Back to Journals » Infection and Drug Resistance » Volume 14
Clinical Features and Factors Associated with Occult Gastrointestinal Bleeding in COVID-19 Patients
Authors Zhao X, Tao M , Chen C , Zhang Y , Fu Y
Received 26 August 2021
Accepted for publication 6 October 2021
Published 14 October 2021 Volume 2021:14 Pages 4217—4226
DOI https://doi.org/10.2147/IDR.S335868
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Xi Zhao,* Meihui Tao,* Chaoyue Chen, Ying Zhang, Yu Fu
Department of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yu Fu
Department of Gastroenterology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, 430022, People’s Republic of China
Tel +8613907194622
Email [email protected]
Background: There has been an increasing number of COVID-19 patients around the world. Since some patients developed with gastrointestinal bleeding, our study focused on the clinical features and gastroscopic findings of these patients, and factors associated with occult gastrointestinal bleeding.
Patients and Methods: In this retrospective, observational study, we collected 368 COVID-19 patients who performed fecal or gastric occult blood from Wuhan Tongji Hospital, Jin Yin-tan Hospital, and Wuhan Union Hospital between February 1, 2020 and March 6, 2020. Clinical features were compared between patients with or without occult gastrointestinal bleeding, and gastroscopic findings of seven patients were described. Logistic regression analyses were performed to explore the factors associated with occult gastrointestinal bleeding.
Results: In total, 43 (11.7%) patients presented occult gastrointestinal bleeding, whereas 35 (81.4%) of severe cases. CRP level, prothrombin time and D-dimer were higher, while lymphocyte count and albumin levels were decreased in patients with occult gastrointestinal bleeding. Gastroscopy in seven COVID-19 patients showed mucosal congestion, erosion or scattered bleeding at different sites. Albumin levels (OR, 0.856 [95% CI 0.793– 0.924]; p < 0.001), prothrombin time (OR, 1.267 [1.089– 1.475]; p = 0.002) on admission and severe disease (OR, 4.157 [1.765– 9.791]; p = 0.001) were independent factors associated with GIB in COVID-19 patients, while antiviral drugs and glucocorticoid therapy were not associated with it.
Conclusion: COVID-19 patients with occult gastrointestinal bleeding suffered from worse prognosis. Patients with decreased serum albumin levels or prolonged prothrombin time, and severe cases were at higher risk of occult gastrointestinal bleeding.
Keywords: COVID-19, occult gastrointestinal bleeding, clinical characteristics, related factors
Introduction
The global pandemic of SARS-CoV-2 has posed a huge challenge to the world.1 As of August 23, 2021, the number of confirmed cases worldwide has risen to more than 200,000,000, with an alarming mortality rate of 2%.2 The typical clinical symptoms of COVID-19 are fever, cough, dyspnea, fatigue and myalgia.3,4 Digestive symptoms are in the range of 2% to 18.6%, including nausea, vomiting, diarrhea, abdominal pain, and the incidence of digestive symptoms was higher in the late epidemic period than early.5 Gastrointestinal bleeding (GIB) is in the range of 4% to 13.7%, which is one of the complications in critically ill patients.6,7 Previous studies reported that patients with GIB during the hospitalization had a higher mortality risk.8 Patients with COVID-19 sometimes received treatments, which could potentially cause damage to the digestive tract, including glucocorticoids, antiviral agents, mechanical ventilation, extracorporeal membrane oxygenation (ECMO) and anticoagulants.
As we know, the angiotensin converting enzyme 2 (ACE2) receptor is also expressed in the epithelial cells of the gastrointestinal, and SARS-CoV-2 binds to ACE2 to exert an effect.9 Treatment of GIB in patients with SARS-CoV-2 presents unique challenges. Although there are some studies on gastrointestinal bleeding in COVID-19 patients, the direction and specific content of the studies are different from ours. Here, we discuss the clinical features and gastroscopic findings of COVID-19 patients with occult GIB, and factors associated with occult GIB. We identify potential gastrointestinal bleeding patients, which is conducive to the formulation of related diagnosis and treatment plans.
Methods
Patients and Study Design
In this multicenter, retrospective study, the subjects included were laboratory-confirmed COVID-19 patients from Wuhan Tongji Hospital (designated hospital for COVID-19), Jin Yin-tan Hospital (designated hospital for COVID-19) and Wuhan Union Hospital main district (non-designated hospital) between February 1, 2020 and March 6, 2020. Throat swab specimens were collected, and SARS-CoV-2 RNA was detected by real-time reverse transcription PCR (rRT-PCR). All patients received a standard treatment based on “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7)”.10 Data were extracted for demographic characteristics, laboratory values, comorbidities, complications, treatments, outcomes and gastroscopic findings using electronic medical records.
