2DM (65.5% males) cases and equal number of healthy subjects (62.5% males), Health data like hypertension, increased urination, weight loss, fatigue, wound healing, blue vision, loss of appetite, smoking status, alcoholism and obesity were also collected (Table 1).
 |
Table 1 Population Descriptive of Study Participants |
Evaluation of Biochemical Factors Among T2DM Cases and Healthy Controls
Biochemical parameters were compared between T2DM cases and healthy controls. Significant differences were observed in Fasting blood glucose (mg/dl), Post prandial glucose (mg/dl), HbA1c (%), Systolic blood pressure (mmHg), Diastolic blood pressure (mmHg), HDL (mg/dl), LDL (mg/dl), TG (mg/dl), Cholesterol (mg/dl), VLDL (mg/dl) between T2DM patients and healthy control (Table 2).
 |
Table 2 Differences in Biochemical Parameters Among the T2DM Patients and Healthy Controls |
Comparison of Biochemical Parameters of T2DM Patients with and without Obesity
Two groups were made such as T2DM patients with and without obesity based on BMI, Further, biochemical parameters were compared among the T2DM patients with and without obesity (Table 3). It was observed that the fasting blood glucose (p<0.0001) and HbA1c (p<0.0001) were high in T2DM patients with obesity compared to the non-obese T2DM patients. T2DM patients with obesity exhibited significantly higher systolic blood pressure compared to non-obese (p=0.001). Serum levels of LDL (p=0.02), TG (p=0.02) and cholesterol (p=0.01) were also significantly higher among the T2DM patients with obesity compared T2DM without obesity (Table 3).
 |
Table 3 Differences in Biochemical Parameters Among the T2DM with and without Obesity |
Comparison of FNDC-5 and Selectin-E mRNA Expression in T2DM Patients with and without Obesity
Present study observed overall fold change in FNDC-5 mRNA expression was 0.54 fold (SD±0.39) and selectin-E mRNA expression was 1.56 fold (SD±0.60) among the T2DM cases. FNDC-5 and selectin-5 mRNA was analyzed among two groups such as T2DM patients with and without obesity (Figure 1A and B). It was observed that FNDC-5 mRNA expression was lower in T2DM cases with obesity (0.28 fold) (p<0.0001) while selectin-E mRNA expression was higher in T2DM cases with obesity (1.64 fold) when compared without obesity (p=0.28).
 |
Figure 1 mRNA expression (A) FNDC-5 expression in T2DM patients with and without obesity (B) Selectin-E expression in T2DM patients with and without obesity. |
Comparison of FNDC-5 and Selectin-E Among Vitamin-D Sufficient, Insufficient and Deficient Patients with T2DM
T2DM patients were divided in 3 groups namely based on serum vitamin-D levels such as vitamin-D deficient, insufficient and sufficient. The mRNA expression levels of FNDC-5 and selectin-E were compared among the patients of each group (Figure 2A and B). It was observed that the T2DM cases with vitamin-D deficiency had low FNDC-5 mRNA expression (0.28 fold) compared to insufficient (0.56 fold, p=0.03) and sufficient (0.67 fold, p<0.0001) vitamin-D level. T2DM cases with vitamin-D deficiency had low selectin-E mRNA expression (1.44 fold) compared to insufficient (1.66 fold) and sufficient (1.60 fold) serum vitamin-D level.
Comparison of FNDC-5 and Selectin-E with Respect to Vitamin B12 Status
T2DM patients were divided in 2 groups based on their serum Vitamin B12 levels. It was observed that the T2DM cases with normal B12 level had 0.60 fold FNDC-5 mRNA expression while B12 deficient T2DM had 0.28 fold FNDC-5 mRNA expression (Figure 3A), (p=0.005). T2DM cases with B12 normal level had 1.52 fold selectin-E mRNA expression while B12 deficient T2DM cases had 1.69 fold mRNA expression (Figure 3B) (p=0.20).
