Back to Journals » Clinical Ophthalmology » Volume 14
Clinical Evaluation of a Novel Electromechanical Topical Ocular Drug Delivery System: Two Phase 1 Proof of Concept Studies
Authors Quiroz-Mercado H, Ivri E, Gonzalez-Salinas R, Kourtis IC, Gilbert J, Pérez-Vázquez JF, Blumenkranz M , Jiménez-Román J, Marcellino G
Received 3 July 2019
Accepted for publication 29 November 2019
Published 20 January 2020 Volume 2020:14 Pages 139—147
DOI https://doi.org/10.2147/OPTH.S221749
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Hugo Quiroz-Mercado, 1 Ehud Ivri, 2 Roberto Gonzalez-Salinas, 1 Iraklis C Kourtis, 2 Joseph Gilbert, 2 José Francisco Pérez-Vázquez, 1 Mark Blumenkranz, 2 Jesús Jiménez-Román, 1 George Marcellino 2
1Association to Prevent Blindness, Hospital Sanchez-Bulnes, Mexico City, Mexico; 2Kedalion Therapeutics Inc., Menlo Park, CA, USA
Correspondence: Hugo Quiroz-Mercado Vicente García Torres #46, Coyoacan, CDMX CP 04330, Mexico
Tel/Fax +52-5-10841-400
Email [email protected]
Purpose: Self-administration of topical ophthalmic therapies remains challenging for many patients as errors due to improper technique are common. The aim of the current studies was to evaluate a novel electromechanical topical ocular drug delivery device designed to facilitate precise dosing and accurate delivery with substantially lower drug exposure than conventional eye drops.
Patients and Methods: Two randomized Phase 1 studies were performed to evaluate the efficacy and safety of a single dose of a topical ophthalmic solution administered as a ∼ 9 μL microfluid stream via the test device compared with a ∼ 30– 40 μL drop delivered via conventional dropper in healthy subjects (Trial 1) and glaucoma patients (Trial 2). In Trial 1, a 1% tropicamide/2.5% phenylephrine solution was administered via the test device in one eye and by conventional dropper in the contralateral eye. Pupil dilation was measured at 30 min intervals post-instillation and subject comfort was assessed using a visual analogue scale (range, 0– 100). In Trial 2, patients were randomized to receive latanoprost 0.005% via the test device or conventional dropper. Intraocular pressure was measured at baseline and 4– 8 hrs post-instillation.
Results: In Trial 1 (N=20), mean (SD) pupil diameter 30 mins post-instillation increased by 3.4 (0.9) and 3.5 (1.0) mm in the test and control eyes, respectively. The mean comfort score was 81.7 for the test device versus 57.3 for conventional dropper delivery. In Trial 2 (N=18), the mean change in intraocular pressure following administration of latanoprost was – 5.0 (1.8) and – 4.3 (3.3) mm Hg in the test and control groups, respectively. No serious adverse events were observed in either study.
Conclusion: Administration of a single dose of topical ophthalmic therapy via an electromechanical drug delivery device resulted in comparable effects on pupil dilation and intraocular pressure with lower drug exposure and increased patient comfort compared with conventional dropper delivery.
