Back to Journals » Clinical Ophthalmology » Volume 17
Clinical Features Associated with Acute Elevated Intraocular Pressure After Intravitreal Anti-VEGF Injections
Authors LoBue SA , Gindina S, Saba NJ, Chang T, Davis MJ, Fish S
Received 17 April 2023
Accepted for publication 2 June 2023
Published 13 June 2023 Volume 2023:17 Pages 1683—1690
DOI https://doi.org/10.2147/OPTH.S414212
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Stephen A LoBue,1,2 Sofya Gindina,2 Nicholas J Saba,2 Tom Chang,2 Michael J Davis,2 Steven Fish2
1Department of Ophthalmology, Acuity Eye Group, Pasadena, CA, USA; 2Department of Ophthalmology, SUNY Downstate Medical Center, Brooklyn, NY, USA
Correspondence: Tom Chang, Department of Ophthalmology, Acuity Eye Group, 100 E California Blvd, Pasadena, CA, 91105, USA, Email [email protected]
Purpose: To study the effects of intravitreal injection (IVI) of anti-VEGF (vascular endothelial growth factor) agents on intraocular pressure (IOP) and find associations with acute pressure spikes.
Methods: This was a three-month, prospective study of patients receiving outpatient IVI of anti-VEGF agents for diabetic retinopathy (DR), age-related macular degeneration (AMD), and retinal vein occlusion (RVO) at the Acuity Eye Group Medical Centers. IOP was measured pre- and post-injection at 10-minute intervals up to 50 minutes after injection with a handheld tonometer. Patients with an IOP greater than 35 mmHg at 30 minutes received an anterior chamber paracentesis (ACP), while patients below 35 mmHg were monitored without intervention.
Results: A total of 617 patients (51% female, 49% male) received IVI for DR (n = 199), AMD (n = 355), and RVO (n = 63). ACP was performed in 17 patients. Average pre-injection IOP was 16 ± 4 compared to 24 ± 7 mmHg for the non-ACP vs ACP group, respectively (mean ± standard deviation), p < 0.0001. IOP returned to baseline in 98% of patients at 50 minutes. A diagnosis of glaucoma and glaucoma suspect was more prevalent in the ACP group compared to the non-ACP group, 82.3% vs 14.2% and 17.6% vs 9.0%, respectively, p < 0.0001 and p > 0.05. Patients with a pre-injection IOP > 25 mmHg and a history of glaucoma had a 58.3% rate of ACP. A 31-gauge needle had a higher mean increase in IOP from baseline compared to 30-gauge needle, p < 0.0001.
Conclusion: IOP spikes are most significant in the first 10 minutes after IVI but typically resolve within the first hour. However, utilizing a smaller 31-gauge IVI in patients with a glaucoma history and pre-injection IOP > 25 mmHg may be associated with significant IOP spikes lasting longer than 30 minutes.
Keywords: intravitreal injection, anti-VEGF, glaucoma, acute pressure spikes, anterior chamber paracentesis
Introduction
Macular edema secondary to retinal vein occlusion, diabetic retinopathy, and age-related macular degeneration are some of the most common causes of visual impairment and blindness worldwide.1 Vascular endothelial growth factor (VEGF) over-expression has been identified as a major pathologic process in these disorders.
As a result, Anti-VEGF has revolutionized the treatment of certain retinal pathologies with macular edema. Landmark trials have proven the efficacy of anti-VEGF in improving vision and reversing retinal edema.2–4 Although anti-VEGF had a favorable safety profile in these trials, each injection poses the risk of adverse events such as endophthalmitis, ocular hemorrhage, retinal detachment, and elevation of intraocular pressure (IOP).5
Several studies have documented a significant but transient elevation of IOP immediately after intravitreal injection (IVI). Saatci et al performed a prospective study analyzing 229 eyes regarding the immediate IOP changes after the IVI of ranibizumab compared to 2 versus 4mg triamcinolone acetonide. IOP measured immediately after injection was >25 mmHg in 48.1% in the ranibizumab group, 31.9% in the 2mg, and 51.9% in 4mg triamcinolone group.6 In one study, IOP was documented to be >50 mmHg in 35% of eyes immediately after injection which decreased to 4% >50 mmHg at 5 minutes.7 In most cases, IOP returned to baseline within 30 minutes after injection.6,8–11
Yet in some instances, IOP spikes have been documented to persist even longer. In a study of 104 patients, three patients (2.9%) had an IOP of 25 mm Hg or higher at 30 minutes which normalized by 2 hours in two patients but required a 1-week course of topical glaucoma drops in the last patient.11 Some authors have documented that glaucoma may be a risk factor for prolonged pressure spikes after IVI.12
Understanding potential clinical characteristics for pressure spikes after IVI is important as significant rises in IOP, repeated monthly for many years, may lead to permanent optic nerve damage. Thus, we created a prospective study analyzing the immediate effect of IOP after IVI to determine clinical features associated with significant pressure spikes.
