Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Clinical Efficacy and Safety of an Immune Checkpoint Inhibitor in Combination with Regorafenib Therapy as Second-Line Regimen for Patients with Unresectable Hepatocellular Carcinoma
Authors Li J, Jia Y, Shao C, Li Y, Song J
Received 9 December 2022
Accepted for publication 19 March 2023
Published 5 April 2023 Volume 2023:19 Pages 329—339
DOI https://doi.org/10.2147/TCRM.S400079
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Deyun Wang
Jinpeng Li,1,* Yuntao Jia,1,* Changdong Shao,2,* Yuanming Li,3 Jinlong Song1
1Intervention Ward 1, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, People’s Republic of China; 2Qixia Hospital of Traditional Chinese Medicine Hospital, Qixia, Shandong, People’s Republic of China; 3Laizhou Hospital of Traditional Chinese Medicine, Laizhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jinpeng Li, Intervention Ward 1, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan, Shandong, 250117, People’s Republic of China, Tel +86 531 67626412, Fax +86 531 67626411, Email [email protected]
Purpose: This study aimed to evaluate the safety and efficacy of a combination of programmed death-1 (PD-1) inhibitor and regorafenib as second-line treatment for advanced hepatocellular carcinoma (HCC).
Patients and Methods: We retrospectively analyzed the data of 38 patients with unresectable HCC who were treated with PD-1 inhibitor in combination with regorafenib as a second⁃line therapy as well as the data of 32 patients treated with regorafenib only therapy as a control. The clinical data, previous treatment strategies, follow-up imaging results, and adverse events during follow-ups were recorded. The mRECIST Criteria were used to evaluate the treatment outcome of intrahepatic lesions, and the Kaplan–Meier method was used to evaluate survival time.
Results: Up to the last follow-up, the rego-PD-1 group had higher objective response rate (39.5% vs 15.6%, P = 0.028), longer progression-free survival (median 5.9 vs 4.6 months; P = 0.044), and better overall survival (OS) (median 14.5 vs 9.5 months; P = 0.041) than the regorafenib only group. Among the 38 patients in rego-PD-1 group, 1 patient (2.7%) achieved complete response, 14 patients (36.8%) achieved partial response, 14 patients (36.8%) achieved stable disease, and 9 patients (23.7%) achieved progressive disease. Among the 32 patients in regorafenib alone, 5 (15.6%) achieved partial response, 12 (37.5%) achieved stable disease, and 15 (46.9%) achieved progressive disease. Regorafenib alone, Child–Pugh B, and tumors > 3 were independent prognostic factors for poor OS. The difference in the incidence of grade 3/4 adverse events between the two groups was not statistically significant (36.8% vs 28.1%; P = 0.439). Grade ≥ 3 treatment-related adverse events included hypertension and diarrhea.
Conclusion: PD-1 inhibitor combined with regorafenib is a promising regimen in treating patients with unresectable HCC owing to its safety and effectiveness as well as low incidence of serious adverse events with its use.
Keywords: hepatocellular carcinoma, immune checkpoint inhibitor, regorafenib, anti-PD-1 antibody, second-line treatment, overall survival
Introduction
Hepatocellular carcinoma (HCC) is currently the fourth most common malignant tumor and the second leading cause of tumor deaths in China. The onset of HCC is insidious, and in most cases it will already be in advanced stages when diagnosed. The decision on its treatment depends on the clinical stage and liver function reserve.1 The multikinase inhibitors sorafenib, lenvatinib, and donafenib are currently recommended in China and abroad as targeted drugs for the first-line treatment of advanced liver cancer. However, the median progression-free survival (PFS) of first-line targeted drugs is only 3.7–7.3 months,2,3 and more effective second-line therapies are urgently needed clinically after disease progression.