Keywords: mydriasis, phenylephrine, tropicamide, glaucoma, intraocular pressure, latanoprost, topical ocular drug delivery, safety
Introduction
Self-administration of topical ophthalmic medications remains a continuing challenge for many patients. Conventional dropper bottles require the patient to tilt their head backward, position the dropper directly over the eye without a reliable guidance system, and then deposit the prescribed number of drops on the surface of the eye—all without contacting the eye or eyelid with the tip of the dropper.1,2 As a consequence, errors due to poor technique are common and range from missing the eye completely or delivering an excessive dose to contaminating the dropper bottle by contacting the eye or eyelid.3,4 Additionally, physiologic factors such as an exaggerated blink reflex, lid spasm, and reflex tearing can prevent proper deposition and retention of the medication on the surface of the eye.5
Proper administration and strict patient adherence to the prescribed therapeutic regimen are important to ensure safe and efficacious use of topical ocular therapies and to preserve vision in chronic conditions such as glaucoma, dry eye, and ocular inflammatory and infectious disease.1,6–8 Improper instillation techniques can lead to treatment failure, adverse events, and a potential risk of serious infectious complications.9–13 Despite the importance of proper administration, studies evaluating instillation technique in glaucoma patients receiving topical antihypertensive therapy via a conventional eye dropper show that the proportion of patients using improper technique ranges from 34% to 92%.3,8,12–17 Patients’ self-perception of their proficiency in correctly instilling eye drops is frequently inconsistent with their actual proficiency based on objective measures of proper technique.3,16 In a prospective study evaluating self-administration of topical ocular antihypertensive medications, 93% of patients reported using good instillation technique, but evaluation of video evidence using objective performance criteria revealed that less than one-third were able to successfully instill a single drop without contacting the eye with the dropper bottle and 17–25% missed the eye completely.16
To compensate for suboptimal instillation technique and imprecise delivery, standard eye droppers typically contain supratherapeutic drug concentrations or deliver supratherapeutic volumes of solution, with the delivered dose often exceeding the therapeutic dose by a factor of four to five.18 While much of the delivered solution is washed out of the eye, up to 80% of the administered dose can enter the systemic circulation via transport into surface blood vessels or passage through the nasal lacrimal duct and into the digestive system, leading to an increased risk of systemic adverse events.8,16 Any excess solution remaining in the eye can lead to local adverse events, including hypersensitivy reactions to the drug or excipients.16 In addition to the potential adverse health consequences, the use of supratherapeutic doses of topical ophthalmic drugs results in excess waste and increased cost.16 Collectively, these observations suggest an unmet need for a convenient, user-friendly device that reliably, accurately, and comfortably delivers topical ocular therapy with improved efficiency and patient comfort and reduced reliance on patient skill and instillation technique.
We tested a novel, electromechanically actuated, topical ocular drug delivery device designed to facilitate precise dosing and accurate delivery of low-dose topical ophthalmic medications. The active pharmaceutical ingredient is delivered as a low-velocity microfluidic stream with the patient in a seated position and looking straight ahead rather than tilting the head backward. Activation of the electromechanical delivery system initiates the release of a metered dose of drug which can be delivered with precision to a specific area on the surface of the eye via a novel aiming mechanism. The volume of drug can be varied as a function of the pulse duration of the electromechanical system, which is preset. In contrast to conventional eye droppers which deliver a 30–50 μL volume of solution that cannot be easily reduced or accurately measured, the electromechanically acutated system can reliably and precisely deliver a volume of 8–10 μL or less, which more closely corresponds with the fluid volume capacity of the surface of the eye and associated fornices.
The objective of the two Phase 1 proof of concept studies reported herein was to evaluate the acute clinical efficacy and safety of a single dose of a topical ophthalmic solution administered with the novel electromechanical drug delivery system compared with a conventional eye dropper in two different clinical scenarios. A secondary objective was to assess the comfort of administration using the test device compared with conventional dropper delivery.
Materials and Methods
Device Design
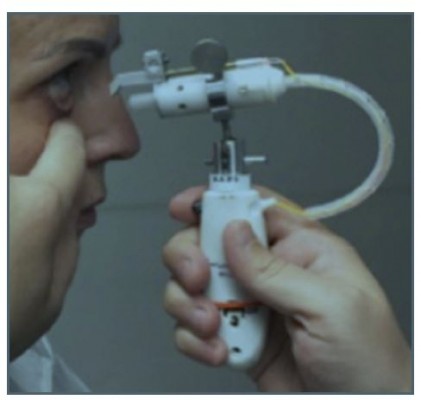
The prototype test device is a handheld, electromechanically actuated device (Figure 1). The handpiece houses a battery-powered circuit board that connects to a piezoelectric actuator and a disposable ampule with a reservior that can accommodate a 30-day supply of drug solution. The ampule attaches to a 0.1 mm-diameter nozzle which is calibrated to deliver a metered dose of 8–10 μL of solution as a microfluidic stream with low impact velocity to the area between the upper and lower eyelids.
