Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Efficacy, Antibiotic Resistance Genes, Virulence Factors and Outcome of Hospital-Acquired Pneumonia Induced by Klebsiella pneumoniae Carbapenemase 2-Producing with Tigecycline Treatment in the ICU
Authors Bai XR, Cao JR, Wang ZZ, Li WC, Chen DD, Lou R, Qu X, Yan SY
Received 7 July 2022
Accepted for publication 7 September 2022
Published 21 September 2022 Volume 2022:15 Pages 5545—5555
DOI https://doi.org/10.2147/IDR.S381280
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Xiang-Rong Bai,1,* Jing-Rong Cao,2,* Zhi-Zhou Wang,1 Wen-Chao Li,1 Dian-Dian Chen,2 Ran Lou,3 Xin Qu,4 Su-Ying Yan1
1Department of Pharmacy, Xuan Wu Hospital Capital Medical University, National Gerontic Disease Clinical Research Center, Beijing, 100053, People’s Republic of China; 2Department of Clinical Laboratory, Xuan Wu Hospital Capital Medical University, National Gerontic Disease Clinical Research Center, Beijing, 100053, People’s Republic of China; 3Department of Intensive Medicine, Xuan Wu Hospital Capital Medical University, National Gerontic Disease Clinical Research Center, Beijing, 100053, People’s Republic of China; 4Intensive Care Unit, Department of Neurosurgery, Xuan Wu Hospital Capital Medical University, National Gerontic Disease Clinical Research Center, Beijing, 100053, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Su-Ying Yan, Department of Pharmacy, Xuan Wu Hospital Capital Medical University, National Gerontic Disease Clinical Research Center, No. 45 Changchun Street, Xi Cheng District, Beijing, 100053, People’s Republic of China, Tel/Fax +86 10-63012837, Email [email protected]
Purpose: Tigecycline is an agent for carbapenemase-producing Klebsiella pneumonia (KPC-KP), given its penetration into lung tissues. Our study focused on the molecular and clinical efficacy of tigecycline for hospital-acquired pneumonia (HAP) in the ICU.
Patients and Methods: A retrospective cohort study of 52 adult KPC-KP HAP patients by searching hospital medical records from January 2018 to December 2020 was established to investigate the epidemiology of KPC-KP infections for tigecycline treatment and the associated clinical efficacy of tigecycline. The KPC-KP isolates underwent multilocus sequence typing. Molecular typing, antimicrobial resistance, and virulence profiling were also analyzed by whole-genome sequencing of KPC-KP.
Results: Among 52 patients with KPC-KP, the ICU mortality rate was 14/52 (27%), and there was no significant statistical difference in mortality between the effective group and failure group (p = 0.754). However, the duration of tigecycline was statistically different between the two groups of patients (14.4 vs 10 days, p=0.046). The total bacterial clearance rate was 6/52 (11.5%). There was no significant statistical difference in both groups (p=0.416). Antibiotic resistance genes (aac3iia) and virulence gene (AREO-iutA, Capsule-wzc) were negatively correlated with clinical efficacy (p = 0.011, OR = 1.237).
Conclusions: Blakpc was the main carbapenemase in all K. pneumoniae strains. ST11-KL64 KPC-KP was the most common virulence factors in KPC-KP isolates. This study suggested that antibiotic resistance genes (aac3iia) and virulence gene (AREO-iutA, Capsule-wzc) were independent mortality risk factors for patients with Klebsiella pneumoniae carbapenemase-2 producing K. pneumoniae infections, when during the tigecycline treatment. Molecular analysis of K. pneumoniae may provide an option when choosing the antimicrobial treatment.
