Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 12
Clinical evidence on the efficacy and tolerability of a topical medical device containing benzoylperoxide 4%, retinol 0.5%, mandelic acid 1% and lactobionic acid 1% in the treatment of mild facial acne: an open label pilot study
Authors Garofalo V, Cannizzaro MV , Mazzilli S, Bianchi L, Campione E
Received 2 August 2018
Accepted for publication 7 January 2019
Published 15 May 2019 Volume 2019:12 Pages 363—369
DOI https://doi.org/10.2147/CCID.S182317
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Virginia Garofalo, Maria Vittoria Cannizzaro, Sara Mazzilli, Luca Bianchi, Elena Campione
Division of Dermatology, Department of Systems Medicine, University of Rome Tor Vergata, Rome, Italy
Background: Acne is a debilitating disorder that requires proper treatment depending on the clinical manifestations and pathogenetic factors, among which hyper-keratinization, seborrhea and bacterial proliferation. Combining active ingredients targeting the different mediators of acne pathogenesis may yield optimal outcomes.
Purpose: The purpose of this study was to evaluate the clinical effectiveness, safety and tolerability of a new topical medical device in cream containing benzoylperoxide 4%, pure retinol 0.05%, palmitate retinol 0.5%, mandelic acid 1% and glycyrrhetic acid on patients with mild acne.
Patients and methods: Twenty consecutive patients of both sexes with mild acne were included in the study. The topical treatment was self-applied twice a day for 12 weeks. Evaluations included: Global Acne Grading System (GAGS); inflammatory and non-inflammatory lesions count; reflectance confocal microscopy; seborrhea and hydration degree; photographic documentation; a questionnaire to assess tolerability.
Results: The GAGS score showed a 39% reduction from T0 to T1 and 69.20% from T0 to T2. The count of comedonic lesions showed a 44% reduction from T0 to T1 and 65% from T0 to T2. The count of papular lesions diminished by 49.4% from T0 to T1 and by 62% from T0 to T2. The count of pustular lesions decreased by 43% from T0 to T1 and by 80% from T0 to T2. Improvement of hydration and a decrease of seborrhea degree were even observed. These clinical results were confirmed by reflectance confocal microscopy exam.
Conclusion: The topical medical device has shown to be clinically effective and well tolerated for the treatment of mild acne. Side effects were mild, transient and well tolerated. The results of our study demonstrated a high tolerability of this new combination of benzoylperoxide 4% and retinol. Furthermore, our results suggested that the studied compound could be considered as a “maintenance treatment” after specific pharmacological treatment, even in more severe types of acne.
Keywords: acne vulgaris, therapeutics, benzoyl peroxide, alpha hydroxide acid, vitamin A
Introduction
Acne is the most prevalent skin disorder, affecting over 90% of adolescents between 16 and 18 years old.1
It is a distressing condition, which has a negative impact on the patients’ quality of life.2
Four major pathological processes are implicated in the development of acne: increased sebum production, alteration of the follicular keratinization process, bacterial colonization by Cutibacterium acnes (C. Acnes) and inflammation.3
Acne vulgaris is a chronic disease characterized by the presence of non-inflammatory and inflammatory lesions. It has been traditionally thought that comedones are the non-inflammatory lesions, which are present either in open or closed form. Inflammatory lesions of acne are papules, pustules, and nodules.4
Besides the disease itself, acne scarring is a common and persistent sequela that often occurs in highly visible areas such as the face, thus have both an undesirable cosmetic appearance and are a potential impairment of mental health, social functioning, and overall well-being.5
Treatments of mild to moderate acne include therapies based on the combination of topical benzoylperoxide (BPO), antibiotics, alpha and beta hydroxid acids, and/or retinoids, as well as oral antibiotics and isotretinoin in refractory cases.6,7
The BPO is a lipophilic drug. Once it enters into the pilosebaceous unit, it decomposes into benzoic acid and hydrogen peroxide (HPO) and generates free radicals that exert a bactericidal action.8
Alpha hydroxy acids (AHA) have been recognized as commonly used therapy for acne through chemical peels, inducing exfoliation in hydrophilic areas.9 Mandelic acid is one of the largest AHAs used in mild to moderate acne for its keratolytic, anti-inflammatory and comedolytic effects: it penetrates the epidermis more slowly and uniformly, breaking down follicular plugs and causing desquamation of the stratum corneum.10
Topical retinoid compounds have several effects on acne treatment and act on more than one element involved in the acne pathogenesis.11 These drug classes have anti-inflammatory activity as well as a comedolytic effect, normalizing epidermal proliferation and differentiation. They normalize epidermal proliferation and differentiation, exert their anti-inflammatory effect via inhibition of proinflammatory cytokines release, as well as via inhibition of neutrophil chemotaxis and the expression of toll-like receptors.12