 |
Figure 3 Comparison of mRNA expression of (A) FNDC-5 in T2DM patients with normal and deficient Vitamin B12. (B) Selectin-E in T2DM patients with normal and deficient serum Vitamin B12 level. |
Prognostic Importance of FNDC-5, Selectin-E mRNA Expression and T2DM with and without Obesity
Prognostic importance of FNDC-5 and selectin-E were analyzed with T2DM cases with and without obesity (Figure 4, Table 4). It was observed that AUC for FNDC-5 was 0.75 and at the 0.30-fold cutoff, sensitivity, specificity was 70% and 60% (p<0.0001) suggested that the expression of FNDC-5 ≤ 0.30-fold may be an indicator of T2DM in patients with obesity. Similarly, AUC for selectin-E was 0.54 and at the 1.35-fold cutoff, sensitivity, specificity was 61% and 46% (p=0.28).
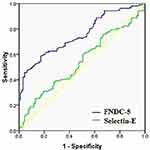 |
Figure 4 ROC curve for FNDC5 and Selectin-E among the T2DM patients with obesity vs without obesity. |
 |
Table 4 ROC Curve to Presenting the AUC for FNDC-5 and Selectin-E Among the T2DM with Obesity Vs Without Obesity |
Correlation of FNDC5 and Selectin-E with Biochemical Parameters Among the T2DM Patients with and without Obesity
Correlation analysis for FNDC5 and selectin-E with biochemical parameters were done and observed that the FNDC-5 mRNA expression was found to have significant negative correlation with Post prandial glucose (mg/dl) (p=0.04) and TG (mg/dl) (p=0.02) (Table 5).
 |
Table 5 Correlation Analysis of FNDC-5 and Selection-E with Biochemical Parameters |
Discussion
Obesity, insulin resistance, dyslipidemia, hyperglycemia, and hypertension are among the medical conditions that make up the metabolic syndrome.14 Metabolic syndrome affects about one-fourth of the adult population in both developed and developing countries, which causes diabetes, cardiovascular disease, and all-cause mortality, as well as significant financial costs associated with hospitalization and long-term prescription drug use for blood glucose, lipids, and blood pressure management.15 Obesity and diabetes, unfortunately, have been steadily increasing in recent years and have become a major public health concern worldwide.16 The biochemical parameters of T2DM patients with and without obesity showed significant differences. It has been observed higher fasting blood glucose, HbA1c, systolic blood pressure, LDL, TG, cholesterol among the T2DM patients with obesity. Overall mRNA expression of FNDC-5 (0.54-fold±0.39) and selectin-E (0.98-fold±0.67) was significantly decreased. It was found that the T2DM patients with obesity had lower FNDC-5 mRNA expression compared to T2DM cases without obesity. Vitamin-D deficient T2DM patients had lower mRNA level of FNDC-5 and selectin-E compared to patients with insufficient and sufficient serum vitamin-D level. It was also observed that the T2DM cases with normal B12 deficient had lower FNDC-5 mRNA expression when compared with T2DM patients with those having normal B12 level in serum.