Keywords: Klebsiella pneumoniae, carbapenemase, virulence factors, resistance genes, hospital-acquired pneumonia
Introduction
Klebsiella pneumoniae is the most clinically relevant Klebsiella species, often exhibiting multidrug resistance and high virulence. Globally, K. pneumoniae infections are leading healthcare-associated infection.1 The most common are pneumonia, urinary tract and abdominal infections, any of which can progress to bacteremia. In the last 10 years, carbapenem-resistant K. pneumoniae (CR-Kp) had increased from 3% in 2005 to 25.3% in 2019 in China.2
The management of nosocomial infections due to CR-Kp infections has become challenging. The mortality rates of CR-Kp were between 23% and 75%, which are attributed to inappropriate empirical therapy and underlying comorbidities of patients.3 A cohort study in ICU suggested that empirical antibiotic therapy, the use of piperacillin-tazobactam, was associated with mortality, and CR-Kp increased 20% of adjusted mortality.4
The most mechanism of carbapenem resistance was carbapenem production in Klebsiella pneumoniae, particularly KPC in China.5 Based on understanding of carbapenem resistance mechanisms can guide the selection of treatment option for CR-Kp.6,7
The clinical evidence seems to indicate tigecycline has promising for KPC- Klebsiella pneumoniae (KPC-Kp) infections, but its clinical efficacy is varied.3 The current study believes that the insufficient dose recommended by the manual is one of the reasons for the poor efficacy of tigecycline. A large number of studies show that high-dose (100mg bid) tigecycline is an effective treatment option.8 A meta analysis study indicated that the monotherapy tigecycline had a higher mortality compared to the combination therapy group.9 When new treatment options were not available, high dose tigecycline combination therapy could be an option.10 In vitro and vivo data indicated that tigecycline combination with carbapenem can decrease bacterial loads.11 A multicenter retrospective cohort study suggested that combination of tigecycline and meropenem was associated with lower mortality.12 Therefore, the current lack of alternative treatment option suggests that tigecycline benefit–risk continues to be positive.
In addition, genomic studies provide a clue for the interpretation of studies aimed at the pathogenicity and/or virulence of K. pneumoniae.13 Yersiniabactin (ybt, irp, fyuA), which form the Yersinia high-pathogenicity island (HPI), was the most prevalent virulence-associated locus and strongly predictive of infection. Similarly, all K. pneumoniae encoding aerobactin (iucABCD, iutA), salmochelin (iroN, iroBCD), and rmpA/rmpA2 were isolated from human infections. The combination of salmochelin, aerobactin, and rmpA was frequently linked to the presence of genes from the known K. pneumoniae virulence plasmids pLVPK and pK2044.14 In China, sequence type (ST) 11 carbapenem-resistant hypervirulent K. pneumoniae is still the dominant clone.15 ST23 has also been identified in China.16 A study indicated that an ST16 clone is associated with high mortality for adult KPC-KP bloodstream infection.17 However, it is unknown for HAP. Thus, we conducted a study to understand the molecular of KPC-KP infections for tigecycline treatment, and associated with the clinical efficacy of tigecycline with HAP.
Materials and Methods
Study Population
We conducted a retrospective cohort study of adult KPC-KP HAP cases in Xuanwu hospital from January 2018 to December 2020. We retrieved the case by searching the microbiology database adults aged >18 years old whose respiratory tract specimens (Bronchoalveolar Lavage or Endotracheal Aspirates) were KPC-KP who had taken more than three days of tigecycline. Respiratory tract secretion test was performed daily. Data were extracted from the medical records in our hospital computer health-care system. The variables collected included patient demographic characteristics (gender, age, BMI, comorbidities), and Charlson's comorbidity index, medical history, surgery during hospitalization. We also collected medication, including tigecycline dosage: High Dose (HD) (loading dose 200mg, maintenance dose 100mg q12h), Standard Dose (SD) (loading dose 100mg, maintenance dose 50mg q12h) and course of treatment, and the combined use of antibacterial drugs. Vital signs, such as body temperature, respiration rate, pulse, and laboratory test, drug sensitivity results (tigecycline, amikacin and compound trimethoprim) were also collected within 48 hours before and after tigecycline treatment. The endpoint was 72 hours after tigecycline withdrawal or transfer from the ICU ward. Patients were excluded if they had combined other sites of infection, bacteremia or adjusted the dose of tigecycline during the study, the main outcome indicators cannot be evaluated, liver or kidney insufficiency. Hospital-Acquired Pneumonia was diagnosis based on IDSA guideline published in 2016.18
Patient group: All patients were divided into effective (body temperature, symptoms and signs improved, and lung imaging improved or not progressed) group and failure group (body temperature, vital signs have not improved or worsened, lung imaging progressed or the patient died due to infection).19 The outcomes were evaluated by two authors separately. When the results were inconsistent, another two authors negotiated together.
Outcomes
All-cause death in the ICU: The patient died during the whole follow-up.
