Back to Journals » Clinical Ophthalmology » Volume 16
Clinical Characteristics of Idiopathic Orbital Inflammation Syndrome in Relation to Intraocular Pressure
Authors Otsuka M , Yunoki T , Ozaki H, Hayashi A
Received 16 February 2022
Accepted for publication 9 May 2022
Published 11 May 2022 Volume 2022:16 Pages 1467—1473
DOI https://doi.org/10.2147/OPTH.S361645
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Mitsuya Otsuka,1 Tatsuya Yunoki,1 Hironori Ozaki,2 Atsushi Hayashi1
1Department of Ophthalmology, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, Toyama, Japan; 2Department of Ophthalmology, Kurobe City Hospital, Toyama, Japan
Correspondence: Atsushi Hayashi, Department of Ophthalmology, Graduate School of Medicine and Pharmaceutical Sciences, University of Toyama, 2630 Sugitani, Toyama, 930-0194, Japan, Tel +81-76-434-7363, Fax +81-76-434-5037, Email [email protected]
Purpose: To investigate the clinical characteristics of idiopathic orbital inflammation and changes in intraocular pressure (IOP) before and after its treatment.
Patients and Methods: We retrospectively studied 20 eyes from the medical records of 19 patients who were diagnosed with idiopathic orbital inflammation between April 1, 2004, and April 30, 2019, at Toyama University Hospital. The inflammation site (type of disease), treatment provided, IOP before and after treatment, and the symptoms (proptosis, decreased ocular movements or diplopia, periorbital edema, and ocular pain) were analyzed.
Results: The types of idiopathic orbital inflammation were dacryoadenitis in 14, myositis in 7, diffuse-type in 3, and posterior periscleritis in 1 case. The mean IOP after treatment was 15.4± 3.9 mm Hg, which was significantly lower than the mean pretreatment IOP of 19.0± 5.3 mm Hg (p = 0.009). Before treatment, all cases with the diffuse-type had high IOPs of 21 mm Hg or more. Ocular pain and eye movement disorders were present in 86% and 100% of subjects in the group with an IOP of 21 mm Hg or higher, but 38% and 31% in the group with an IOP of 20 mm Hg or lower, respectively.
Conclusion: Diffuse-type of idiopathic orbital inflammation is prone to develop high IOP. Patients with idiopathic orbital inflammation and high IOP exhibit many symptoms such as decreased ocular movements, diplopia, and ocular pain.
Keywords: idiopathic orbital inflammation, intraocular pressure, decreased ocular movements, ocular pain
Introduction
Idiopathic orbital inflammation is a disease that comprises inflammation of unknown origin in the orbit, with symptoms such as conjunctival injection, periorbital edema, proptosis, and diplopia.1–4 It is necessary to differentiate this disease from other orbital diseases such as immunoglobulin G4 (IgG4)-related eye disease, thyroid ophthalmopathy, and lymphoma.5 Local or systemic steroids,6,7 immunosuppressants,8 which suppress inflammation; and radiotherapy are administered,9,10 but the disease often relapses, in which case long-term steroid treatment may be administered.1,5
In general, orbital diseases are known to often cause increased intraocular pressure (IOP),11 and the management of increased IOP is also a therapeutic issue. An increase in IOP due to idiopathic orbital inflammation is caused by an increase in episcleral vein pressure,11 which is a type of open angle. However, cases of idiopathic orbital inflammation with a closed angle have been reported.12–15 In these cases, the IOP decreased after systemic steroid administration. However, the relationships between IOP, clinical features such as symptoms and inflamed sites, and changes in IOP before and after treatment of idiopathic orbital inflammation have not been sufficiently studied. This study retrospectively investigated the clinical features, IOP values, and treatment details of idiopathic orbital inflammation.
Materials and Methods
We retrospectively studied 20 eyes from the medical records of 19 patients who were diagnosed with idiopathic orbital inflammation between April 1, 2004, and April 30, 2019, at Toyama University Hospital. This study was approved by the institutional review board of Toyama University Hospital (Approval no. R2019060), and the procedures used conformed to the tenets of the Declaration of Helsinki. Informed consent was obtained from all individual participants included in the study.