Among retinoid compounds, retinol and retinyl palmitate are converted into the active retinoic acid (tretinoin) to perform its function in the skin. While retinyl palmitate is an earlier form of retinol, it does not have the same effects. Retinyl palmitate is converted into retinol by retinyl palmitate hydrolase, an enzyme that is normally found in the skin. Retinyl palmitate requires one extra step in the conversion pathway before it becomes tretinoin, prior to exerting effects similar to those of retinol. However, retinyl palmitate does not have the same exfoliating effects as retinol; it can deliver benefits to the skin in boosting collagen fiber production in a more gentle way with less irritation.13 It has been shown that Vitamin A derivatives degrade significantly when exposed to UV radiation or are combined with BPO. However, when microsphere-encapsulated retinol is combined with BPO, it is only minimally degraded. Microsphere encapsulation protects the stability of drugs, makes Vitamin A derivatives photostable and enables the use of convenient topical combination regimens with BPO.14
Glycyrrhetinic acid is a bioactive compound extracted from licorice that has potential anti-cancer, anti-inflammatory and anti-microbial activities.15
Phytosphingosine is part of the natural immune defense system of the body. It has an anti-inflammatory activity through the inhibition of NF-κB, JAK/signal transducer and activator of transcription (JAK/STAT), and mitogen-activated protein kinase (MAPK) signaling.16
In this study, we evaluate the effectiveness, safety and tolerability of a topical medical device in cream - available for commerce in Italy - for the treatment of acne, composed by benzoilperoxide, mandelic acid, vitamin A derivative in microsphere encapsulation, glycyrrhetinic acid and phytosphingosine. Nowadays, topically applied semi-solid preparations licensed as medicals devices and not as topical drugs are progressively used for the treatment of acne, although they have caused much confusion to regulators, manufacturers and consumers alike. Medical devices are defined as:
objects or substances that serve the recognition, prevention, monitoring, treatment and alleviation of diseases that achieve this purpose (“intended main effect”) by physical means, not by pharmacological/immunological means or through metabolic effects. The physical effects of medical devices may, nonetheless, be supported by pharmacological, immunological or metabolic effects.
A less complex marketing authorization process compared to topical drugs may at least partly justify the increased placing of medical devices on the dermatological market. If the requirements are fulfilled to certify a product as a medical device, the opportunity will be offered to quickly introduce innovations onto the market and propagate them.17
Patients and methods
An uncontrolled pilot study with an open-label design was conducted in our Division of Dermatology of the University of Rome Tor Vergata.
The complete formulation of the cream tested was composed by: benzoylperoxide 4%, mandelic acid 1%, lactobionic acid 1%, pure retinol 0.05%, palmitate retinol 0.5%, sulfate zinc 1%, glycyrrhetinic acid, undecylenic acid and phytosphingosines (Kurac, from Braderm®). Patients referring to our out-patients clinic for the treatment of acne were evaluated for the study.
Twenty consecutive patients of both sexes were included in the study, after signing informed consent for participation and publication of their photographs. For patients <18 years of age parents or guardians provided the informed consent.
Inclusion criteria were: age between 13–30 years old and clinical diagnosis of mild acne, mainly located on the face.
Exclusion criteria were: previous treatment with antibiotics, BPO or topical retinoids (suspended at least for 4 weeks), oral retinoids (suspended at least for 6 months); endocrine disease, pregnancy and therapy with oral corticosteroids.
Each patient was instructed to apply a thin film of the topical medical device cream as a monotherapy twice a day for 12 weeks. Before topically applying the medication, patients were invited to thoroughly wash their facial skin using a gentle, non-medicated cleanser, rinse with warm water and gently pat dry.
Clinical and instrumental evaluations were performed at the beginning of the treatment (T0), after 4 weeks (T1) and after 12 weeks (T2).
This is a post-marketing observational study on a medical device just approved for topical treatment of acne, for this reason no ethical approval was needed in order with an Italian law decree (Dgs. 46/97) in compliance with European legislation on medical devices (93/42/CEE).
The study complied with the tenets of the Declaration of Helsinki.
Efficacy and safety evaluation
The main outcome of this study was a change in the acne lesion number.