Clearance of pathogens: The microbiology efficacy was divided into clearance (bacterial culture results are negative), hypothetical clearance (clinically effective, not checked due to the inability to obtain respiratory specimens), and replacement (carbapenemase 2–Producing K was clear, other bacteria are detected) and re-infection (carbapenemase 2–Producing K was still detected in the culture). The microbiology clearance rate = (number of cleared cases + assumed number of cleared cases + number of replacement cases)/total number of cases ×100%. The duplicated cases (multiple isolates in the same patient) were not counted.20
Microbiological Analysis
The isolates of K. pneumoniae were cultivated overnight on MacConkey agar media at 37°C for further sequence typing. The K. pneumoniae strains were subjected to antimicrobial susceptibility tests comprising 13 different antibiotics by VITEK-2 compact system (bioMérieux, France). The minimum inhibitory concentration (MIC) was routinely determined in accordance with the general guidance of the Clinical and Laboratory Standards Institute (CLSI).21 Tigecycline susceptibility breakpoints were determined according to the FDA standard (MIC <2mg/L, sensitive; MIC = 4 intermediate, MIC ≥8mg/L, resistant) by using the E-test method (BIO-KONT, Wenzhou, China).22
Carbapenemase Test
The VITEK2 Compact system (bioMérieux, France) was used for antimicrobial susceptibility test and further confirmed by using E-test. The MIC results of carbapenems were interpreted strictly followed the guidelines of the Clinical and Laboratory Standards Institute (CLSI). Escherichia coli ATCC25922 was used as quality control strain. Confirmation of suspected carbapenemase production in K. pneumoniae isolates was performed using a modified carbapenem inactivation method (mCIM). Phenotypic screening for the presence of carbapenemases was performed using a double-disk synergy test (DDST) with phenylboronic acid or ethylenediaminetetraacetic acid with meropenem as previously described. The mCIM analysis and DDST are biochemical assay for carbapenemase activity, and the results were positive. The blaKPC gene was detected in all isolates with PCR and CARBA NP test.
Genome sequencing
Extraction of Genome DNA
Bacterial genomic DNA was extracted and quantified by Qubit® 2.0 Fluorometer (Thermo Scientific).
Library Construction and Sequencing
Sequencing libraries were generated using a NEBNext Ultra DNA Library Prep Kit for Illumina (NEB, USA) following the manufacturer’s recommendations, and index codes were added to attribute sequences to each DNA sample fragmented by sonication to a size of 350 bp. Libraries were analyzed for size distribution by an Agilent2100 Bioanalyzer and quantified using real-time PCR. The GeneMarkS program was used to retrieve the related coding gene. The whole genome of K. pneumoniae was sequenced using Illumina NovaSeq PE150 at the Beijing Novogene Bioinformatics Technology Co., Ltd. Genomes were assembled with SOAP denovo, SPAdes, Abyss and gapclose software. Antibiotic Resistance Genes Database (ARDB), Pathogen Host Interactions (PHI) and Virulence Factor Database (VFDB) were used to perform the pathogenicity and drug resistance analyses. Then, multilocus sequence typing (MLST) database (https://www.pasteur.fr/fr/mlst) with various allelic profiles was performed to determine the genetic diversity of K. pneumoniae. In addition, we also used Kaptive to search for the K-loci of K. pneumoniae, which allowed for defining the capsule serotype (K and KL), thus narrowing the isolate classification. Finally, the results obtained by the programs mentioned above are summarized and presented in a concise tabular form.
Statistical Analysis
Categorical variables was used χ2 or Fisher’s exact test and t-test or Mann–Whitney U-test for continuous variables. Multivariate analysis to determine the impact of covariates on clinical efficacy was performed by binary logistic regression, adjusting for confounding factors, and using IBM SPSS Statistics 25.0 (Armonk, New York). The critical value of p < 0.2 in univariate analysis is used to select the covariates of the multivariate model, and the Horner-Lemeshow goodness-of-fit test is applied. The entire study (two-tailed) showed precise p-values. The statistical significance was established at p < 0.05.