The expression of FNDC5 necessary for a healthy metabolic status in humans.17 Dysregulation of FNDC5 can cause a systemic metabolism imbalance, which can lead to metabolic disorders.18 Furthermore, studies using cell cultures and animal models revealed that FNDC5 is involved in insulin resistance and adipose tissue inflammation, suggesting that FNDC5 could be useful in averting inflammation and insulin resistance in obesity and diabetes.19 A research study in animal model of chronic heart failure, and ischemic cardiomyopathy reported reduced FNDC5 mRNA expression in skeletal muscle.20 Duan et al reported that streptozotocin-induced diabetic mice, treated with recombinant FNDC-5 (irisin) exhibited significantly reduced blood glucose levels.21 It has been observed that patients with T2DM have lower levels of FNDC-5 expression.4 Moreno-Navarrete et al reported that FNDC5 gene expression was significantly lower in obese individuals.22 In both T1DM and T2DM, FNDC5 mRNA expression was found to be lower in animal models.23
Overall, more than 1.1-fold selectin-E expression was observed among the T2DM cases compared to control. It was found that the T2DM patients with obesity had lower selectin-E mRNA expression compared to those without obesity Based on vitamin-D level, deficient and insufficient T2DM patients had lower selectin-E mRNA expression when compared with those with sufficient levels of serum vitamin-D. Serum B12 deficient T2DM patients B12 deficient had lower selectin-E mRNA expression when compared with their counterparts with normal B12 levels Selectin-E upregulation has been known to play an active role in the high glucose condition, suggesting a new mechanism by which diabetes accelerates arterial disease.24 Increased selectin-E expression has also been implicated as a factor in the development of diabetes-related comorbidities.25 Elevated E-selectin levels have been described as a key marker of endothelial dysfunction in T2DM patients,26 which increases the risk of diabetes-related micro- and macrovascular complications.27 Scientists have also reported that selectin-E gene expression is comparatively higher in obese T2DM patients.28 It was also found that the FNDC-5 mRNA expression was more down-regulated among the obese T2DM cases and as per ROC curve analysis it shows a more decreased in expression (≤0.30 fold). This can be used as a predictor of the development of obesity in T2DM cases.
Conclusion
This present study suggested that T2DM patients with obesity had higher fasting blood glucose, HbA1c, Systolic blood pressure, LDL, TG and cholesterol. Significantly reduction in expression of FNDC-5 mRNA expression was found in T2DM cases with obesity. Vitamin-D and B12 level greatly affect the expression of FNDC-5 mRNA expression among the T2DM with obesity. ROC analysis suggested that the FNDC-5 mRNA down-regulation could be used as an indicator for development of obesity among the T2DM patients.
Data Sharing Statement
We confirm that the data used during the research will not be shared with anybody/broadcasted in any public domain. The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics and Informed Consent
This research study was ethically approved (12/9/176/JMI/IEC/2018) by the ethical committee board, Jamia Millia Islamia, New Delhi, India and all participants gave their written informed consent before the study began, and all the ethical principles regarding human experimentation were followed and the study was conducted in accordance with the Declaration of Helsinki.
Acknowledgment
The authors Irfan Ahmad grateful to the Deanship of Scientific Research, King Khalid University, Abha, Saudi Arabia, for monetarily support to this work through a Small Research Group Project under grant number (R.G.P.1/48/42).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hu FB. Obesity Epidemiology. Oxford; New York: Oxford University Press; 2008:498.
2. Reinehr T, Elfers C, Lass N, Roth CL. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. J Clin Endocrinol Metab. 2015;100:2123–2130. doi:10.1210/jc.2015-1208
3. Chen N, Li Q, Liu J, Jia S. Irisin, an Exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev. 2016;32:51–59. doi:10.1002/dmrr.2660
4. Perakakis N, Triantafyllou GA, Fernandez-Real JM, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–337. doi:10.1038/nrendo.2016.221
5. Park KH, Zaichenko L, Brinkoetter M, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:4899–4907. doi:10.1210/jc.2013-2373
6. Crujeiras AB, Zulet MA, Lopez-Legarrea P, et al. Association between circulating irisin levels and the promotion of insulin resistance during the weight maintenance period after a dietary weight-lowering program in obese patients. Metabolism. 2014;63:520–531. doi:10.1016/j.metabol.2013.12.007
7. McEver RP. Selectins: initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc Res. 2015;107:331–339. doi:10.1093/cvr/cvv154
8. Yago T, Shao B, Miner JJ, et al. E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin αLβ2-mediated slow leukocyte rolling. Blood. 2010;116:485–494. doi:10.1182/blood-2009-12-259556
9. Antoine M, Tag CG, Gressner AM, Hellerbrand C, Kiefer P. Expression of E-selectin ligand-1 (CFR/ESL-1) on hepatic stel- late cells: implications for leukocyte extravasation and liver metastasis. Oncol Rep. 2009;21(2):357–362.