Diagnosis of Idiopathic Orbital Inflammation
The diagnosis of idiopathic orbital inflammation was made comprehensively based on clinical symptoms, magnetic resonance imaging (MRI), and blood tests. Blood tests measuring IgG4, interleukin 2 receptor, thyroid autoantibodies (thyroid-stimulating hormone, receptor antibodies, anti-thyroid peroxidase antibodies, and anti-thyroglobulin antibodies), blood counts, C-reactive protein, angiotensin-converting enzyme, Serine proteinase 3-anti-neutrophil cytoplasmic antibody, and myeloperoxidase-anti-neutrophil cytoplasmic antibody were performed. We investigated the possibility of diseases such as lymphoma, thyroid eye disease,16 orbital cellulitis,17 ANCA-related vasculitis,18,19 and sarcoidosis.20,21 We confirmed the presence of dilatation of the superior ophthalmic vein, the presence of an orbital mass on MRI, and the presence of diseases such as orbital tumors and carotid-cavernous fistulae. If diagnosis by MRI and blood sampling was difficult, a biopsy was performed.5
Clinical Symptoms of Idiopathic Orbital Inflammation
Clinical symptoms were classified into proptosis, decreased ocular movements or diplopia, periorbital edema, and ocular pain. Proptosis was determined based on a left-right asymmetry of 2 mm or more, or an exophthalmos of 15 mm or more from the orbital lateral bone margin observed on Hertel Exophthalmometry.22 When an asymmetry between left and right exophthalmos was observed on an MRI, proptosis was considered. The restriction of extraocular movement was determined using a Hess screen. The restriction was detected when the 15° grating of the left and right eye was unequal. Diplopia was judged if it was seen in the primary position or in peripheral gaze. Periorbital edema was judged based on the patient’s complaint and the difference between the left and right eyelids. Ocular pain was judged based on the patient’s complaint.
The Types of Idiopathic Orbital Inflammation
The sites of inflammation were determined on the MRIs. The types of idiopathic orbital inflammation were divided into dacryoadenitis, myositis, diffuse-type, and posterior periscleritis. Other types of idiopathic orbital inflammation that have been reported include anterior apical periscleritis, perineuritis, and focal inflammatory mass,2,10,23,24 but these were not observed in the present study. Overlapping sites of inflammation were also considered.
The average IOP value of three measurements from a non-contact tonometer (NCT) (FT-01, TOMEY, Japan) was used to obtain each IOP value.
Statistical Analysis
Statistical analysis was performed using JMP 14 software (SAS, Cary, NC, USA). The t-test was used to compare the continuous variables, and Fisher’s exact test was used to compare the ratios of the two groups. P-values less than 0.05 were considered statistically significant.
Results
The clinical characteristics of the 20 cases are shown in Table 1. The patients’ mean age was 55.4 ± 13.7 years. One was male, and the others were females. The type of idiopathic orbital inflammation was dacryoadenitis in 14, myositis in 7, diffuse in 3, and posterior periscleritis in 1 case. There was 1 case of dacryoadenitis type with bilateral onset. One case showed a combined type of myositis and diffuse types. Two cases showed a combined type of myositis and dacryoadenitis types. A biopsy was performed in 5 patients. All 5 had the dacryoadenitis-type, 3 of whom received oral steroids, and 2 received local steroid injections. One case showed a shallow anterior chamber in the acute phase.
 |
Table 1 Clinical Characteristics of the 20 Cases |
Table 2 shows the treatments administered for idiopathic orbital inflammation. Sixteen patients were treated with oral steroids, one with steroid pulse, and three with local steroid injections. Oral steroids were started at 30 to 50 mg per day of prednisolone and tapered off after approximately three months. In the steroid pulse, 1000 mg per day of methylprednisolone sodium succinate was administered for three days. Six patients who received increased doses of oral steroids developed recurrent orbital inflammation after treatment, two of whom received additional immunosuppressant treatment at the time of recurrence.
 |
Table 2 Treatment for Idiopathic Orbital Inflammation |
The mean IOP after treatment was 15.4±3.9 mm Hg (range 9–27), which was significantly lower than the pretreatment IOP of 19.0±5.3 mm Hg (range 14–31) (p = 0.009) (Figure 1). Anti-glaucoma eye drops and oral medication were used for increased IOP in two cases before steroid treatment. The mean observation period after treatment was 2.3 ± 1.1 months. There was no case who showed a rise of IOP secondary to steroid treatment. Figure 2 shows the change in IOP for each site of idiopathic orbital inflammation. The diffuse-type had the highest IOP before treatment at 25 ± 4.2 mm Hg and was significantly higher than that of the dacryoadenitis-type (p = 0.01). The mean IOP after treatment was normal for all types of idiopathic orbital inflammation. Figure 3 shows the percentage of cases with high IOP before treatment. Of the dacryoadenitis-type cases, 21% (3/14) had an elevated IOP of 21 mm Hg or higher, and 60% (3/5) of the myositis-type cases had an elevated IOP of 21 mm Hg or higher. On the other hand, in all diffuse-type cases, the IOP before treatment was 21 mm Hg or higher. However, there was no significant difference between the diffuse-type and the other types.