Methods
A prospective study was carried out at the Acuity Eye Group medical centers in Southern California, United States of America. The study was approved by the Advarra Institutional Review Board (IRB). The study population included consecutive eligible patients with macular edema from neovascular age-related macular degeneration (AMD), diabetic retinopathy (DR), or retinal vein occlusions (RVO), receiving intravitreal anti-vascular endothelial growth factor (anti-VEGF) injections. Patients were included in the study within a three-month window, from 3/1/2020 to 6/1/2020.
IOP was measured using a handheld tonometer (Tono-Pen XL, Haag-Streit, Köniz, Switzerland) prior to intravitreal injection and in 10-minute intervals after injection. Patients were administered either 0.05 mL of bevacizumab (Avastin; Genentech, San Francisco, California, USA) or 0.05 mL of ranibizumab (Lucentis; Genentech). Intravitreal injections were performed by one provider, using a 30 or 31-gauge needle, with a standard technique described below.
Patients were prepped with 10% povidone-iodine on the eyelids followed by the instillation of anesthetic drops (proparacaine hydrochloride), insertion of a lid speculum, and a 5% povidone-iodine solution on the eye and fornices. Using a cotton-tipped applicator soaked with a topical anesthetic, the injection site at the inferotemporal region of the sclera was anesthetized. The injection site was marked with a caliper measuring 4 mm from the limbus for phakic patients and 3.5 mm for pseudophakic patients. The conjunctiva was displaced slightly with a sterile cotton-tipped applicator just before entering the eye with a needle. The injection site was then occluded temporarily and was massaged with a sterile cotton-tipped applicator.
After injection, the lid speculum was removed and IOP was measured at 10-minute intervals up to 50 minutes after injection. The post-injection vision was assessed by the examiner performing hand movements at 6 inches from the treated eye, while the contralateral eye was occluded. Anterior chamber paracentesis (ACP) was performed using a 30-gauge needle on a TB syringe in instances of post-injection IOP greater than 35mmHg at 30 minutes. Prior to performing an ACP, the eye was anesthetized and prepped with topical proparacaine and 5% povidone-iodine solution, respectively. Patients with an IOP below 35mmHg were monitored without further intervention. Elevation in baseline IOP was defined as >4 mmHg.
Patients included in the study involved men and women over 18 years of age who were eligible for an anti-VEGF injection of either bevacizumab or ranibizumab. Patients excluded from the study involved patients receiving IVI triamcinolone (steroid), neovascular glaucoma, and a history of glaucoma surgery (eg, trabeculectomy or valve implant).
Statistical tests were performed using SPSS software version 14.0 (SPSS Inc, Chicago, Illinois, USA). A two-tailed Student’s t-test was used to determine the significance between groups. Chi-square test was used to compare the proportion of treated eyes (ACP Group) and untreated eyes. Statistical significance was classified as p values <0.05.
Results
A total of 617 patients (51% female, 49% male) received IVI of anti-VEGF agents of ranibizumab (n = 243) or bevacizumab (n = 374). The average age of patients was 73 ± 14 years of age (mean ± standard deviation). Intravitreal injections were administered for the treatment of macular edema from DR (n = 199), AMD (n = 355), and RVO (n = 63) (Table 1).
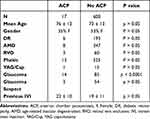 |
Table 1 Demographic Data Comparing Differences Between ACP and Non-ACP Group |
For the entire study, the mean baseline IOP was 16 ± 5 mmHg pre-injection which increased to 25 ± 10 mmHg post-injection (mean ± standard deviation, p < 0.0001, Figure 1). Post-injection IOP returned to baseline in 85% and 98% of patients at 30 and 50 minutes, respectively (Figure 2). At 30 minutes post-injection, an IOP greater than 35mmHg was observed in 17 eyes (2.8%), thus requiring ACP intervention. No patients in the study required an urgent ACP before 30 minutes due to a severe loss of vision.