Institutional Review Board Statement: The study was carried out in accordance with the Declaration of Helsinki criteria and was approved by the Ethics Committee of Xuanwu Hospital Capital Medical University (IRB, protocol number: 2020038 6/20/2020). All patients gave written informed consent. The clinical trial registration number is ChiCTR2000041036
Results
In total, 3477 patients were observed from January 2018 to December 2020 in ICU. Among them, 3.5% (120/3477) of the cases were Klebsiella pneumoniae with Tigecycline treatment. A total of 87 patients were diagnosis of Hospital-Acquired Pneumonia. Twenty-four patients were carbapenem-sensitive Klebsiella pneumoniae. Tigecycline dose adjustment was performed in 11 patients. At last, 52 patients were included in the final analysis. The distribution of these patients is shown in Figure 1.
 |
Figure 1 Flowchart of patient inclusion. |
The mean ± standard deviation age of all patients was 62.3 ± 15.2 years, with 44.2% (23/52) was female. Mechanical ventilation patients were 65.4% (34/52). Combining therapy with tigecycline was a major treatment option, with 46.2% (24/52) of patients being on carbapenems in our study. 38.5% (20/52) patients were treated with high-dose tigecycline. The treatment days of tigecycline were 10 in the treatment failure group and 14.5 days for the patients in the effective treatment group, p = 0.046. The clinical characteristics are listed in Table 1.
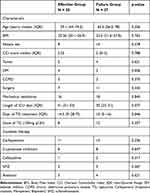 |
Table 1 Characteristics of Patients with HAP Caused by KPC with TG in Both Groups |
AST and Isolated Antibiotic Resistance Genes
All isolates were all resistant to IPM: (Imipenem), MEM: (meropenem), AMP (ampicillin), CAZ: (ceftazidime), CTX (cefotaxime), CRO (ceftriaxone), PIP (piperacillin), TZP (piperacillin and tazobactam), MNO (minocycline), and ATM (aztreonam) except CT (Clostin) and AMK (amikacin). Sixteen isolates (30.8%) showed MIC=1mg/L, 15 (28.4%) MICs = 2 mg/L, 6 (11.5%) MIC = 0.5 mg/L, 8 (15.4%) MIC = 0.25 mg/L, 5 (9.6%) MIC ≤0.125 mg/L; among them, 50 (96.0%) was susceptible, and 2 (4%) was intermediate to tigecycline (MIC = 4 mg/L).
The phenotype studies showed that 52 isolates were ampicillin-resistant, which corresponds well with their possession of SHV-type beta-lactamase genes known to be chromosome-encoded. All isolates were also resistant to other beta-lactam antibiotics and carbapenems. The prevalent blaKPC-2 gene was detected in all 52 isolates, whereas the blaNDM genes were not detected. The prevalent β-lactamase genes blaSHV, blaCTX-M, and blaTEM were mostly expressed by the CRKP isolates at ratios of 100%, 100%, and 90.4% (47/52), respectively. Each CRKP isolate in this study expressed at least three resistance genes, whereas 63.5% (33/52) of the CRKP strains co-expressed the blaKPC-2, blaCTX-M-15, blaSHV, and blaTEM genes. The β-lactamase resistance genes blaOXA-1 and blaTEM-1B, the phenicol resistance gene catB4, the sulphonamide resistance gene sul2, and the trimethoprim resistance gene dfrA14 were also expressed in the CRKP isolates. Three isolates expressed blaTET (5.8% each). The data obtained are summarized in Figure 2.
Virulence Factors
All strains had aero, salmonellin- and yersiniabactin-related genes, and 69% of the isolates contained the rmpA (regulator of mucoid phenotype A) gene. In addition, almost all of the isolates contained genes of the mrk and fim clusters, which are involved in types 3 and 1 fimbriae formation, respectively. Most isolates contained four or more virulence factors, suggesting that they could be dangerous in clinical settings, requiring further investigation. The main virulence factors given above are shown in Figure 3.
Typing and Classification
Two STs were identified among the 52 KPC-KP isolates, which included 37 isolates for ST11 (71.1%), the most prevalent ST, belonged to CC258, while the others (40%) were novel sequence types with phoE gene allelic profiles 419.
There were five different KL types among the isolates, with two isolates belonging to the KL47 type. The KL types identified were KL47, KL64, k20, k50, and k25, respectively. The most frequent K type was KL64 (88%), which is usually considered to be associated with high virulence in China.23 However, there was no significant difference between the two groups in the mortality in ICU for ST11-KL64 KPC-KP (P > 0.05).