Regorafenib, a novel oral multikinase inhibitor, was approved by the United States Food and Drug Administration (FDA) in 2017 through the international multicenter Phase III RESORCE trial for the second-line treatment of patients with advanced HCC after the failure of sorafenib.4 In the RESORCE trial, a phase III trial demonstrated that regorafenib significantly improve the overall survival (OS) of HCC patients who progressed after sorafenib treatment, becoming the first TKI approved for second-line therapy.4 Clinical trials in many countries have also verified the effectiveness of regorafenib as a second-line treatment after sorafenib resistance.5,6 However, more effective second-line treatment is needed given the low objective response rate (ORR) and modest improvement in OS.7
In the past decade, systemic therapy has made a breakthrough in the treatment of liver cancer owing to the application of targeted drugs and the rise of immunotherapy. In particular, the combination of targeted drugs and immune checkpoint inhibitors can achieve an ORR of up to about 30% in the treatment of advanced liver cancer, and the median survival time can also reach about 20 months. Immunotherapy combined with targeted therapy has gradually become a new treatment option.8 It also brings new methods and new ideas for the second-line treatment of advanced HCC. Regorafenib is a VEGFR inhibitor which inhibits JAK1/2-STAT1 and MAPK signaling, which could subsequently increase PD-L1 expression in tumors and increase intratumoral CD8+ T-cell infiltration by normalizing the tumor vasculature and improving the efficacy of the PD-1 antibody.9,10 All these provide the theoretical principle for the combined strategy of regorafenib and PD-1 inhibitors.
Currently, there are few studies on PD-1 inhibitor combined with regorafenib in the treatment of liver cancer in China (eg). In this study, the clinical data of domestic PD-1 inhibitor combined with regorafenib as second-line treatment for patients with unresectable hepatocellular carcinoma were retrospectively analyzed, and the efficacy and safety of the two-drug combination regimen in Chinese HCC population were preliminarily explored.
Materials and Methods
Patient Selection
We reviewed the electronic medical records of consecutive patients with advanced HCC who received rego-PD-1 or regorafenib after disease progression on sorafenib or lenvatinib treatment, from April 2019 to December 2021 in Shandong Cancer Hospital and Institute. Patients were assigned to receive either rego-PD-1 or regorafenib, according to the patient’s decision
and the attending physician’s suggestion. This retrospective study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review boards of the Ethics Committee of Shandong Cancer Hospital and Institute. Written informed consent was obtained from each patient prior to treatment.
HCC was diagnosed according to the European Association for the Study of Liver and American Association for the study of Liver Disease guidelines. The inclusion criteria for the study population were as follows: 1) age between 18 and 75 years; 2) Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; 3) BCLC stage C HCC; 4) Child–Pugh A or B liver function, and 5) radiographic disease progression on first-line treatment with sorafenib or lenvatinib. Patients were excluded from this study if they: 1) had previously received targeted therapy in addition to sorafenib or lenvatinib monotherapy; 2) had previously received immunotherapy; 3) currently or previously had any malignant tumor in addition to HCC; 4) had severe medical comorbidities including severe organ dysfunction and coagulation disorders, such as creatinine≥1.5 upper limit of normal or international normalized ratio≥1.5, or 5) had a follow-up less than 3 months.
Observed Indicators and Efficacy Evaluation
Response to treatment was assessed based on dynamic CT or MR imaging according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.111 and modified RECIST.12 ORR was defined as the incidence of complete response and partial response. Disease control rate (DCR) was defined as the incidence of complete response, partial response, and stable disease.
PFS was defined as the time from initiation of regorafenib to confirmation of tumor progression or death or the last follow-up in the reviewed data. OS was defined as the time from initiation of regorafenib to death or the last follow-up in the reviewed data.
Evaluation of adverse reactions: Adverse reactions are classified into grades 1 to 5 according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0.13 During the treatment period, each patient’s vital signs, blood routine, liver function, renal function, urine routine, stool routine, and other indicators were closely observed through telephone, WeChat, and outpatient reexamination after discharge. Other adverse reactions were evaluated and recorded in a timely manner.
Statistical Analyses
The measurement data showing normal distribution were expressed as mean ± standard deviation. Groups were compared by the t-test. The enumeration data were expressed as case (%). The comparison was performed by χ2 test. Nonnormal data were expressed by M (P25, P75). The Mann–Whitney U-test was used for comparing the two groups. The corresponding survival curves were created using the Kaplan–Meier method. All tests were two-sided and P < 0.05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics, version 22.0 (SPSS, Chicago, IL, USA).