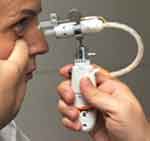 |
Figure 1 Photograph of the hand-held, physician administered, prototype drug delivery system in position for administration to a patient. |
Trial 1
Study Design
Trial 1 was a prospective, randomized, examiner blinded, single-dose study evaluating the mydriatic effect of a 1% tropicamide/2.5% phenylephrine solution delivered via either the test device or a conventional eye dropper in subjects attending an academic ophthalmic clinic in Mexico City. Subjects were randomly assigned to receive a single dose of a combination formulation of 1% tropicamide/2.5% phenylephrine (T-P®, Laboratorios Sophia, Guadalajara, Mexico) administered as a ~9.2 μL microfluid stream via the test device in either the right or left eye. A single dose of the of the same solution was administered as one drop (approximately 30–40 μL) via a conventional dropper bottle to the contralateral eye, which served as the control. The study drug was administered to each eye by a single investigator.
Study Population
Eligible subjects were adult males and females (age >21 years) who required bilateral pupil dilation for fundus examination by indirect ophthalmoscopy. Subjects were required to have a baseline pupil diameter ≤3.5 mm in both eyes and a clear cornea on slit lamp examination. The following criteria mandated exclusion from enrollment: pupillary defects; corneal abnormalities or diminished aqueous clarity preventing accurate assessment of pupil diameter; irregular pupil shape; closed- or narrow-angle glaucoma; use of an anticholinergic agent for the treatment of open-angle glaucoma; moderate to severe dry eye; and known allergy or contraindication to the study treatment.
Study Procedures
The schedule of study assessments is summarized in Table 1. Prior to instillation of study drug, subjects completed a screening evaluation including slit lamp examination and administration of fluorescein to the cornea to test for any abnormalities of the epithelium. Pupil diameter was measured at baseline and at 30 min intervals post-instillition until a decrease in diameter was observed on two consecutive measurements or until a maximum of 4 hrs. Pupillometry was performed by a single blinded examiner using an automated NeurOptics PLR® 3000 pupillometer (NeurOptics, Laguna Hills, CA). Measurements were performed in accordance with the manufacturer’s instructions under static lighting conditions using standard pupillometer settings. Subject comfort was evaluated using a visual analogue comfort scale (VACS) administered within 5 mins after study drug instillation. Subjects were asked to indicate their level of comfort during drug administration for each eye separately using a linear scale ranging from 0 (least comfort) to 100 (most comfort). Other safety assessments included evaluation of adverse events, serious adverse events, and corneal epithelial integrity. Slit lamp examination of the corneal surface and anterior chamber was performed using the Haag-Streit 900 BQ slit lamp (Haag-Streit Diagnostics, Bern, Switzerland). Additionally, administration of study drug was digitally recorded with a video camera for analysis of subject response to the instillition procedure.
 |
Table 1 Schedule of Study assessments—Trial 1 |
Trial 2
Study Design
Trial 2 was a prospective, randomized, examiner blinded, active control, single-dose study evaluating the effect of latanoprost on intraocular pressure in patients with glaucoma after instillition using the test device versus a conventional eye dropper. Following a minimum two-week drug washout period, eligible patients were randomly assigned (1:1) to receive a single dose of latanoprost 0.005% topical ophthalmic solution (Xalatan®, Pfizer Inc., New York, NY) in each eye via either the test device or a conventional eye dropper. Study treatment was administered by the investigator as a ~9.2 μL microfluid stream using the test device or as a ~30–40 μL drop using a conventional eye dropper. Following instillation of study drug, patients completed assessments of intraocular pressure and corneal surface integrity.