Definitions
Laboratory confirmed cases referred to patients who were tested positive for SARS-CoV-2 in throat swab samples. Sepsis and ARDS were diagnosed according to WHO interim guidance and acute kidney injury (AKI) was determined based on KDIGO clinical practice guidelines.11,12 Acute cardiac injury was diagnosed according to serum levels of cardiac biomarkers, electrocardiography, and echocardiography.4 Acute liver injury was confirmed if abnormalities were seen in alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), alkaline phosphatase (ALP) or γ-glutamyl transpeptidase (GGT). The date of symptom onset was defined as the day when initial symptoms were noticed. The baseline values of laboratory examinations were the results on admission, while the extremum values were the maximum or minimum results after hospitalization.
Statistical Analysis
Continuous variables and categorical variables were expressed as median (interquartile range, IQR) and number (%), respectively. Independent t-test, Mann–Whitney U, χ2 test and Fisher’s exact test were used under appropriate conditions. Logistic regression model was adopted to determine the independent factors associated with occult GIB, and adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated. Given that a total of 43 patients with occult blood tested positive in our study, we enrolled five variables for multivariate logistic regression analysis. Previous studies have shown that gastrointestinal bleeding is more common in critically ill or dead patients, C-reactive protein is a risk factor for severe COVID-19, and coagulation disorder is a risk factor for gastrointestinal bleeding.6,13,14 The use of glucocorticoids and antiviral drugs may increase the risk of gastrointestinal bleeding. Therefore, disease severity, antiviral therapy, glucocorticoid therapy, albumin level and prothrombin time were entered into logistic regression models. SPSS (Statistical Package for the Social Sciences) version 22.0 software was applied for all statistical analyses. The results were two-tailed, and p value <0.05 was considered statistically significant.
Results
Baseline Characteristics, Treatments, and Outcomes
We initially collected 558 patients with COVID-19, but a total of 368 patients were included in the final analyses after exclusion of those who had not taken fecal or gastric juice occult blood tests. As outlined in Table 1, the median age of all subjects was 57.0 years (IQR 39.0–68.0), and 191 were men. There were 43 (11.7%) cases with occult GIB (38 cases with fecal occult blood test, 5 cases with gastric juice occult blood test) that had a median age of 67.0 years (57.0–71.0), while those without occult GIB were much younger (55.0 years [38.5–67.0], p<0.001). The percentage of males in patients with occult GIB was significantly higher relative to those without occult GIB (69.8% vs 49.5%, p=0.013). Pre-existing comorbidities were more common in occult GIB group, though only hypertension (17 [39.5%]), cardiovascular disease (8 [18.6%]), chronic liver disease (9 [20.9%]) and malignancy (6 [14.0%]) showed significant difference between two groups. The most common symptoms were fever (40 [93.0%]), followed by cough (31 [72.1%]), anorexia (22 [51.2%]), fatigue (10 [23.3%]), and nausea or vomiting (6 [14.0%]). Except for myalgia (p=0.048), the difference in clinical symptoms between the two groups was not statistically significant.
 |
Table 1 Baseline Characteristics, Treatments, and Outcomes of Patients with COVID-19 with and without Occult Gastrointestinal Bleeding |
Compared to patients without occult GIB, severe cases accounted for a larger proportion in occult GIB group (34.5% vs 81.4%, p<0.001) and were prone to other complications, including sepsis (1.5% vs 11.6%, p=0.003), ARDS (7.1% vs 39.5%, p<0.001), acute liver injury (36.3% vs 65.1%, p<0.001), acute kidney injury (11.7% vs 37.2%, p<0.001), acute cardiac injury (4.3% vs 46.5%, p<0.001) and coagulopathy (8.6% vs 46.5%, p<0.001). The proportions of patients who received glucocorticoid therapy and mechanical ventilation were higher (74.4% vs 37.2%, p<0.001 and 74.4% vs 9.5%, p<0.001, respectively), while antiviral therapy accounted for a lower proportion (86.0% vs 97.8%, p=0.002). The mortality rate was significantly higher in patients with occult GIB (74.4% vs 10.2%, p<0.001) where 32 out of 43 patients were dead. Among the deceased patients with occult GIB, the median time from symptoms onset to death was 19.0 days (18.0–24.8), and the median time from positive occult blood tests to death was 5.0 days (2.0–9.0).
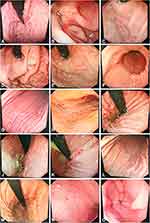
We performed gastroscopy on seven COVID-19 patients (Figure 1). Gastric mucosal lesions were mainly manifested as congestion, erosion or scattered bleeding, while esophageal mucosa, pylorus and duodenum were normal.