Results
Study Population
A total of 96 patients with advanced HCC who received rego-PD-1 or regorafenib only as a second-line treatment were assessed for eligibility during the study period. Twenty-six patients were excluded because they met the exclusion criteria (Figure 1). At the end of follow-up, 70 patients were enrolled in this study, including 54 males and 16 females. The mean age was 51.2 ± 7.7 years, ranging from 26 to 73 years. According to Child–Pugh classification, 48 cases (68.6%) were A, 22 cases (31.4%) were B. By the BCLC staging: 15 patients (34.1%) were in stage B, 29 patients (65.9%) were in stage C, 28 patients (40.0%) received lenvatinib and 42 patients (60.0%) received sorafenib in the first-line treatment. There was no statistically significant difference in baseline data between the two groups (Table 1).
 |
Table 1 Baseline Characteristics of Two Groups (n) |
 |
Figure 1 Flow chart of patient screening. |
Treatment Response
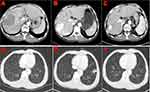
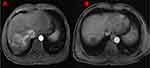
Tumor responses of the two groups are shown in Table 2. Two patients in the rego-PD-1 group versus no patients in the regorafenib group showed complete response (Figures 2 and 3). The ORR for the rego-PD-1 group was significantly higher than that of the regorafenib group (28.9% vs 9.4%, by RECIST 1.1, P = 0.041; 39.5% vs 15.6% by mRECIST, P = 0.028). Similarly, the DCR for the rego-PD-1 group was significantly higher than that of the regorafenib group (76.3% vs 50.0% by RECIST 1.1, P = 0.022; 76.3% vs 53.1% by mRECIST, P = 0.042).
 |
Table 2 Treatment Responses |
Safety
Treatment-related AEs are shown in Table 3. No treatment-related mortality occurred. The overall incidence of AEs was similar between the rego-sintilimab group and the regorafenib group (any grade: 94.7% vs 87.5%, P = 0.516; grade 3/4:36.8% vs 28.1%, P = 0.439). There were higher incidences of hypothyroidism and rash of any grade in the rego-PD-1 group than in the regorafenib group (42.1% vs 15.6%, P = 0.016 and 34.2% vs 9.4%, P = 0.029). Incidences of other AEs were not significantly different between the two groups. Adverse events with an incidence of more than 10% and grade ≥3 adverse events are shown in Table 3. Treatment was discontinued because of AE in 13.2% (5/38) of patients in the rego-PD-1 group and in 9.4% (3/32) in the regorafenib group (P = 0.906).
 |
Table 3 Adverse Events in the Two Groups |
Survival Profiles
The median follow-up time was 12.1 months (IQR, 7.9–18.8) in the regorafenib-PD-1 group and 8.1 months (IQR, 6.2–13.1) in the regorafenib group. The median PFS in the rego-PD-1
group was 5.9 months (95% confidence interval (CI),2.6–9.7) compared with 4.6 months (95% CI, 2.7–6.4) in the regorafenib group (P =0.044) (Figure 4). The 6-, 12-, and 24-month OS rates were 86.8%, 69.4%, and 20.6% (median OS, 14.5 months [95% CI, 10.2–16.8]), respectively, in the rego-PD-1 group, and regorafenib 83.4%, 55.6%, and 19.3%, respectively, (median OS, 9.5 months [95% CI, 6.3–14.9]), (P = 0.041) (Figure 5). According to the univariate and multivariate analyzes, regorafenib alone (HR, 1.738; 95% CI, 1.025–2.947; P = 0.040), Child–Pugh B liver function (HR, 1.486; 95% CI, 1.079–2.431; P = 0.032), and number of tumors > 3 (HR, 1.897; 95% CI, 1.075–3.348; P = 0.027) were independent prognostic factors for OS (Table 4).