Among the 52 patients with KPC-KP, the ICU mortality rate was 22/52 (27%), and there was no significant difference in mortality between the two groups (p = 0.754). The total bacterial clearance rate was 6/52 (11.5%), but there was no statistical difference between the two groups of patients (p = 0.578). See Table 2 for more information.
 |
Table 2 The Mortality, Clearance of Pathogens and Distribution of Antibiotic Resistance Genes, Virulence Factors in Both Groups |
Clinical Efficacy Predictor Analysis
We performed an analysis for factors expected to influence clinical efficacy. See Table 3. The results showed that the dose, course of treatment and combination of tigecycline had no significant correlation with clinical efficacy. As for the antibiotic resistance gene, ant2ia was correlated with clinical efficacy, p=0.024. In the detection of virulence genes, AREO-iutA and Capsule-wzc were found to be related to the clinical efficacy.
 |
Table 3 Univariate and Multivariate Analyses of the Risk Factors Associated with Clinical Efficacy in Patients with Klebsiella Pneumoniae Carbapenemase 2-Producing K. Pneumoniae Infections |
Discussion
In this study, we conducted a clinical efficacy and molecular biology study on the treatment of KPC Klebsiella pneumonia with tigecycline. Our study found there were no significant differences in mortality between the two groups.
Studies reported that KPC Klebsiella pneumonia patients, who had chronic diseases or immunosuppressive status, has high mortality.24 An animal study has shown that tigecycline alone or in combination can achieve effective treatment of experimental KPC Klebsiella pneumonia.11 However, a meta-analysis study indicated that there were no statistically significant differences in the pooled likelihood of mortality, clinical or microbiological responses between combination therapy.25 Therefore, Charlson Comorbidity Index, combination therapy with tigecycline were included in our study. We found that these factors were not statistically different between two groups. Overall, the baseline characteristics of participants between two groups were comparable.
Food and Drug Administration (FDA) recommend Minimum inhibitory concentration (MIC) breakpoints for tigecycline susceptible (MIC ≤ 2 μg/mL), intermediate (MIC 4 μg/mL), and resistant (MIC ≥ 8 μg/mL)26. Matthaios Papadimitriou-Olivgeris’s study showed 32 (10.6%) MICs of tigecycline <0.5 mg/L, whereas 177 (58.6%) showed MICs that were 0.75–2 mg/L. The 30-day mortality rate of the latter was higher than that of the former.27 It was inconsistent with our findings. This may be due to the study being mainly monotherapy with tigecycline. Current research reports that high-dose tigecycline is one of the treatment options. A main concern is the suboptimal concentrations of tigecycline, which could be overcome by increasing the dose, leading to better outcomes.28 However, none of these studies mentioned the drug sensitivity data of tigecycline. Monte Carlo simulation study indicated that high dose tigecycline may be appropriate for patients with MIC >0.48μg/mL.29
Shelenkovis et al’s study analyzed 36 cases of KPC-Kp for virulence factors and resistance genes. The results showed that 32 cases were multi-drug resistant strains and 5 cases were polymyxin-resistant strains. The rare type was ST 377.30 Our study showed that all strains are resistant to carbapenem, β-lactamase inhibitor combinations, third-generation cephems, aminoglycosides, and levofloxacin. The detection rate of the key enzyme of the carbapenem resistance gene blaKPC-2 and the extended-spectrum β-lactamases blaSHV, blaCTX-M, and blaTEM were ≥90%, which support the contention of the expression of multiple resistance genes of the CRKP strains. In the study, ST11 was the predominant clone (60%) in CRKP strains, but emerging novel ST with phoE gene allelic profiles 419 worthy of continued attention.31 This was consistent with numbers of study in China and other countries.32,33 ST11 type CRKP strains, which belong to one of clonal group 258 (CG258) type of CRKP, is a high risk resistant clone with transmissible characteristic of carrying co-resistance determinants and virulence factors.34 It poses enormous challenges to infection control. In our study, the ST11- KL64 K. pneumoniae strains (88%) were the dominant K types of the most important clinical pathogens, which is consistent with previous studies.35 The KL64 strains contained the most virulence genes, including iucA and rmpA. ST11-KL64 K. pneumoniae strains seem to have no higher mortality and poor clinical efficacy. This may be due to the small sample size in our study.