Laboratory Findings
Baseline laboratory values are shown in Table 2. Patients with occult GIB showed higher levels of C-reactive protein (CRP), white blood count, AST, TBil, lactate dehydrogenase (LDH), creatine kinase, creatinine, prothrombin time (PT) and D-dimer. However, lymphocyte count, platelet and albumin levels decreased compared with those in the patients without occult GIB. Proportions of hypoalbuminemia and prolonged PT in occult GIB group were significantly higher than those in the patients without occult GIB (79.1% vs 37.0%, p<0.001 and 23.8% vs 2.2%, p<0.001, respectively). For extremum laboratory values during the disease course (Table 2), statistically significant indicators were similar to those in baseline laboratory values, whereas patients with occult GIB showed higher levels of ALP (p<0.001), GGT (p=0.003) and activated partial thromboplastin time (p<0.001), and decreased hemoglobin (p=0.008). With the progress of disease, although the number of patients with hypoalbuminemia or prolonged PT increased in both groups, the proportion of hypoalbuminemia and the proportion of prolonged PT was always significantly higher in patients with occult GIB (90.7% vs 51.1%, p<0.001 and 51.2% vs 8.1%, p<0.001, respectively).
 |
Table 2 Laboratory Findings of Patients with COVID-19 with and without Occult Gastrointestinal Bleeding |
Factors Associated with Occult Gastrointestinal Bleeding
As shown in Table 3, when analyzed by univariable analysis, age, sex, disease severity, hypertension, cardiovascular disease, chronic liver disease, malignancy, CRP, white blood cell count, lymphocyte count, platelet, AST, albumin, TBil, LDH, creatine kinase, creatinine, PT, D-dimer, antiviral therapy, and glucocorticoid therapy were associated with occult GIB. However, in the multivariable logistic regression model, albumin levels (OR, 0.856 [95% CI 0.793–0.924]; p<0.001), prothrombin time (OR, 1.267 [1.089–1.475]; p=0.002) on admission and severe disease (OR, 4.157 [1.765–9.791]; p=0.001) were independent factors associated with occult GIB in COVID-19 patients.
 |
Table 3 Multivariable Logistic Regression Identifying Independent Factors Associated with Occult Gastrointestinal Bleeding |
Discussion
To our knowledge, this is the first retrospective cohort study to discuss the clinical features of patients with occult gastrointestinal bleeding and factors associated with it. In particular, severe cases, decreased serum albumin levels and prolonged PT on admission were associated with occult gastrointestinal bleeding. Of all subjects, 43 (11.7%) had occult gastrointestinal bleeding, while Yang et al found only 2 (4%) cases with GIB in their study.6 This may be related to the fact that we enrolled patients with fecal or gastric juice occult blood test and the relatively large sample size. A majority of patients performed occult blood tests because of diarrhea, abdominal discomfort, or anemia.
In our study, we found that the baseline level of albumin was an independent related factor for occult GIB, and 90.7% patients with occult GIB suffered from hypoalbuminemia throughout the course of the disease. Serum albumin is one of the most important proteins in human plasma, the function of which is to maintain stabilization of blood colloid osmotic pressure and nutrition. In animal experiments, it was proved that serum albumin has protective effects, including improving arterial hyporeactivity in patients with endotoxemia, reducing ischemia-reperfusion injury, and exerting anti-inflammatory effects.15–17 Hypoalbuminemia is associated with poor prognosis in many diseases and patients with hypoalbuminemia had higher mortality rate and longer length of hospital stay.18 Low serum albumin levels may cause mucosal edema through decreasing the plasma colloid osmotic pressure, which may exacerbate mucosal damage caused by stress or other factors. Previous study has demonstrated that serum albumin is a significant predictor of short-term mortality in patients with upper gastrointestinal bleeding, and intravenous infusion of albumin shortens the duration of hospitalization for patients with peptic ulcer bleeding complicated with hypoalbuminemia.19
Based on our results, the risk for occult gastrointestinal bleeding was much higher in severe patients, which was possibly because of stress-related mucosal disease (SRMD) in critical cases. SRMD may be caused by hypotension, hypovolaemia, high levels of catecholamines, release of proinflammatory cytokine or vasoconstriction.20 In our study, seven critically ill patients showed SRMD under gastroscopy. In intensive care unit, the frequency of clinically important bleeding from SRMD was 2.6%. For patients with risk factors for SRMD bleeding, including mechanical ventilation for over 48 hours and coagulopathy, stress-ulcer prophylaxis (SUP) can be used to inhibit gastric acid secretion.21 Previous study reported that 2 (4%) patients with GIB all died.6 Our results showed that patients with occult GIB had higher mortality rate (32 [74.4%]), and the median time from positive occult blood test to death was 5.0 days. Moreover, the proportions of organ injury and mechanical ventilation were remarkably higher in cases with occult GIB. All these results indicated that patients with occult GIB had a poor prognosis.