Study Population
Eligible patients were adult males and females (age >18 years) with a diagnosis of bilateral primary open-angle glaucoma or ocular hypertension and an intraocular pressure ≤18 mm Hg during treatment with a topical ocular antihypertensive agent or untreated intraocular pressure ≥22 mm Hg and ≤32 mm Hg on initial screening. Additionally, patients were required to complete a washout period of at least two weeks during which topical antihypertensive therapy was withheld. The following criteria mandated exclusion from enrollment: current treatment for glaucoma with more than one drug (including fixed-combination therapies); current or anticipated use of agents with a known effect on intraocular pressure; corneal abnormalities likely to interfere with accurate assessment of intraocular pressure using applanation tonometry; use of oral or topical ophthalmic corticosteroids within 14 days of screening or an anticipated need for ocular corticosteroid therapy during the study; intravitreal or peribulbar steroid injections or placement of an intravitreal steroid implant within the preceding three months; any active ocular disease; closed-angle glaucoma; and significant optic nerve abnormality or an inability to visualize the patient’s optic nerve.
Study Procedures
Scheduled study assessments in Trial 2 are summarized in Table 2. Prior to randomization, patients completed a screening evaluation including slit lamp examination, topical administration of fluorescein to the cornea, and assessment of best corrected visual acuity (BCVA). Intraocular pressure was measured at baseline and 4–8 hrs post-instillation by a single investigator who was masked to treatment assignment. Measurements were performed using a precalibrated Goldmann applanation tonometer coupled to a Haag-Streit 900 BQ slit lamp (Haag-Streit Diagnostics, Bern, Switzerland). Evaluation for fluorescein staining was repeated immediately post-instillation and slit lamp examination of the corneal surface and anterior chamber was performed 4–8 hrs post-instillition. The examiner assessed the corneal surface and anterior chamber using a dichotomous scoring system (normal/abnormal). Patients were monitored for adverse events and serious adverse events from the time of randomization until the end of the observation period.
 |
Table 2 Schedule of Study assessments—Trial 2 |
Statistical Methods
Trial 1 and Trial 2 were exploratory proof of concept studies; therefore, no formal hypothesis testing was planned. In Trial 1, the primary outcome variable was the mean change from baseline in pupil diameter during the post-instillation period. Differences in the mean change in pupil diameter between test and control eyes were evaluated using parametric and non-parametric methods. Subject comfort was evaluated based on comparison of the least squares (LS) mean score on the VACS in the test and control eyes; comparison of the between-group difference in the LS mean VACS score was performed using the Wilcoxon ranked sum test.
In Trial 2, the primary outcome variable was the mean change from baseline in intraocular pressure measured 4–8 hrs post-instillition. The mean change from baseline in each treatment group was evaluated using the student’s t-test. Between-group comparison of the LS mean change from baseline in intraocular pressure was performed using the Wilcoxon ranked sum test. Intraocular pressure is reported as the average value of three measurements performed for each eye.
Data are summarized descriptively as the mean, standard deviation (SD), median, minimum, and maximum values for continuous variables and as the distribution (number and percentage) of subjects or patients for categorical variables. All available data are included in the descriptive summaries; missing data were not replaced with imputed values. Analyses were performed using SAS statistics software, version 9.4 (SAS Institute Inc., Cary, NC).
All patients in both studies provided written informed consent prior to enrollment. The study protocols were approved by the institutional review board of the participating site (Association to Prevent Blindness, Hospital Sanchez-Bulnes, Mexico City, Mexico), and the studies were conducted in compliance with the Declaration of Helsinki and all applicable legal and regulatory requirements.
Results
Trial 1
A total of 40 eyes in 20 subjects were treated with a combination formulation of 1% tropicamide and 2.5% phenylephrine delivered via either the test device or a conventional dropper. Subject demographic and baseline characteristics are summarized in Table 3. The median age was 65 years (range, 21–85 years) and the mean (SD) pupil diameter in the test and control eyes was 3.34 (0.87) mm and 3.35 (1.0) mm, respectively.