 |
Table 4 Univariate and Multivariate Analyzes Affecting Overall Survival |
Discussion
Despite better understanding of the pathogenesis of HCC, sorafenib, lenvatinib, donafenib, and combination immunotherapy with bevacizumab and atilizumab have been approved as first-line systemic therapies for HCC patients with advanced HCC, which has greatly improved the prognosis of patients with advanced HCC. However, development of drug resistance is inevitable with prolonged treatment.3,8,14
Currently, results of the studies on second-line drugs for HCC are not conclusive. The multi-kinase inhibitor regorafenib, cabozantinib, VEGFR-2 inhibitor ramucirumab, and the immune checkpoint inhibitor have been approved by FDA.15 Regorafenib appears to be the best option in terms of OS among the current standard second-line treatments for HCC. However, considering the low ORR and modest improvement in OS, a more effective second-line treatment is warranted. The emergence of immunotherapy has greatly changed the prospect of liver cancer treatment. According to Checkmate – 040 study16 and Keynote-224 study,17 Nivolumab and Pembrolizumab are effective in liver cancer after sorafenib treatment. Following the publication of the research results of Imbrave 15018 and Keynote-524,19 immunotherapy combined with targeted therapy has gradually become a new treatment option as it also brings new methods and ideas for the second-line treatment of advanced HCC. Therefore, this retrospective study aimed to investigate the efficacy and safety of the PD-1 inhibitor plus regorafenib in patients with advanced HCC who had progressed after first-line therapy.
In this study, our findings showed that after failure of first-line treatment for HCC, the combination of PD-1 inhibitor and regorafenib had significantly better ORR and DCR than regorafenib alone (ORR, 28.9% and 9.4%, respectively, p = 0.041; DCR was 76.3% and 50.0%, respectively. p=0.022). The Kaplan–Meier survival analysis showed no significant difference in median PFS and OS between the two groups (PFS: 5.3 months vs 4.0 months, p = 0.512; OS: 14.1 months vs 13.7 months, p = 0.764 for PL vs PR), with a significant survival benefit. However, our results differ somewhat from those of the RESCUE trial.20 The study showed that carrellizumab in combination with apatinib (selective VEGF 2 inhibitor) as second-line treatment for advanced HCC, median follow-up of 29.1 months, ORR of 22.5% (RECIST v1.1 criteria), the mOS was 21.8 months (17.3–26.8), and the 2-year OS rate was 44.6%, which may be due to the fact that only patients with Child A, ECOG 0–1 score and large vessel invasion (24.2%) were included in RESCUE. However, the patients included in our study had relatively more advanced cancer, with the majority of patients in the PR/R group [Child B (31.6%/31.3%), BCLC (78.9%/ 65.6%), and extrahepatic dissemination (63.2%/53.1%), reflecting real-world data and more clinical.
In multivariate analysis, treatment modality (in combination with anti-PD-1 antibody) was an independent predictor of better OS and PFS. This clinical benefit may be attributed to the synergistic antitumor effects of PD-1 inhibitors and TKI agents, which not only reshape the immune microenvironment,21 but also promote the normalization of immunocompetent cell function.22,23 Regorafenib effectively inhibits JAK 1/2 STAT 1 and MAPK signaling, thereby attenuating PD-L1 expression in tumors 22 and may enhance the efficacy of anti-PD-1 antibodies by targeting VEGFR 2/3 and increasing intratumoral CD 8 + T cell infiltration through vascular normalization.
Tumor burden is a negative factor in the prognosis of uHCC patients undergoing TACE or immunotherapy.24,25 In our study, Cox multivariate analysis showed that patients with >3 tumors had a higher risk of cause-of-death and tumor progression than patients with ≤3 tumors. Liver function status is closely related to the prognosis of patients with hepatocellular carcinoma. TACE, antiangiogenic therapy and immunotherapy all require a certain liver function status. In this study, in the regorafenib-PD 1 group, patients with Child–Pugh classification (especially Child–Pugh class A) and good liver function had obvious OS benefit. Patients with advanced liver cancer with good liver function status reserve can benefit from immune 3.0 treatment. In our study, approximately 31.4% of patients had Child–Pugh B HCC. In a multicenter study, it was reported that the OS of Child–Pugh B patients treated with regorafenib was significantly lower than that of Child–Pugh A patients, which is consistent with our study results.26 Nevertheless, there remains a need for an effective treatment for patients with Child–Pugh B. A previous study showed that 35% of patients treated with Child–Pugh AHCC prior to sorafenib treatment may develop Child–Pugh B liver function deterioration after sorafenib discontinuation.27 The latest phase I/II study by Kudo et al showed that immunotherapy with immune checkpoint inhibitors had good safety in Child–Pugh B HCC patients, which was comparable to that in Child–Pugh A patients.28 Whether patients with Child–Pugh B liver function benefit from the combination of regorafenib and pd-1 antibody requires further investigation.