Evaluation included:
- Global Acne Grading System (GAGS) to assess acne severity.18 This is a quantitative scoring system in which the final score is calculated by the sum of six regional subscores, which in turn derives by multiplying an established factor for each facial region (factor each cheek and forehead is 2, nose and chin is 1 and chest and upper back is 3) by the most representative lesion within each region (1 for ≥ one comedone, 2 for ≥ one papule, 3 for ≥ one pustule and 4 for ≥ one nodule). The scale values range from 0 to 44. Only patients suffering from mild facial acne with a GAGS Score between 1 and 18 were enrolled.
- We defined as “almost clear” the patients with a reduction of at least 30% of the initial lesions (evaluated using the GAGS score), and as “clear” the patients with reduction of 60% of the lesions from baseline (T0) to the end of the treatment (T2).
- Lesions count, through which major acne lesions are independently assessed: comedons, papules/pustules and nodules/cysts.17The face is divided into several regions such as nose, forehead, each cheek, and perioral region.
- Photographic assessment, performed at each visit.
- Seborrhea and hydration degree were measured using a specific software (SoftPlus-Callegaris®), through two different skin probes, at T0 and T2. Degrees of seborrhea ranging from 30 to 60 were defined as normal range by the software system, while a good level of hydration was considered for values over 59.
- Main histopathologic aspects of the different acne lesion and clinical improvement were investigated through reflectance confocal microscopy (RCM) Vivascope 1500®, evaluating dermal inflammation and infundibular hyperkeratinization. This assessment gives the opportunity to acquire optical biopsies of the skin at cellular level resolution with no tissue damage. Moreover, RCM permits to repeat the imaging over time on the same target lesion at each visit.19
- A questionnaire filled by the patients to assess the tolerability of the product (excellent, good, fair, poor) and the presence of side effects.
All evaluations requested for the study were made by a dermatologist certified by the board.
Statistical analysis
The treatment effects were evaluated through the following analysis: comparing the GAGS scores after 4 weeks of treatment to the baseline scores, and after 12 weeks of treatment to the baseline scores using the paired t-student test. Statistical significance was assumed for a p-value less than 0.05. Statistical analysis was performed by using Excel (Microsoft) ® vers. 2011
Results
A total of 20 consecutive patients (9 male and 11 female) were enrolled for the study. Patients were assessed from September 2017 to January 2018. The mean age was 19.5 years. All patients completed the study and there were no drops out.
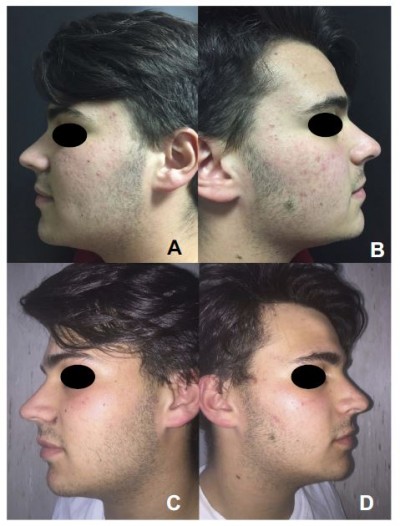
The GAGS score showed a 39% reduction from T0 to T1 (considered as almost clear) and 69,20% from T0 to T2 (mean values 11,55 at T0; 7 at T1; 3,55 at T2; p-value < 0.05) (considered as clear) (Figures 1 and 2B).
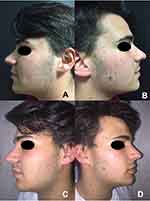 | Figure 1 Efficacy of the treatment. Clinical evaluation before (A and B) and after 12 weeks of treatment (C and D) .The mean GAGS score decreased significantly over the course of the study period. |
A regular decrease in the number of acne lesions was observed during the study (Figure 2A).
The count of comedonic lesions showed a 44% reduction from T0 to T1 and 65% from T0 to T2 (mean values at T0 16.3; 9.1 at T1; 5.7 at T2).
The count of papular lesions diminished by 49.4% from T0 to T1 and by 62% from T0 to T2 (mean values 8.7 at T0; 4.4 at T1; 3.3 at T2).
The count of pustular lesions decreased by 43% from T0 to T1 and by 80% from T0 to T2 (mean values 2.3 at T0; 1.3 at T1; 0.45 at T2).
The measurement of seborrhea using SoftPlus® showed a 15% reduction from T0 to T2 (mean values 72.7 at T0 and 61.6 at T2) (Figure 2C). Hydration showed a 32% improvement from T0 to T2 (mean values 45.2 at T0 and 60 at T2) (Figure 2D).