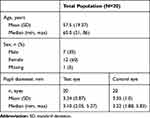 |
Table 3 Demographics and Baseline characteristics—Trial 1 |
Pupillometry results are summarized in Figure 2. Mean (SD) pupil diameter at 30 mins post-instillition increased from 3.34 (0.87) mm to 6.82 (1.07) mm in eyes treated with ~9.2 μL of the tropicamide/phenylephrine solution delivered via the test device (LS mean difference [SD], 3.4 [0.9] mm) and from 3.35 (1.0) mm to 6.97 (1.04) mm in eyes treated with ~30–40 μL of the tropicamide/phenylephrine solution delivered via a conventional dropper (LS mean difference [SD], 3.5 [1.0] mm; Figure 2A). The magnitude of pupil dilation was sustained for 120 mins post-instillation in both the test and control eyes, with peak values observed at 60 mins post-instillation. Comparison of the mean change from baseline in pupil diameter between the test and control eyes showed no statistically significant difference during the post-instillation period (Figure 2B).
Analysis of subject comfort according to the VACS showed a 43% improvement in comfort in eyes treated with the test device compared with a conventional dropper (Figure 3). The mean VACS score was 81.7 mm for the test device and 57.3 mm for conventional dropper delivery (LS mean difference, 23.0 mm; 95% confidence interval [CI], 4.22–41.78; p=0.019).
A total of 13 adverse events were reported; of these, 5 (26.3%) occurred in eyes treated using the test device and 8 (40.0%) occurred in eyes treated using a conventional dropper. Eyelid pain or discomfort was the only reported adverse event in both groups. No serious adverse events were reported during the study. Post-instillation slit lamp examination and topical fluorescein administration showed no evidence of corneal epithelial damage in either the test or control eyes.
Trial 2
A total of 36 eyes from 18 patients were treated with a single dose of latanoprost 0.005% topical ophthalmic solution administered via either the test device or a conventional dropper. Demographic and baseline characteristics are summarized in Table 4. The median age was 61 years (range, 45–76) in the test group and 65 years (range, 58–70) in the control group. The mean (SD) baseline intraocular pressure was 18.6 (2.71) mm Hg and 17.7 (4.12) mm Hg in the test and control groups, respectively. All patients in both groups had completed a washout period of at least two weeks prior to instillation of study treatment.
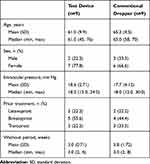 |
Table 4 Demographics and Baseline characteristics—Trial 2 |
Administration of latanoprost resulted in a statistically significant reduction in intraocular pressure in both treatment groups. In the test group, mean (SD) intraocular pressure decreased by 5.0 (1.8) mm Hg following administration of ~9.2 μL of latanoprost 0.005% (p<0.001); in the active control group, mean (SD) intraocular pressure decreased by 4.3 (3.3) mm Hg following administration of ~30–40 μL of latanoprost 0.005% with a conventional dropper (p<0.001). Between-group comparison showed no statistically significant difference in the mean change from baseline in intraocular pressure following administration of study treatment using either the test device or a conventional dropper (LS mean difference, –0.7 mm Hg; 95% CI, –2.44 to 1.10; p=0.345; Figure 4).
The greater variability in the mean change in intraocular pressure observed in the control group was attributable to a single outlier with a baseline intraocular pressure of 30 mm Hg (OS) and a post-instillation pressure of 18 mm Hg. A post hoc sensitivity analysis of the mean change from baseline using the pre-instillation intraocular pressure measured in the patient’s contralateral eye (OD, 20 mm Hg) yielded a result that was consistent with the primary analysis. The mean (SD) change in intraocular pressure was –5.0 (1.8) mm Hg and –3.8 (2.7) mm Hg in the test and control groups, respectively (LS mean difference, –1.2 mm Hg; 95% CI, –2.75 to 0.31; p=0.139).
Safety results in Trial 2 were consistent with observations from Trial 1. Slit lamp examination and fluorescein administration showed no evidence of corneal trauma or epithelial disruption following administration of study treatment. Additionally, no adverse events and no serious adverse events were reported in either treatment group.
Discussion
The electromechanical topical ocular drug delivery system was developed to help address the challenges associated with self-administration of topical ocular therapies via conventional eye droppers, including poor instillation technique and non-adherence and the corresponding risks of treatment failure, contamination, and adverse events. In addition, the device could represent an alternative to extended release inserts because it does not require an invasive procedure. To our knowledge, the current studies are the first to evaluate the use of a single-stream electromechanical drug delivery system in human subjects. The studies demonstrated that administration of a single dose of a topical ocular agent by a physician using this novel delivery system was safe and more comfortable than a conventional dropper and resulted in comparable effects on pupil diameter and intraocular pressure at a substantially lower dose than a conventional eye dropper.