 |
Figure 1 Across both groups, the mean baseline IOP was 16 ± 5 mmHg pre-injection which increased to 25 ± 10 mmHg post-injection. *P < 0.0001. |
 |
Figure 2 Percentage of patients within 4 mmHg of baseline IOP after intravitreal injection. Post-injection IOP returned to baseline in 85% and 98% of patients at 30 and 50 minutes, respectively. |
The average pre-injection IOP was 16 ± 4 mmHg in the non-ACP group compared to 24 ± 7 mmHg in the ACP group (p < 0.0001, Figure 3). Overall, 33% of patients with a pre-injection IOP greater than 25 mmHg and 50% with a pre-injection IOP greater than 30 mmHg required an ACP. The average number of prior IVI was similar between the ACP and non-ACP groups at 22 ± 10 vs 19 ± 11 injections, respectively (p > 0.05, Table 1). Furthermore, a diagnosis of glaucoma was more prevalent in the ACP group compared to the non-ACP group, 82.3% vs 14.2% (p < 0.0001, Figure 4). A diagnosis of glaucoma suspect was also higher in the ACP vs non-ACP group, 17.6% vs 9.0%, respectively (p > 0.05, Figure 4).
There was no significant age difference between the ACP group at 76 ± 11.7 years versus the non-ACP group at 73 ± 13.1 years of age (p > 0.05, Table 1). There was also no significant difference when comparing gender, lens status, presence of YAG capsulotomy, or number of IVI between the ACP and non-ACP groups (p > 0.05, Table 1).
Patients with a pre-injection IOP greater than 25 mmHg and a history of glaucoma had a 58.3% rate of ACP. Using a 31-gauge needle had a higher incidence of ACP when pre-injection IOP >25 when compared to using a 30-gauge needle, 42.9% vs 29.4%, respectively, p > 0.05. The smaller bore 31-gauge needles used for Avastin injections had a higher mean increase in IOP from baseline compared to the 30-gauge Lucentis injections. The average IOP increase for the 31-gauge IVI was 10 ± 8 mmHg compared to 7 ± 5 mmHg for the 30-gauge IVI (p < 0.001, Figure 5).
 |
Figure 5 The increase in IOP from baseline was significantly higher in the 31-gauge at 10 ± 8 mmHg vs the 30-gauge needles at 7 ± 5 mmHg (mean ± standard deviation). *P < 0.0001. |
Discussion
Published literature has consistently shown that transient rises in IOP occur immediately after IVI. These pressure spikes are due to the additional volume of fluid added to the closed space of the globe. The concern of these pressure spikes is that not all patients are affected equally and that repetitive spikes may lead to retinal nerve fiber layer (RNFL) loss and visual field defects consistent with glaucoma.
Our data are consistent with the literature that IVI led to transient IOP changes which resolved in 98% of patients at 50 minutes. Across all patients, the greatest increase in IOP was seen within 10 minutes with a mean post-injection IOP increase of 9 ± 10 mmHg. However, initial IOP changes were likely to be higher as most authors found the greatest pressure spike immediately after injection.13 We did not take an IOP at time zero because there is ample evidence to support an immediate increase in IOP after IVI. Our focus was determining types of patients that had meaningful elevations of IOP >35mmHg after 30 minutes which we classified as our intervention group, receiving an ACP.
As expected, patients who required an ACP had the greatest mean increase in post-IVI IOP with an increase of 23 ± 10 and 20 ± 9 mmHg at 10 and 20 minutes, respectively, p < 0.0001. The ACP group also had a significantly higher pre-injection IOP of 24 ± 7 mmHg compared to the overall group of 16 ± 4 mmHg, p < 0.0001. Our data suggest an association between pre-injection IOP and pressure spikes. Within our cohort, 33% of patients with a pre-injection IOP greater than 25 mmHg and 50% with a pre-injection IOP greater than 30 mmHg required an ACP.
Several studies have shown that patients with glaucoma demonstrate higher and more prolonged IOP spikes.12,14,15 Kim et al examined 102 eyes receiving IVI and found eyes with pre-existing glaucoma take longer to recover from the acute spikes. Fewer patients with glaucoma had reached an IOP <30 mmHg at 5, 10, and 15 minutes after injection compared with non-glaucomatous eyes.12 In contrast, patients with prior history of incisional glaucoma surgery have been found to have a lower pressure spike that recovers more quickly.16
Our data mirrors the literature as pressure spikes were more significant and prolonged in patients with pre-existing glaucoma. Glaucoma suspects were also a higher percentage in the ACP group but were not statistically significant. Specifically, in our data, patients with a pre-injection IOP greater than 25 mmHg and a history of glaucoma had a 58.3% rate of IOP elevation >35 mmHg at 30 minutes.