Hypervirulent Klebsiella pneumoniae (HvKP) strains are characterized by the presence of capsular polysaccharides (K antigen), fimbriae, lipopolysaccharides (O antigen), and siderophores (aerobaction and yersiniabactin).36 Herein, we investigated 42 virulence-associated genes in 52 KPC-KP isolates. According to the aforementioned criteria, these isolates were identified as carbapenem-resistant hypervirulent K. pneumonia strains with a ST11 KL64/KL47 serotype. The mrkD gene encodes type 3 fimbriae, which play critical roles in adhesion to the respiratory tract, often detected in cases of ventilator-associated pneumonia caused by K. pneumoniae.37 A previous study reported that biofilm formation requires the type 3 fimbriae and adhesion factor mrkD.38 Biofilm formation inhibits the penetration of drugs, thus increasing antibiotic resistance, which further complicates the clinical treatment of K. pneumonia infections.39 Our study found that ant2ia gene was correlated with clinical efficacy. Nucleotidyltransferase (ant2ia) gene regions suggested it was resistance to aminoglycosides. However, all Klebsiella pneumoniae were sensitive to amikacin, and 9 patients were treated with amikacin, accounting for 17.3% (9/52), and the detection rate of ant2ia gene was 36.5% (19/52). It is suggested that the selection of drugs based solely on the drug susceptibility results has certain limitations, and the monitoring of antibiotic resistance genes has certain significance for the selection of antibiotics. However, this still needs to be verified by large-scale clinical studies. Our results suggested that virulence gene (AREO-iutA, Capsule-wzc) was related to the clinical efficacy. The AREO-iutA gene encodes a membrane protein receptor involved in biofilm formation in Klebsiella pneumoniae.40 Capsule-wzc is produced through a Wzy-dependent process for which the conserved machinery is encoded by genes that are present in most K-antigen biosynthesis loci.41
The outbreak of KPC-KP strains and the emergence of hypervirulence forces us to promote awareness and to strengthen epidemiological surveillance and infection control measures in our hospital, especially regarding ST11 KPC-producing hypervirulent strains.
Conclusion
In conclusion, we found that there was no significant difference in mortality for effective group and failure group. Antibiotic resistance genes (aac3iia) and virulence gene (AREO-iutA, Capsule-wzc) were independent mortality risk factors for patients with KPC-KP. However, there was no significant difference between the two groups in the mortality in ICU for ST11-KL64 KPC-KP. ST11-KL64 KPC-Kp had a significantly higher mortality rate than other CRKP-infected patients in the ICU. There is a need for further study to confirm the association between ST11-KL64 KPC-KP and the dose of tigecycline.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by Beijing Municipal Science and Technology Commission Special Funding Project (D181100000218002); National Clinical Research Center for Geriatric Disorders, Beijing, China. Beijing Municipal Commission of Health and Family Planning, No. PXM2018_026283_000002; Beijing Municipal Administration of Hospitals Incubating Program (PX2020038); Beijing High-level Public Health Technical Talent Development Plan (2022-01-023).
Disclosure
The authors declare no competing interests.
References
1. Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521–S528. doi:10.1093/cid/ciz824
2. Hu F, Wang M, Zhu D, Wang F. CHINET efforts to control antimicrobial resistance in China. J Glob Antimicrob Resist. 2020;21:76–77. doi:10.1016/j.jgar.2020.03.007
3. Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother. 2015;59(10):5873–5884. doi:10.1128/AAC.01019-15
4. Bertolini G, Nattino G, Tascini C, et al. Mortality attributable to different Klebsiella susceptibility patterns and to the coverage of empirical antibiotic therapy: a cohort study on patients admitted to the ICU with infection. Intensive Care Med. 2018;44(10):1709–1719. doi:10.1007/s00134-018-5360-0