The results were considered statistically significant as demonstrated by a p-value less than 0.05 for each score.
These results were confirmed by RCM showing a reduction of dermal inflammation and exocytosis, and improvement of infundibular hyperkeratinizazion of the target lesions (Figure 3).
After 4 weeks of treatment (T1) the tolerability of the treatment was considered excellent according to 40% of subjects; good according to 45% of subjects, and fair according to 15% of subjects.
At T2 it was: excellent according to 70% of patients, good according to 25% of patients and fair according to 5% of patients.
No severe side effects were reported. 5% of patients complained about erythema and desquamation during the treatment, evaluated as mild and transient.
Discussion
Acne vulgaris of the face is one of the most commonly encountered diseases in all dermatology and primary care practice. Even in its mild form, acne causes a high degree of psychosocial anguish in terms of social interactions, self-confidence, self-esteem, and employment opportunities.20
Although the use of successful medications and adjunctive procedures has radically improved outcomes in many patients with severe acne, clinical improvements in the treatment of patients with mild or moderate acne are still a challenge. In these patients, meticulous individualization of the therapy and persistence by both patients and clinicians is crucial.21
Acne vulgaris is characterized by hormonally-mediated sebum overproduction, follicular hyperkeratinization, and chronic inflammation of the pilosebaceous unit. Microbes, genetic susceptibilities, and various environmental factors have been linked to the pathogenesis of this condition.22 Therefore, in order to effectively and rapidly reduce acne lesions, treatments need to address as many of these underlying factors as possible. Combination therapies with a topical retinoid and an antimicrobial agent are the favourite approach to target more pathogenic factors and reach faster results with a more complete clearing of acne lesions. Fixed-dose combinations are also more convenient than applying two medications separately and lead to improved adherence with the regimen.23
The association of BPO, retinol, mandelic acid and glycyrrhetic acid is a rational therapeutic option. BPO has antimicrobial and anti-inflammatory effects. Pure retinol, palmitate retinol and mandelic acid have keratolytic and comedolytic effects and through the desquamation of the stratum corneum allow other topical active ingredients to penetrate more effectively through the epidermidis. Glycyrrhetic acid has an anti-inflammatory effect which contrasts the irritating action of other ingredients (BPO, retinoids).
Our study demonstrates an improvement in acne lesions over a 12-weeks treatment period. Using this cream formulation, all patients experienced a significant reduction of both inflammatory and non-inflammatory lesions. No one reported major side effects; a small percentage complained of dryness and slight erythema; the overall product tolerability was defined as “excellent” by the 70% of the subjects at the end of the treatment.
The progressive reduction in the clinical scores of acne severity was associated with the improvement of all RCM parameters related with acne. Indeed, at the end of the treatment, the inflammatory RCM parameters, including inflammatory lesions (papule and pustules) and exocytosis/dermal inflammation, were significantly reduced as well as the number of comedones and infundibula, with hyper-reflecting borders characterized by the accumulation of keratin.
The effectiveness of this new topical compound was demonstrated especially on comedonic lesions, showing a 65% reduction after 12 weeks of treatment (from a mean lesion count of 16.3 at T0 to a mean of 5.7 at T2). The instrumental analysis of seborrhea and hydration degree allowed an objective assessment, otherwise not applicable through self-assessed evaluation.
Conclusion
Despite the scientific evidence on the pathogenesis of acne is constantly enriched, acne treatments, even for mild forms, in some young patients remain a challenge, requiring the recovery with systemic treatments. The possibility of using a new topical formulation containing the most effective active ingredients in acne, improves the dermatologist’s therapeutic armamentarium. Notwithstanding the significant limitations inherent the study design, our data indicate that this innovative topical medical device is an effective, safe and well-tolerated treatment for mild acne with a synergistic mode of action that inhibits C. acnes proliferation, reduces seborrhea while improving skin texture and hydration.
Limitations of the study
Some limitations should be considered in evaluating our results. First of all, this research has an exploratory pilot nature and lacks a placebo arm. Moreover, our study was not designed as a comparative trial of different treatment strategies.
A second limitation could be the relative short (ie 12 weeks) observation and treatment period. Further studies must be focused on a long-term follow-up to ensure the improvements herein showed are stable. Moreover, the sample size was very limited. Thus, studies on larger populations should be performed.
However, we believe that the external validity of this study could be considered good considering that inclusion and exclusion criteria were not particularly strict, offering therefore a significant generalization of the results obtained.
Acknowledgments
We thank Denis Mariano for his technical editing assistance.