Combination immunotherapy brings clinical benefit to patients with advanced liver cancer and also shows a good safety profile. The safety of regorafenib-PD 1 mAb was basically consistent with that in the previous historical data.29 Although palmoplantar redness, diarrhea, fatigue, decreased appetite, increased AST, and hypertension are the most common adverse events, most of these events are grade 3 and can be managed with dose modification and optimal supportive care.30,31 In addition, combination therapy with PD-1 mAb did not increase the overall incidence of any grade or grade 3/4 AEs relative to regorafenib monotherapy. In the regorafenib-PD-1 group, 12.1% of patients discontinued treatment due to adverse events, which was similar to the incidence reported previously for target immunotherapy (14%).32
Limitations
This study had several limitations. First, this is a retrospective study, with patients coming from a single medical center and possibly influenced by specific treatment practices. Second, as a retrospective study, the comparison of regorafenib-tirilizumab to regorafenib may have selection bias, and due to the small sample size, paired analysis between the two groups was not performed. Third, the sample size of this study is relatively small, and the results of subgroup analysis should be interpreted with caution and need to be verified by further studies.
Conclusion
In conclusion, the regorafenib-PD-1 combination is effective and safe in the second-line treatment of patients with advanced HCC. Patients seem to benefit from regorafenib-PD-1 treatment more than patients treated with regorafenib alone, which may lead to a survival benefit for patients. In addition, the small sample size of this retrospective study necessitates more large-scale studies to explore and validate the efficacy of this combination regimen.
Abbreviations
PD-1, Programmed death-1; CI, Confidence interval; DCR, Disease control rate; ECOG, Eastern Cooperative Oncology Group; FDA, Food and Drug Administration; HAIC, Hepatic arterial infusion chemotherapy; ORR, Objective response rate; OS, Overall survival; PFS, Progression-free survival; RECIST, Response Evaluation Criteria in Solid Tumors.
Data Sharing Statement
The data that support the findings of this study are available via a data access agreement. Please contact the corresponding author for this request.
Ethics Approval and Informed Consent
This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute. All trial participants provided informed consent and the trial was conducted in accordance with the Declaration of Helsinki.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rimassa L, Wörns M-A. Navigating the new landscape of second-line treatment in advanced hepatocellular carcinoma. Liver Int. 2020;40:1800–1811.
2. Llovet JM, Ricci S, Mazzaferro V, et al; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857
3. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised Phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi:10.1016/S0140-6736(18)30207-1
4. Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56–66. doi:10.1016/S0140-6736(16)32453-9
5. Elseud YA, Shaaban A, Mohanty A, Albarrak J. Safety and tolerability of regorafenib: a real-life experience. J Gastrointest Cancer. 2022;53(1):187–191. doi:10.1007/s12029-020-00570-1
6. Lee MJ, Chang SW, Kim JH, et al. Real-world systemic sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Korea. Invest New Drugs. 2021;39(1):260–268. doi:10.1007/s10637-020-00977-4
7. Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv238–iv255. doi:10.1093/annonc/mdy308
8. Bruix J, Chan SL, Galle PR, Rimassa L, Sangro B. Systemic treatment of hepatocellular carcinoma: an EASL position paper. J Hepatol. 2021;75:960–974. doi:10.1016/j.jhep.2021.07.004
9. Shigeta K, Matsui A, Kikuchi H, et al. Regorafenib combined with PD1 blockade increases CD8 T-cell infiltration by inducing CXCL10 expression in hepatocellular carcinoma. J Immunother Cancer. 2020;8(2):e001435. doi:10.1136/jitc-2020-001435
10. Tai WT, Chu PY, Shiau CW, et al. STAT3 mediates regorafenib-induced apoptosis in hepatocellular carcinoma. Clin Cancer Res. 2014;20(22):5768–5776. doi:10.1158/1078-0432.CCR-14-0725
11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30(1):52–60. doi:10.1055/s-0030-1247132