Coagulation disorder can be observed in severe infections, sepsis, septic shock and COVID-19, some of whom present prolonged PT.22,23 In our study, PT was another factor associated with occult gastrointestinal bleeding, and every 1s increase in PT will increase the risk of occult GIB by 1.267 times. Previously, coagulopathy has been reported as a risk factor for gastrointestinal bleeding, prolonged PT may aggravate gastrointestinal bleeding caused by mucosal damage.14 Inflammatory response and coagulation are two important host defense mechanisms, and the response will increase with the severity of the disease, which may cause potential damage to the host.24 Coagulation abnormality is associated with an increased risk of death in patients with COVID-19. Autopsy results of COVID-19 patients show typical platelet-rich thrombus deposits in the small vessels of the lungs and other organs. It suggests that coagulopathy associated with COVID-19 is the combination of low-grade disseminated intravascular coagulation and localised pulmonary thrombotic microangiopathy, which may have a significant impact on organ dysfunction.25 In critically ill patients, cytokine storm characterized by high concentrations of proinflammatory cytokines and chemokines can be observed, and the release of tumor necrosis factor (TNF-α) and interleukin can affect coagulation function.26 It was demonstrated in a study of rheumatoid arthritis that the use of glucocorticoids may cause gastric mucosal damage.27 Clinically, antiviral therapy may induce gastrointestinal adverse effects, including nausea, vomiting, anorexia, diarrhea, abdominal pain, and rare gastrointestinal bleeding. However, our results did not support glucocorticoid and antiviral drugs correlation with gastrointestinal bleeding in COVID-19 patients.
Among the patients with occult GIB, there were only 3 (7.0%) developed with moderate anemia and one patient had severe anemia. Besides, hematemesis and melena were not found in all participants, and there was no significant difference in the baseline level of hemoglobin and the proportion of severe anemia between patients with or without occult GIB. Thus, there was only minimal hemorrhage in the gastrointestinal tract in COVID-19 patients. Therefore, we consider that gastrointestinal mucosal damage is caused by stress gastric mucosal microcirculation disorder or hypoalbuminemia induced mucosal repair dysfunction.
This study has several limitations. First, patients with COVID-19 were unable to perform comprehensive gastrointestinal examination to clarify gastrointestinal mucosal damage and bleeding owing to the restriction of clinical conditions. Second, due to the limitation of retrospective study design, the time of occult blood tested positive may be affected by discontinuous detection of gastrointestinal bleeding. Third, this is a retrospective study that relies on GIB documentation provided by healthcare providers; therefore, some GIB cases may have been missed. The number of patients with GI bleed is small in our study, and we suggest that larger studies need to be carried out in future. Fourth, SARS-CoV-2 was not detected in stool samples; therefore, it is hard to assess the relationship between viral loads and gastrointestinal bleeding.
In general, we found that severe cases, albumin and PT upon admission were independent related factors for occult gastrointestinal bleeding in COVID-19 patients. This may help physicians identify patients with GIB and take enteral nutrition or histamine-2 receptor blockers or proton-pump inhibitors treatment in the early stage. Mucosa erosion in gastrics was observed under gastroscopy. Although SARS-CoV-2 may not cause massive hemorrhage in the gastrointestinal tract, it is worth noting that patients with gastrointestinal bleeding still had worse prognosis.
Ethics Approval
The study received approval from the ethics commission of Wuhan Tongji Hospital, Wuhan Union Hospital and Jin Yin-tan Hospital (S2020-055, S2020-056, 2020-YJ-047), and was conducted in accordance with the Declaration of Helsinki. Due to the specific nature of the disease, the requirement of informed consent was waived, and all data were anonymized to maintain participants’ privacy.
Acknowledgment
This study is funded by Natural Science Foundation of China (grant number 82070572, 81770554, 81570501). We acknowledge that all of the medical workers participated in the diagnosis and treatment of patients in Wuhan, and the patients involved in this study.
Author Contributions
All authors made substantial contributions to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing for important intellectual content; have agreed on the journal to which the article will be submitted; gave final approval of the version to be published; agree to take responsibility and be accountable for the contents of the article.
Disclosure
None of the authors have declared any potential conflicts of interest.