Another factor that has been shown to contribute to immediate pressure spikes is the amount of vitreous reflux.17–19 A decrease in vitreous reflux post-IVI has been associated with greater pressure spikes. A reduction in vitreous reflux has been documented with tunneled injection technique and smaller 32-gauge needles.17,18 This technique of tunneling was not utilized in our patient group, and all patients had standard injection techniques with perpendicular entry into the sclera. Our study also demonstrated a higher rate of pressure spikes resulting in ACP in patients receiving IVI from 31-gauge vs 30-gauge needles. We also documented a statistically higher mean increase in IOP from 31-gauge vs 30-gauge needles.
Mixed data exist on lens status and axial length associated with transient rises in IOP after IVI. Some hypothesized that short-term post-injection IOP levels were high in eyes with short axial length because of the smaller intraocular volume and scleral rigidity.20 However, other researchers have found no association between short-term IOP increase and axial length.21,22 Increased ocular rigidity and history of Nd:YAG capsulotomy have been postulated to be associated with rises in IOP after IVI, possibly due to ease of communication of fluid between the posterior and anterior chambers.23 Nevertheless, our data did not reflect a significant difference between age, lens status, and YAG capsulotomy rate in patients who developed significant IOP spikes (Table 1).
More evidence is suggesting that repeatable pressure spikes after IVI may increase the risk for RNFL loss and glaucoma.13,24 Large studies have shown a dose-related relationship between anti-VEGF injections and the development of open-angle glaucoma. Patients with 7 or more injections per year compared to eyes with 3 or fewer injections per year have been shown to have a higher incidence of glaucoma surgery.25 Additionally, eyes receiving 14 or more injections a year have higher odds of initiating IOP-lowering therapy or having a new diagnosis of glaucoma.26
It should also be noted that several studies do not document long-term IOP changes after IVI.27,28 A recent report from the Academy of Ophthalmology on the effect of anti-VEGF IVI on IOP and glaucoma reaffirms that a strong relationship exists between immediate IOP changes and IVI. However, the presence of sustained IOP elevation and glaucoma is unclear as most studies measure IOP before and after an IVI intravitreal injection but not in between visits or at regular intervals after cessation of injections.29 Thus, more studies are needed to determine if a true causality exists.
Nevertheless, clinicians should consider closely monitoring post-injection pressures in patients with elevated pre-injection IOP and glaucoma. In these patients, consideration could be given to pretreat IOP prior to receiving an IVI in order to mitigate a significant IOP spike. Pretreatment with topical eye drops including brimonidine/timolol, brimonidine, and acetazolamide reduced post-injection IOP compared to the control but did not prevent the occurrence of an IOP spike.30–32 However, pre-treating with ACP not only prevented acute IOP spikes but was also associated with a decrease in RNFL loss over time compared to controls.33
While there is no consensus among ophthalmologists around pre-injection protocols to prevent acute IOP elevations, our group recommends that clinicians should strongly consider an ACP in patients with a pre-injection IOP >25mmHg with a history of glaucoma. However, patients with a history of glaucoma in the setting of normal IOP could be justified with ACP prior to IVI in order to mitigate an acute IOP spike and potential RNFL loss from repeat IVI over an extended period of time.
Conclusion
Transient rises in IOP occur immediately after IVI. IOP spikes are most significant in the first 10 minutes after IVI but typically resolve within the first hour. However, patients with a pre-injection IOP greater than 25mmHg, combined with a history of glaucoma or glaucoma suspect, had a higher rate of prolonged IOP elevation following IVI. The use of smaller gauge needles and less vitreous reflux was also associated with a greater increase in post-injection IOP. As a result, we recommend that clinicians should, at the minimum, consider close observation of IOP after IVI in patients with high pre-injection IOP and glaucoma. In these patients, there can also be consideration for prophylactic reduction of IOP spikes with an ACP.
Consent
All participants provided informed consent, in accordance with the Declaration of Helsinki.
Acknowledgment
The abstract of this paper was presented at the Association for Research in Vision and Ophthalmology (ARVO) as a poster presentation with interim findings. The poster’s abstract was published in ARVO Annual Meeting Abstracts within the journal of Investigative Ophthalmology and Visual Science (IOVS).
Funding
The work was supported by the Acuity Eye Group. No outside funding or grants were necessary for this project.
Disclosure
No conflicting relationship/interests exists for any author.