13. National Cancer Institute. Common terminology criteria for adverse events, version 5.0; 2017.
14. Qin S, Bi F, Gu S, et al. Donafenib versus sorafenib in first-line treatment of unresectable or metastatic hepatocellular carcinoma: a randomized, open-label, parallel-controlled Phase II–III trial. J Clin Oncol. 2021;39(27):3002–3011. doi:10.1200/JCO.21.00163
15. Liu Z, Lin Y, Zhang J, et al. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38(1):447. doi:10.1186/s13046-019-1412-8
16. El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocel-lular carcinoma (CheckMate 040): an open-label, non- comparative, Phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi:10.1016/S0140-6736(17)31046-2
17. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): anon-randomised, open-label Phase 2 trial. Lancet Oncol. 2018;19:940–952. doi:10.1016/S1470-2045(18)30351-6
18. Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894–1905. doi:10.1056/NEJMoa1915745
19. Finn RS, Ikeda M, Zhu AX, et al. Phase ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol. 2020;8(26):2960–2970. doi:10.1200/JCO.20.00808
20. Mei K, Qin S, Chen Z, Liu Y, Wang L, Zou J. Camrelizumab in combination with apatinib in second-line or above therapy for advanced primary liver cancer: cohort a report in a multicenter phase Ib/II trial. J Immunother Cancer. 2021;9(3):e002191. doi:10.1136/jitc-2020-002191
21. Aspriţoiu VM, Stoica I, Bleotu C, Diaconu CC. Epigenetic regulation of angiogenesis in development and tumors progression: potential implications for cancer treatment. Front Cell Dev Biol. 2021;9:689962. doi:10.3389/fcell.2021.689962
22. Xu F, Jin T, Zhu Y, Dai C. Immune checkpoint therapy in liver cancer. J Exp Clin Cancer Res. 2018;37(1):110. doi:10.1186/s13046-018-0777-4
23. Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–174. doi:10.1038/nri2506
24. Wang YK, Bi XY, Li ZY, et al. A new prognostic score system of hepatocellular carcinoma following hepatectomy. Zhonghua Zhong Liu Za Zhi. 2017;39:903–909. doi:10.3760/cma.j.issn.0253-3766.2017.12.005
25. Samstein RM, Lee C-H, Shoushtari AN, et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet. 2019;51:202–206. doi:10.1038/s41588-018-0312-8
26. Kim HD, Bang Y, Lee MA, et al. Regorafenib in patients with advanced Child-Pugh B hepatocellular carcinoma: a multicentre retrospective study. Liver Int. 2020;40(10):2544–2552. doi:10.1111/liv.14573
27. Casadei Gardini A, Frassineti GL, Foschi FG, et al. Sorafenib and regorafenib in HBV- or HCV-positive hepatocellular carcinoma patients: analysis of RESORCE and SHARP trials. Dig Liver Dis. 2017;49(8):943–944. doi:10.1016/j.dld.2017.04.022
28. Kudo M, Matilla A, Santoro A, et al. CheckMate 040 cohort 5: a phase I/II study of nivolumab in patients with advanced hepatocellular carcinoma and Child-Pugh B cirrhosis. J Hepatol. 2021;75(3):600–609. doi:10.1016/j.jhep.2021.04.047
29. Huang J, Guo Y, Huang W, et al. Regorafenib combined with PD-1 blockade immunotherapy versus regorafenib as second-line treatment for advanced hepatocellular carcinoma: a multicenter retrospective study. J Hepatocell Carcinoma. 2022;9:157–170. doi:10.2147/JHC.S353956
30. Yoo C, Park JW, Kim YJ, et al. Multicenter retrospective analysis of the safety and efficacy of regorafenib after progression on sorafenib in Korean patients with hepatocellular carcinoma. Invest New Drugs. 2019;37:567–572. doi:10.1007/s10637-018-0707-5
31. Ogasawara S, Ooka Y, Itokawa N, et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Japan. Invest New Drugs. 2020;38:172–180. doi:10.1007/s10637-019-00801-8
32. Ren Z, Xu J, Bai Y, et al; ORIENT-32 study group. Sintilimab plus a bevacizumab biosimilar (IBI305) versus sorafenib in unresectable hepatocellular carcinoma (ORIENT-32): a randomised, open-label, phase 2–3 study. Lancet Oncol. 2021;22(7):977–990. doi:10.1016/S1470-2045(21)00252-7
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.