References
1. World Health Organization. Director-General’s opening remarks at the media briefing on COVID-19-11 March 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. Accessed January 29, 2021.
2. World Health Organization. Coronavirus disease (COVID-19) outbreak situation. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019.
3. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi:10.1016/S0140-6736(20)30211-7
4. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi:10.1016/S0140-6736(20)30183-5
5. Gulen M, Satar S. Uncommon presentation of COVID-19: gastrointestinal bleeding. Clin Res Hepatol Gastroenterol. 2020;44(4):e72–e76. doi:10.1016/j.clinre.2020.05.001
6. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi:10.1016/S2213-2600(20)30079-5
7. Tian Y, Rong L, Nian W, et al. Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. doi:10.1111/apt.15731
8. Trindade AJ, Izard S, Coppa K, et al. Gastrointestinal bleeding in hospitalized COVID-19 patients: a propensity score matched cohort study. J Intern Med. 2021;289(6):887–894. doi:10.1111/joim.13232
9. Gu J, Han B, Wang J. COVID-19: gastrointestinal manifestations and potential fecal-oral transmission. Gastroenterology. 2020;158(6):1518–1519. doi:10.1053/j.gastro.2020.02.054
10. Commission CNH. Chinese clinical guidance for COVID-19 pneumonia diagnosis and treatment. Available from: http://kjfy.meetingchina.org/msite/news/show/cn/3337.html.
11. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–c184. doi:10.1159/000339789
12. World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected-13 March 2020. Available from: https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected.
13. Lu J, Hu S, Fan R, et al. ACP risk grade: a simple mortality index for patients with confirmed or suspected severe acute respiratory syndrome coronavirus 2 disease (COVID-19) during the early stage of outbreak in Wuhan, China. medRxiv. 2020. doi:10.1101/2020.02.20.20025510
14. Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. N Engl J Med. 1994;330(6):377–381. doi:10.1056/NEJM199402103300601
15. Meziani F, Kremer H, Tesse A, et al. Human serum albumin improves arterial dysfunction during early resuscitation in mouse endotoxic model via reduced oxidative and nitrosative stresses. Am J Pathol. 2007;171(6):1753–1761. doi:10.2353/ajpath.2007.070316
16. Lang JD, Figueroa M, Chumley P, et al. Albumin and hydroxyethyl starch modulate oxidative inflammatory injury to vascular endothelium. Anesthesiology. 2004;100(1):51–58. doi:10.1097/00000542-200401000-00012
17. Lee JH, Kim J, Kim K, et al. Albumin and C-reactive protein have prognostic significance in patients with community-acquired pneumonia. J Crit Care. 2011;26(3):287–294. doi:10.1016/j.jcrc.2010.10.007
18. Franch-Arcas G. The meaning of hypoalbuminaemia in clinical practice. Clin Nutr. 2001;20(3):265–269. doi:10.1054/clnu.2001.0438
19. Cheng HC, Chang WL, Chen WY, et al. Intravenous albumin shortens the duration of hospitalization for patients with hypoalbuminemia and bleeding peptic ulcers: a pilot study. Dig Dis Sci. 2013;58(11):3232–3241. doi:10.1007/s10620-013-2821-8
20. Bardou M, Quenot JP, Barkun A. Stress-related mucosal disease in the critically ill patient. Nat Rev Gastroenterol Hepatol. 2015;12(2):98–107. doi:10.1038/nrgastro.2014.235
21. Garcia-Rayado G, Lanas A. Upper gastrointestinal bleeding in critically ill patients: proton-pump inhibitors, histamine-2 receptor antagonists or placebo? Many questions remain unanswered. Curr Med Res Opin. 2018;34(11):1881–1883. doi:10.1080/03007995.2018.1510830
22. Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi:10.1111/jth.14768
23. Vervloet MG, Thijs LG, Hack CE. Derangements of coagulation and fibrinolysis in critically ill patients with sepsis and septic shock. Semin Thromb Hemost. 1998;24(1):33–44. doi:10.1055/s-2007-995821
24. Iba T, Levy JH, Connors JM, et al. The unique characteristics of COVID-19 coagulopathy. Crit Care. 2020;24(1):360. doi:10.1186/s13054-020-03077-0
25. Levi M, Thachil J, Iba T, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi:10.1016/S2352-3026(20)30145-9
26. Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi:10.1016/S0140-6736(20)30628-0
27. Tsujimoto S, Mokuda S, Matoba K, et al. The prevalence of endoscopic gastric mucosal damage in patients with rheumatoid arthritis. PLoS One. 2018;13(7):e0200023. doi:10.1371/journal.pone.0200023
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.