5. Han R, Shi Q, Wu S, et al. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant enterobacteriaceae isolated from adult and children patients in China. Front Cell Infect Microbiol. 2020;10:314. doi:10.3389/fcimb.2020.00314
6. Di Tella D, Tamburro M, Guerrizio G, Fanelli I, Sammarco ML, Ripabelli G. Molecular epidemiological insights into colistin-resistant and carbapenemases-producing clinical Klebsiella pneumoniae isolates. Infect Drug Resist. 2019;12:3783–3795. doi:10.2147/IDR.S226416
7. Karakonstantis S, Kritsotakis EI, Gikas A. Treatment options for K. pneumoniae, P. aeruginosa and A. baumannii co-resistant to carbapenems, aminoglycosides, polymyxins and tigecycline: an approach based on the mechanisms of resistance to carbapenems. Infection. 2020;48(6):835–851. doi:10.1007/s15010-020-01520-6
8. Bai XR, Jiang DC, Yan SY. High-dose tigecycline in elderly patients with pneumonia due to multidrug-resistant Acinetobacter baumannii in intensive care unit. Infect Drug Resist. 2020;13:1447–1454. doi:10.2147/IDR.S249352
9. Bai XR, Liu JM, Jiang DC, Yan SY. Efficacy and safety of tigecycline monotherapy versus combination therapy for the treatment of hospital-acquired pneumonia (HAP): a meta-analysis of cohort studies. J Chemother. 2018;30(3):172–178. doi:10.1080/1120009X.2018.1425279
10. Bassetti M, Peghin M. How to manage KPC infections. Ther Adv Infect Dis. 2020;7:2049936120912049. doi:10.1177/2049936120912049
11. Fergadaki S, Renieris G, Machairas N, et al. Efficacy of tigecycline alone or in combination for experimental infections by KPC carbapenemase-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2021;58(3):106384. doi:10.1016/j.ijantimicag.2021.106384
12. Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis. 2012;55(7):943–950. doi:10.1093/cid/cis588
13. Wyres KL, Lam M, Holt KE. Population genomics of Klebsiella pneumoniae. Nat Rev Microbiol. 2020;18(6):344–359. doi:10.1038/s41579-019-0315-1
14. Holt KE, Wertheim H, Zadoks RN, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A. 2015;112(27):E3574–E3581. doi:10.1073/pnas.1501049112
15. Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem-resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18:37–46. doi:10.1016/S1473-3099(17)30489-9
16. Zeng L, Deng Q, Zeng T, Liu Y, Zhang J, Cao X. Prevalence of carbapenem-resistant Klebsiella pneumoniae infection in Southern China: clinical characteristics, antimicrobial resistance, virulence, and geographic distribution. Microb Drug Resist. 2020;26:483–491. doi:10.1089/mdr.2018.0401
17. Andrey DO, Pereira DP, Martins W, et al. An emerging clone, Klebsiella pneumoniae carbapenemase 2-producing K. pneumoniae sequence type 16, associated with high mortality rates in a CC258-endemic setting. Clin Infect Dis. 2020;71:e141–141e150. doi:10.1093/cid/ciz1095
18. Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated Pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–61e111. doi:10.1093/cid/ciw353
19. Niederman MS. Hospital-acquired pneumonia, health care-associated pneumonia, ventilator-associated pneumonia, and ventilator-associated tracheobronchitis: definitions and challenges in trial design. Clin Infect Dis. 2010;51(Suppl 1):S12–S17. doi:10.1086/653035
20. Smith C, Burley C, Ireson M, et al. Clincial trials of antibacterial agents:a practical guide to design and analysis. J Antimicrob Chemother. 1998;41(4):467-480. doi:10.1093/jac/41.4.467
21. Elcocks E, Adukwu EC. Laboratory evaluation of the Sigma Transwab(R) transport system for the recovery of Candida species using the Clinical and Laboratory Standards Institute (CLSI) document M40-A2. Eur J Clin Microbiol Infect Dis. 2021;40:735–738. doi:10.1007/s10096-020-04062-9
22. Wang HSY, Wang MNY, Ma YJR. Expert consensus on operating procedures for tigecycline in vitro susceptibility testing. Chin J Lab Med. 2013;36:584–587.