In the mydriasis study (Trial 1), administration of a single dose of tropicamide/phenylephrine by a physician via the test device resulted in an equivalent effect on pupil diameter at a dose that was 70% lower than that required with conventional dropper delivery. Similar results were also observed in recent studies evaluating targeted delivery of topical mydriatics using a microdroplet delivery system.19,20 Notably, the test device in the current study was associated with a 43% improvement in subject comfort compared with a conventional dropper. Video analysis of subject responses during and immediately after study drug administration supported the patients’ subjective rating of comfort on the VACS, with fewer physical reactions such as forced eye blink or turning the head observed during treatment with the test device compared with a conventional dropper (data not shown).
In the glaucoma study (Trial 2), administration of a single dose of latanoprost 0.005% via the test device resulted in a statistically significant reduction in intraocular pressure 4–8 hrs post-instillation. Consistent with the findings from Trial 1, the magnitude of treatment effect was comparable to that in patients receiving a >3-fold higher dose of study drug via a conventional dropper. A recent study evaluating microdroplet delivery of reduced doses of latanoprost also reported a reduction in intraocular pressure following study treatment; however, the study was conducted in healthy volunteers and without an active comparator.21 The present study is therefore the first study to demonstrate clinically significant reductions in intraocular pressure in patients with glaucoma following administration of a reduced dose of latanaprost via an electromechanical drug delivery device. Additionally, slit lamp examination and topical fluorescein administration showed no evidence of epithelial disruption due to treatment with the test device.
In patients with glaucoma and ocular hypertension, topical antihypertensive agents are typically the first-line therapy for controlling intraocular pressure.1,11,16 Persistent elevation of intraocular pressure leads to optic nerve head damage and visual field loss; therefore, proper instillation and adherence to the prescribed therapeutic regimen are vitally important to prevent optic nerve damage and preserve vision.1,6–8 Prior studies have shown a significant correlation between both non-adherence and poor instillation technique and adverse clinical outcomes such as increased intraocular pressure and visual field loss.8,12,14,22 Studies evaluating self-administration of eye drops in patients with glaucoma have shown that 11–60% are non-adherent to the prescribed therapy, 8,12,14,15,23–26 34–92% use improper instillation technique,3,8,12–17 7–44% miss the eye completely, 3,12,15,16,27,28 and 18–80% contaminate the tip of the bottle by contacting the eye or periocular tissue.1,3,10–13,15–17,27–31 Additionally, up to 30% of patients instill a stream of fluid rather than the prescribed number of drops, resulting in excessive delivery and increasing the risk of local and systemic adverse events.16 Collectively, these observations underscore the need for improved delivery technology for topical ophthalmic therapies.
The two proof of concept trials reported herein provide initial evidence of device feasibility and suggest that the device could provide important clinical and economic benefits for patients who require topical ocular therapy. However, the findings should be interpreted in the context of certain limitations, including the open-label design, the relatively small sample size, and the limited study duration. In addition, study treatment was administered by a physician in both trials; further research is required to assess the safety and efficacy of self-administration of topical agents using an electromechanical low-dose delivery system. Notably, the device used in this study was not designed for patient self-administration but rather as a flexible research tool to determine whether markedly lower volumes of drug (≥75% reduction) administered to the surface of the eye in the form of a continuous stream from an electromechanical system can induce a physiologic effect comparable to conventional administration from a dropper bottle. Study drug was administered by a physician in both the test and control groups; therefore, the test conditions were comparable and the conclusions related to the effect of lower drug volumes on both safety and efficacy outcomes should be valid.