References
1. GBD 2019 Blindness and Vision Impairment Collaborators, Vision Loss Expert Group of the Global Burden of Disease Study. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the Right to Sight: an analysis for the Global Burden of Disease Study. Lancet Glob Health. 2021;9(2):e144–e160. doi:10.1016/S2214-109X(20)30489-7
2. Sun JK, Glassman AR, Beaulieu WT, et al. Rationale and Application of the Protocol S Anti-Vascular Endothelial Growth Factor Algorithm for Proliferative Diabetic Retinopathy. Ophthalmology. 2019;126(1):87–95. doi:10.1016/j.ophtha.2018.08.001
3. Gross JG, Glassman AR, Jampol LM, et al.; Writing Committee for the Diabetic Retinopathy Clinical Research Network. Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: a Randomized Clinical Trial. JAMA. 2015;314(20):2137–2146. doi:10.1001/jama.2015.15217
4. Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–625. doi:10.1016/j.ophtha.2011.01.031
5. Falavarjani KG, Nguyen QD. Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye. 2013;27(7):787–794. doi:10.1038/eye.2013.107
6. Arikan G, Osman Saatci A, Hakan Oner F. Immediate intraocular pressure rise after intravitreal injection of ranibizumab and two doses of triamcinolone acetonide. Int J Ophthalmol. 2011;4(4):402–405. doi:10.3980/j.issn.2222-3959.2011.04.16
7. Gregori NZ, Weiss MJ, Goldhardt R, et al. Ocular decompression with cotton swabs lowers intraocular pressure elevation following intravitreal injection. J Glaucoma. 2014;23(8):508–512. doi:10.1097/IJG.0b013e318294865c
8. Falkenstein IA, Cheng L, Freeman WR. Changes of intraocular pressure after intravitreal injection of bevacizumab (avastin). Retina. 2007;27(8):1044–1047. doi:10.1097/IAE.0b013e3180592ba6
9. Sharei V, Höhn F, Köhler T, Hattenbach LO, Mirshahi A. Course of intraocular pressure after intravitreal injection of 0.05 mL ranibizumab (Lucentis). Eur J Ophthalmol. 2010;20(1):174–179. doi:10.1177/112067211002000124
10. Gismondi M, Salati C, Salvetat ML, Zeppieri M, Brusini P. Short-term effect of intravitreal injection of Ranibizumab (Lucentis) on intraocular pressure. J Glaucoma. 2009;18(9):658–661. doi:10.1097/IJG.0b013e31819c4893
11. Hollands H, Wong J, Bruen R, Campbell RJ, Sharma S, Gale J. Short-term intraocular pressure changes after intravitreal injection of bevacizumab. Can J Ophthalmol. 2007;42(6):807–811. doi:10.3129/i07-172
12. Kim JE, Mantravadi AV, Hur EY, Covert DJ. Short-term intraocular pressure changes immediately after intravitreal injections of anti-vascular endothelial growth factor agents. Am J Ophthalmol. 2008;146(6):930–934.e1. doi:10.1016/j.ajo.2008.07.007
13. de Vries Victor A, Bassil FL, Ramdas Wishal D. The effects of intravitreal injections on intraocular pressure and retinal nerve fiber layer: a systematic review and meta-analysis. Sci Rep. 2020;10:13248. doi:10.1038/s41598-020-70269-7
14. Foss AJE, Scott LJ, Rogers CA, et al. Changes in intraocular pressure in study and fellow eyes in the IVAN trial. Br J Ophthalmol. 2016;100(12):1662–1667. doi:10.1136/bjophthalmol-2015-307595
15. Good TJ, Kimura AE, Mandava N, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011;95(8):1111–1114. doi:10.1136/bjo.2010.180729
16. Lam J, Luttrell I, Ding L, et al. Effect of prior glaucoma surgery on intraocular pressure immediately after anti-vascular endothelial growth factor injection. Graefes Arch Clin Exp Ophthalmol. 2019;257(11):2489–2494. doi:10.1007/s00417-019-04431-x
17. Uyar E, Ulas F, Sahin S, Celebi S. Major factors affecting intraocular pressure spike after intravitreal ranibizumab injection: vitreous reflux and its amount. Eur J Ophthalmol. 2019;29(4):361–367. doi:10.1177/1120672119836613
18. Özkaya A, Alkin Z, Celik U, et al. Comparing the effects of three different intravitreal injection techniques on vitreous reflux and intraocular pressure. J Ocul Pharmacol Ther. 2013;29(3):325–329. doi:10.1089/jop.2012.0144