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
This is a sponsor free trial. The authors report no conflicts of interest in this work.
References
1. Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(Suppl 1):3–12. doi:10.1111/bjd.13462
2. Gieler U, Gieler T, Kupfer JP. Acne and quality of life impact and management. J Eur Acad Dermatol Venereol. 2015;29(Suppl 4):12–14. doi:10.1111/jdv.13191
3. Dréno B. What is new in the pathophysiology of acne, an overview. J Eur Acad Dermatol Venereol. 2017;31(Suppl 5):8–12. doi:10.1111/jdv.14374
4. Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, et al. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029. doi:10.1038/nrdp.2015.29
5. Ng QX, Koh SSH, Shin D, et al. Use of polymethylmethacrylate (PMMA) microspheres collagen to treat atrophic acne scars. Med Hypotheses. 2017;108:115–116. doi:10.1016/j.mehy.2017.08.016
6. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973. doi:10.1016/j.jaad.2015.12.037
7. Nast A, Dreno B, Bettoli V, et al. European evidence-based (S3) guidelines for the treatment of acne. J Eur Acad Dermatol Venereol. 2012;26:1–29. doi:10.1111/j.1468-3083.2011.04374.x
8. Taylor GA, Shalita AR. Benzoyl peroxide-based combination therapies for acne vulgaris: a comparative review. Am J Clin Dermatol. 2004;5(4):261–265. doi:10.2165/00128071-200405040-00005
9. Castillo DE, Keri JE. Chemical peels in the treatment of acne: patient selection and perspectives. Clin Cosmet Investig Dermatol. 2018;16(11):365–372. doi:10.2147/CCID.S137788
10. Taylor MB. Summary of mandelic acid for the improvement of skin conditions. Cosmet Dermatol. 1999;12:26–28.
11. Pedace FJ, Stoughton R. Topical retinoic acid in acne vulgaris. Br J Dermatol. 1971;84:465–469.
12. Khalil S, Bardawil T, Stephan C, et al. Retinoids: a journey from the molecular structures and mechanisms of action to clinical uses in dermatology and adverse effects. J Dermatolog Treat. 2017;28(8):684–696. doi:10.1080/09546634.2017.1309349
13. Boehnlein J, Sakr A, Lichtin JL, Bronaugh RL. Characterization of esterase and alcohol dehydrogenase activity in skin. Metabolism of retinyl palmitate to retinol (vitamin A) during percutaneous absorption. Pharm Res. 1994;11(8):1155–1159.
14. Kircik LH. Microsphere technology: hype or help? J Clin Aesthet Dermatol. 2011;4(5):27–31.
15. Hung CF, Hsiao CY, Hsieh WH, et al. 18ß-glycyrrhetinic acid derivative promotes proliferation, migration and aquaporin-3 expression in human dermal fibroblasts. PLoS One. 2017;12:8. doi:10.1371/journal.pone.0182981
16. Kim BH, Lee JM, Jung YG, et al. Phytosphingosine derivatives ameliorate skin inflammation by inhibiting NF-κB and JAK/STAT signaling in keratinocytes and mice. J Invest Dermatol. 2014;134(4):1023–1032. doi:10.1038/jid.2013.453
17. Korting HC, Schöllmann C. Medical devices in dermatology: topical semi-solid formulations for the treatment of skin diseases. J Dtsch Dermatol Ges. 2012;10(2):103–109. doi:10.1111/j.1610-0387.2011.07764.x
18. Tan JKL. Current measures for the evaluation of acne severity. Expert Rev Dermatol. 2008;3:395–603. doi:10.1586/17469872.3.5.595
19. Manfredini M, Mazzaglia G, Ciardo S, et al. Acne: in vivo morphologic study of lesions and surrounding skin by means of reflectance confocal microscopy. J Eur Acad Dermatol Venereol. 2015;29(5):933–939. doi:10.1111/jdv.12730
20. Kellett S, Gilbert P. Acne: a biopsychosocial and evolutionary perspective with a focus on shame. Br J Health Psychol. 2001;6(Pt 1):1–24. doi:10.1348/135910701169025
21. Thiboutot D. New treatments and therapeutic strategies for acne. Arch Fam Med. 2000;9:179–187.
22. Das S, Reynolds RV. Recent advances in acne pathogenesis: implications for therapy. Am J Clin Dermatol. 2014;15(6):479–488. doi:10.1007/s40257-014-0099-z
23. Del Rosso JQ. Combination topical therapy in the treatment of acne. Cutis. 2006;78(2 Suppl 1):5–12.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.