Conclusion
Evaluation of a novel, electromechanical, low-dose topical ocular drug delivery device in two Phase 1 proof of concept studies showed that administration of topical ocular medications by a physician using the device was safe and well tolerated. Comparable effects on pupil dilation and intraocular pressure were achieved with doses that were conservatively three times lower than conventional drops. Patient-rated scores on the visual analog comfort scale indicated that the test device was associated with a significant improvement in comfort compared with a conventional dropper when the study drug was instilled by a physician. Based on these findings, continued clinical development and further evaluation of this topical ophthalmic drug delivery system is warranted.
Acknowledgments
We wish to thank Biao Lu (statistical consultant) for assistance with statistical analysis, Ken Glasscock (KFG Scientific Communications) for medical writing and editorial assistance, and the study participants and participating staff at the Association to Prevent Blindness in Mexico City, Mexico.
Disclosure
Hugo Quiroz-Mercado, MD and Roberto Gonzalez-Salinas, MD both received an honorarium from Kedalion Therapeutics Inc. Mark Blumenkranz is the Executive Chairman and Co-founder of Kedalion Therapeutics Inc; in addition, Dr Blumenkranz has a patent device and method for treatment of ocular disease pending. Ehud Ivri is an employee and Co-founder of Kedalion Therapeutics Inc. George Marcellino was an employee of Kedalion Therapeutics Inc at the time this study was conducted. Joseph Gilbert and Iraklis Kourtis have received compensation as a consultant for Kedalion Therapeutics Inc. The authors report no other conflicts of interest in this work.
References
1. Davis SA, Sleath B, Carpenter DM, Blalock SJ, Muir KW, Budenz DL. Drop installation and glaucoma. Curr Opin Ophthalmol. 2018;29:171–177. doi:10.1097/ICU.0000000000000451
2. National Institutes of Health. How to Put in Your Eye Drops. Bethesda, MD: National Institutes of Health; 2008. Available from: https://clinicalcenter.nih.gov/ccc/patient_education/pepubs/eyedrops.pdf.
3. Tatham AJ, Sarodia U, Gatrad F, Awan A. Eye drop instillation technique in patients with glaucoma. Eye. 2013;27:1293–1298. doi:10.1038/eye.2013.187
4. Eaton AM, Gordon GM, Konowal A, et al. A novel eye drop application monitor to assess patient compliance with a prescribed regimen: a pilot study. Eye. 2015;29:1383–1391. doi:10.1038/eye.2015.155
5. Winfield AJ, Jessiman D, Williams A, Esakowitz L. A study of the causes of non-compliance by patients prescribed eyedrops. Br J Ophthalmol. 1990;74:477–480. doi:10.1136/bjo.74.8.477
6. Investigators AGIS. The Advanced Glaucoma Intervention Study (AGIS), 7: the relationship between control of intraocular pressure and visual field deterioration. Am J Ophthalmol. 2000;130:429–440.
7. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:
8. Atey TM, Shibeshi W, Giorgis AT, Asgedom SW. The impact of adherence and instillation proficiency of topical glaucoma medications on intraocular pressure. J Ophthalmol. 2017;2017:1683430. doi:10.1155/2017/1683430
9. Schein OD, Wasson PJ, Boruchoff SA, Kenyon KR. Microbial keratitis associated with contaminated ocular medications. Am J Ophthalmol. 1988;15:361–365.