19. Salem WS, Zagora SL, Nguyen V, Mehta H, Gillies MC, Fraser-Bell S. Effect of intravitreal injection speed on acute rise in intraocular pressure. The SPEED IOP study. Clin Exp Ophthalmol. 2021;49(5):519–521. doi:10.1111/ceo.13934
20. Mathalone N, Arodi-Golan A, Sar S, et al. Sustained elevation of intraocular pressure after intravitreal injections of bevacizumab in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2012;250(10):1435–1440. doi:10.1007/s00417-012-1981-0
21. Karakurt Y, Ucak T, Taslı G, Agcayazı B, Icel E, Yılmaz H. The effects of intravitreal ranibizumab, aflibercept or dexamethasone implant injections on intraocular pressure changes. Med Sci Monit. 2018;24:9019–9025. doi:10.12659/MSM.910923
22. El Chehab H, Le Corre A, Agard E, Ract-Madoux G, Coste O, Dot C. Effect of topical pressure-lowering medication on prevention of intraocular pressure spikes after intravitreal injection. Eur J Ophthalmol. 2013;23(3):277–283. doi:10.5301/ejo.5000159
23. Ge J, Wand M, Chiang R, Paranhos A, Shields MB. Long-term effect of Nd:YAG laser posterior capsulotomy on intraocular pressure. Arch Ophthalmol. 2000;118(10):1334–1337. doi:10.1001/archopht.118.10.1334
24. Martinez-de-la-Casa JM, Ruiz-Calvo A, Saenz-Frances F, et al. Retinal nerve fiber layer thickness changes in patients with age-related macular degeneration treated with intravitreal ranibizumab. Invest Ophthalmol Vis Sci. 2012;53(10):6214–6218. doi:10.1167/iovs.12-9875
25. Eadie BD, Etminan M, Carleton BC, Maberley DA, Mikelberg FS. Association of repeated intravitreous bevacizumab injections with risk for glaucoma surgery. JAMA Ophthalmol. 2017;135(4):363–368. doi:10.1001/jamaophthalmol.2017.0059
26. Cui QN, Gray IN, Yu Y, VanderBeek BL. Repeated intravitreal injections of anti-vascular endothelial growth factors and risk of intraocular pressure medication use. Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1931–1939. doi:10.1007/s00417-019-04362-7
27. Effect of pegaptanib sodium 0.3 mg intravitreal injections (Macugen) in intraocular pressure: posthoc analysis from V.I.S.I.O.N. study | British Journal of Ophthalmology. Available from: https://bjo.bmj.com/content/98/11/1543.
28. Gado AS, Macky TA. Dexamethasone intravitreous implant versus bevacizumab for central retinal vein occlusion-related macular oedema: a prospective randomized comparison. Clin Experiment Ophthalmol. 2014;42(7):650–655. doi:10.1111/ceo.12311
29. Hoguet A, Chen PP, Junk AK, et al. The effect of anti-vascular endothelial growth factor agents on intraocular pressure and glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2019;126(4):611–622. doi:10.1016/j.ophtha.2018.11.019
30. Knip MM, Välimäki J. Effects of pegaptanib injections on intraocular pressure with and without anterior chamber paracentesis: a Prospective Study. Acta Ophthalmol. 2012;90(3):254–258. doi:10.1111/j.1755-3768.2010.01904.x
31. Theoulakis PE, Lepidas J, Petropoulos IK, Livieratou A, Brinkmann CK, Katsimpris JM. Effect of brimonidine/timolol fixed combination on preventing the short-term intraocular pressure increase after intravitreal injection of ranibizumab. Klin Monbl Augenheilkd. 2010;227(04):280–284. doi:10.1055/s-0029-1245201
32. Carnota-Méndez P, Méndez-Vázquez C, Otero-Villar J, Saavedra-Pazos JA. Effect of Prophylactic Medication and Influence of Vitreous Reflux in Pressure Rise after Intravitreal Injections of Anti-VEGF Drugs. Eur J Ophthalmol. 2014;24(5):771–777. doi:10.5301/ejo.5000455
33. Soheilian M, Karimi S, Montahae T, Nikkhah H, Mosavi SA. Effects of intravitreal injection of bevacizumab with or without anterior chamber paracentesis on intraocular pressure and peripapillary retinal nerve fiber layer thickness: a prospective study. Graefes Arch Clin Exp Ophthalmol. 2017;255(9):1705–1712. doi:10.1007/s00417-017-3702-1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