10. Vestweber D, Blanks JE. Mechanisms that regulate the function of the selectins and their ligands. Physiol Rev. 1999;79(1):181–213. doi:10.1152/physrev.1999.79.1.181
11. Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced aLb2 integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi:10.1016/j.immuni.2007.04.011
12. Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22(4):477–501. doi:10.1210/edrv.22.4.0437
13. Finkelstein JL, Layden AJ, Stover PJ. Vitamin B-12 and Perinatal Health. Adv Nutr. 2015;6(5):552–563. doi:10.3945/an.115.008201
14. Alberti KG, Zimmet P, Shaw J. Metabolic syndrome–a new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006;23(5):469–480. doi:10.1111/j.1464-5491.2006.01858.x
15. Shrivastava U, Misra A, Mohan V, Unnikrishnan R, Bachani D. Obesity, diabetes and cardiovascular diseases in India: public health challenges. Curr Diabetes Rev. 2017;13(1):65–80. doi:10.2174/1573399812666160805153328
16. Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. doi:10.1161/CIRCRESAHA.115.306825
17. Bonfante IL, Chacon-Mikahil MP, Brunelli DT, et al. Combined training, FNDC5/irisin levels and metabolic markers in obese men: a randomised controlled trial. Eur J Sport Sci. 2017;17(5):629–637. doi:10.1080/17461391.2017.1296025
18. Lecker SH, Zavin A, Cao P, et al. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5(6):812–818. doi:10.1161/CIRCHEARTFAILURE.112.969543
19. Xiong XQ, Geng Z, Zhou B, et al. FNDC5 attenuates adipose tissue inflammation and insulin resistance via AMPK-mediated macrophage polarization in obesity. Metabolism. 2018;83:31–41. doi:10.1016/j.metabol.2018.01.013
20. Matsuo Y, Gleitsmann K, Mangner N, et al. Fibronectin type III domain containing 5 expression in skeletal muscle in chronic heart failure-relevance of inflammatory cytokines. J Cachexia Sarcopenia Muscle. 2015;6(1):62–72. doi:10.1002/jcsm.12006
21. Duan H, Ma B, Ma X, et al. Anti-diabetic activity of recombinant irisin in STZ-induced insulin-deficient diabetic mice. Int J Biol Macromol. 2016;84:457–463. doi:10.1016/j.ijbiomac.2015.12.049
22. Moreno-Navarrete JM, Ortega F, Serrano M, et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J Clin Endocrinol Metab. 2013;98(4):E769–E778. doi:10.1210/jc.2012-2749
23. Jiang S, Piao L, Ma EB, Ha H, Huh JY. Associations of circulating irisin with FNDC5 expression in fat and muscle in Type 1 and Type 2 diabetic mice. Biomolecules. 2021;11(2):322. doi:10.3390/biom11020322
24. Chen TC, Chien SJ, Kuo HC, et al. High glucose-treated macrophages augment E-selectin expression in endothelial cells. J Biol Chem. 2011;286(29):25564–25573. doi:10.1074/jbc.M111.230540
25. Knudsen ST, Foss CH, Poulsen PL, et al. E-selectin-inducing activity in plasma from type 2 diabetic patients with maculopathy. Am J Physiol Endocrinol Metab. 2003;284(1):E1–E6. doi:10.1152/ajpendo.00198.2002
26. Abu-Amero KK, Al-Mohanna F, Al-Boudari OM, Mohamed GH, Dzimiri N. The interactive role of type 2 diabetes mellitus and E-selectin S128R mutation on susceptibility to coronary heart disease. BMC Med Genet. 2007;8:35. doi:10.1186/1471-2350-8-35
27. Hamilton SJ, Watts GF. Endothelial dysfunction in diabetes: pathogenesis, significance, and treatment. Rev Diabet Stud. 2013;10(2–3):133–156. doi:10.1900/RDS.2013.10.133
28. Rana KS, Pararasa C, Afzal I, et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc Diabetol. 2017;16(1):147. doi:10.1186/s12933-017-0627-2
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