10. Schein OD, Hibberd PL, Starck T, Baker AS, Kenyon KR. Microbial contamination of in-use ocular medications. Arch Ophthalmol. 1992;110:82–85. doi:10.1001/archopht.1992.01080130084030
11. Geyer O, Bottone EJ, Podos SM, Schumer RA, Asbell PA. Microbial contamination of medications used to treat glaucoma. Br J Ophthalmol. 1995;79:376–379. doi:10.1136/bjo.79.4.376
12. Sleath B, Blalock S, Covert D, et al. The relationship between glaucoma medication adherence, eye drop technique, and visual field defect severity. Ophthalmol. 2011;118:2398–2402. doi:10.1016/j.ophtha.2011.05.013
13. Gupta R, Patil B, Shah BM, Bali SJ, Mishra SK, Dada T. Evaluating eye drop instillation technique in glaucoma patients. J Glaucoma. 2012;21:189–192. doi:10.1097/IJG.0b013e31820bd2e1
14. Konstas AG, Maskaleris G, Gratsonidis S, Sardelli C. Compliance and viewpoint of glaucoma patients in Greece. Eye. 2000;14:752–756. doi:10.1038/eye.2000.197
15. Kholdebarin R, Campbell RJ, Jin YP, Buys YM. Multicenter study of compliance and drop administration in glaucoma. Can J Ophthalmol. 2008;43:454–461. doi:10.3129/i08-076
16. Stone JL, Robin AL, Novack GD, Covert DW, Cagle GD. An objective evaluation of eyedrop instillation in patients with glaucoma. Arch Ophthalmol. 2009;127:732–736. doi:10.1001/archophthalmol.2009.96
17. Mehari T, Giorgis AT, Shibeshi W. Appropriateness and determinants of proper administration technique of ocular hypotensive agents among glaucoma patients in Menelik II referral hospital, Ethiopia. J Clin Exp Ophthalmol. 2016;7:3. doi:10.4172/2155-9570.1000554
18. Kumar S, Karki R, Meena M, Prakash T, Rajeswari T, Goli D. Reduction in drop size of ophthalmic topical drop preparations and the impact of treatment. J Adv Pharm Technol Res. 2011;2:192–194. doi:10.4103/2231-4040.85540
19. Ianchulev T, Chayet A, Kahook M, Packer M, Pasquale L, Weinreb RN. Pharmacodynamic profile of mydriatic agents delivered by ocular piezo-ejection microdosing compared with conventional eyedropper. Ther Deliv. 2016;7:751–760. doi:10.4155/tde-2016-0061
20. Ianchulev T, Weinreb RN, Sai JC, Lin S, Pasquale LR. High-precision piezo-ejection ocular microdosing: Phase II study on local and systemic effects of topical phenylephrine. Ther Deliv. 2018;9:17–27. doi:10.4155/tde-2017-0095
21. Pasquale LR, Lin S, Weinreb RN, Sai JC, Kramm RL, Ianchulev T. Latanoprost with high precision, piezo-print microdose delivery for IOP lowering: clinical results of the PG21 study of 0.4 μg daily microdose. Clin Ophthalmol. 2018;12:2451–2457. doi:10.2147/OPTH.S185027
22. Rossi GC, Pasinetti GM, Scudeller L, et al. Do adherence rates and glaucomatous visual field progression correlate? Eur J Ophthalmol. 2011;21:410–414. doi:10.5301/EJO.2010.6112
23. Friedman DS, Okeke CO, Jampel HD, et al. Risk factors for poor adherence to eyedrops in electronically monitored patients with glaucoma. Ophthalmology. 2009;116:1097–1105. doi:10.1016/j.ophtha.2009.01.021
24. Kass MA, Meltzer DW, Gordon M, Cooper D, Goldberg J. Compliance with topical pilocarpine treatment. Am J Ophthalmol. 1986;101:515–523. doi:10.1016/0002-9394(86)90939-6
25. Kass MA, Gordon M, Morley RE
26. Rotchford AP, Murphy KM. Compliance with timolol treatment in glaucoma. Eye. 1998;12:234–236. doi:10.1038/eye.1998.56
27. Hennessy AL, Katz J, Covert D, et al. A video study of drop instillation in both glaucoma and retina patients with visual impairment. Am J Ophthalmol. 2011;152:982–998. doi:10.1016/j.ajo.2011.05.015
28. Schwartz GF, Hollander DA, Williams JM. Evaluation of eye drop administration technique in patients with glaucoma or ocular hypertension. Curr Med Res Opin. 2013;29:1515–1522. doi:10.1185/03007995.2013.833898
29. Brown MM, Brown GC, Spaeth GL. Improper topical self-administration of ocular medication among patients with glaucoma. Can J Ophthalmol. 1984;19:2–5.
30. Hosoda M, Yamabayashi S, Furuta M, Tsukahara S. Do glaucoma patients use eye drops correctly? J Glaucoma. 1995;4:202–206. doi:10.1097/00061198-199506000-00011
31. Tsai T, Robin AL, Smith JP
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