23. Zhao J, Liu C, Liu Y, et al. Genomic characteristics of clinically important ST11 Klebsiella pneumoniae strains worldwide. J Glob Antimicrob Resist. 2020;22:519–526. doi:10.1016/j.jgar.2020.03.023
24. Karlsson M, Stanton RA, Ansari U, et al. Identification of a carbapenemase-producing hypervirulent Klebsiella pneumoniae isolate in the United States. Antimicrob Agents Chemother. 2019;63. doi:10.1128/AAC.00519-19
25. Agyeman AA, Bergen PJ, Rao GG, Nation RL, Landersdorfer CB. A systematic review and meta-analysis of treatment outcomes following antibiotic therapy among patients with carbapenem-resistant Klebsiella pneumoniae infections. Int J Antimicrob Agents. 2020;55(1):105833. doi:10.1016/j.ijantimicag.2019.10.014
26. Marchaim D, Pogue JM, Tzuman O, et al. Major variation in MICs of tigecycline in Gram-negative bacilli as a function of testing method. J Clin Microbiol. 2014;52(5):1617–1621. doi:10.1128/JCM.00001-14
27. Papadimitriou-Olivgeris M, Bartzavali C, Nikolopoulou A, et al. Impact of tigecycline’s MIC in the outcome of critically ill patients with carbapenemase-producing Klebsiella pneumoniae bacteraemia treated with tigecycline monotherapy-validation of 2019ʹs EUCAST proposed breakpoint changes. Antibiotics. 2020;9:1–8. doi:10.3390/antibiotics9110828
28. Falagas ME, Vardakas KZ, Tsiveriotis KP, Triarides NA, Tansarli GS. Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents. 2014;44:1–7. doi:10.1016/j.ijantimicag.2014.01.006
29. Kispal B, Walker S. Monte Carlo simulation evaluation of tigecycline dosing for bacteria with raised minimum inhibitory concentrations in non-critically ill adults. Eur J Clin Pharmacol. 2021;77(2):197–205. doi:10.1007/s00228-020-02998-7
30. Shelenkov A, Mikhaylova Y, Yanushevich Y, et al. Molecular typing, characterization of antimicrobial resistance, virulence profiling and analysis of whole-genome sequence of clinical Klebsiella pneumoniae isolates. Antibiotics. 2020;9:261. doi:10.3390/antibiotics9050261
31. Zhou K, Xiao T, David S, et al. Novel subclone of carbapenem-resistant Klebsiella pneumoniae sequence type 11 with enhanced virulence and transmissibility, China. Emerg Infect Dis. 2020;26:289–297. doi:10.3201/eid2602.190594
32. Yang J, Ye L, Guo L, et al. A nosocomial outbreak of KPC-2-producing Klebsiella pneumoniae in a Chinese hospital: dissemination of ST11 and emergence of ST37, ST392 and ST395. Clin Microbiol Infect. 2013;19(11):E509–E515. doi:10.1111/1469-0691.12275
33. Ripabelli G, Sammarco ML, Salzo A, Scutellà M, Felice V, Tamburro M. New Delhi metallo-β-lactamase (NDM-1)-producing Klebsiella pneumoniae of sequence type ST11: first identification in a hospital of central Italy. Lett Appl Microbiol. 2020;71(6):652–659. doi:10.1111/lam.13384
34. Nordmann P, Poirel L, Carrër A, Toleman MA, Walsh TR. How to detect NDM-1 producers. J Clin Microbiol. 2011;49(2):718–721. doi:10.1128/JCM.01773-10
35. Li M, Xiao Y, Li P, et al. Characterization and genome analysis of Klebsiella phage P509, with lytic activity against clinical carbapenem-resistant Klebsiella pneumoniae of the KL64 capsular type. Arch Virol. 2020;165:2799–2806. doi:10.1007/s00705-020-04822-0
36. Russo TA, Marr CM. Hypervirulent Klebsiella pneumoniae. Clin Microbiol Rev. 2019;32. doi:10.1128/CMR.00001-19
37. Panjaitan N, Horng YT, Cheng SW, Chung WT, Soo PC. EtcABC, a putative EII complex, regulates type 3 fimbriae via CRP-cAMP signaling in Klebsiella pneumoniae. Front Microbiol. 2019;10:1558. doi:10.3389/fmicb.2019.01558
38. Huang YJ, Wu CC, Chen MC, Fung CP, Peng HL. Characterization of the type 3 fimbriae with different MrkD adhesins: possible role of the MrkD containing an RGD motif. Biochem Biophys Res Commun. 2006;350:537–542. doi:10.1016/j.bbrc.2006.09.070
39. Gomez-Simmonds A, Uhlemann A. Clinical implications of genomic adaptation and evolution of carbapenem-resistant Klebsiella pneumoniae. J INFECT DIS. 2017;215:S18–18S27. doi:10.1093/infdis/jiw378
40. Zheng JX, Lin ZW, Chen C, et al. Biofilm formation in Klebsiella pneumoniae bacteremia strains was found to be associated with CC23 and the presence of wcaG. Front Cell Infect Microbiol. 2018;8:21. doi:10.3389/fcimb.2018.00021
41. Pan YJ, Lin TL, Chen CT, et al. Genetic analysis of capsular polysaccharide synthesis gene clusters in 79 capsular types of Klebsiella spp. Sci Rep. 2015;5:15573. doi:10.1038/srep15573
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