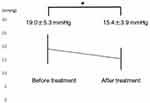 |
Figure 1 Changes in IOP before and after treatment. IOP is shown on the vertical axis. After treatment, IOP decreased significantly. *p = 0.009. |
 |
Figure 2 IOP by inflammatory site. IOP is shown on the vertical axis. The type of idiopathic orbital inflammation is shown on the horizontal axis. *p < 0.05 Dacryoadenitis vs Diffuse type. |
 |
Figure 3 Percentage of high IOP before treatment by inflammation site. The percentage is shown on the vertical axis. The type of idiopathic orbital inflammation is shown on the horizontal axis. |
Ocular symptoms prior to treatment of idiopathic orbital inflammation are shown in Table 3. Ocular pain and decreased ocular movements or diplopia were present in 86% and 100% of subjects in the group with an IOP of 21 mm Hg or higher, but 38% and 31% in the group with an IOP of 20 mm Hg or lower, respectively. The group with an IOP of 21 mm Hg or higher tended to have more symptoms, but there was no statistically significant difference (p = 0.07, p = 0.06).
 |
Table 3 Ocular Symptoms Prior to Treatment of Idiopathic Orbital Inflammation |
Discussion
In this study, we investigated the relationship between changes in IOP before and after treatment, and between clinical symptoms and IOP in cases of idiopathic orbital inflammation. In addition, we classified idiopathic orbital inflammation according to the site of inflammation and showed that the diffuse-type easily developed high IOP. Since there have been few reports on changes in IOP before and after its treatment, our study has clarified the relationship between IOP, symptoms, and the site of inflammation in idiopathic orbital inflammation. Even if IOP is high due to idiopathic orbital inflammation, it can be reduced with existing steroid treatment in many cases.
Elevated IOP in idiopathic orbital inflammation, like other orbital diseases, is thought to be caused by an increase in episcleral venous pressure.11 In this study, idiopathic diffuse-type orbital inflammation tended to show elevated IOP; patients with diffuse inflammation may thus be at increased risk of developing increased venous pressure. Multiple cases with a shallow anterior chamber in the acute phase of inflammation have also been reported,12–15 In this study, one case with a shallow anterior chamber was observed. Increased IOP may also occur due to angle closure.
The first limitation of this study is that it is a retrospective study, in which IOP was measured with an NCT. Although the value obtained from an NCT correlates with that from a Goldmann applanation tonometer (GAT), the deviation of the IOP must also be considered.25 IOP has been measured in cases of thyroid eye disease. In the thyroid eye disease group, the corneal-compensated IOP (20.23 ± 0.54 mm Hg) was significantly higher than the IOP recorded with a GAT (17.54 ± 0.49 mm Hg, p < 0.001).26 The corneal-compensated IOP refers to the IOP adjusted by corneal hysteresis and is considered a more accurate IOP value because it takes corneal stiffness into account. In Graves’ orbitopathy, the compression of the globe by the muscle berry causes increased intraocular pressure. Similarly for the myositis type of idiopathic orbital inflammation, IOP elevation may occur. Although thyroid eye disease was not discussed in our study, the IOP measured by an NCT may be lower than that measured by a GAT.
The rise of IOP secondary to steroid treatment should be considered. In the present study, steroid treatment resulted in a decrease in mean IOP and there was no extreme increase in IOP after steroid treatment. However, young patients may develop steroid glaucoma, and should be carefully followed up.
The second limitation is the diagnosis of idiopathic orbital inflammation. In the present study, only 5 of the 14 dacryoadenitis-type cases were biopsied, so there is some uncertainty as to a definitive diagnosis. However, this was a retrospective study and included cases that were clinically diagnosed by MRI and blood test results. Further studies are needed to confirm the diagnosis of dacryoadenitis-type by performing biopsies in all cases in a prospective study.
The third limitation is our evaluation of clinical symptoms. Periorbital pain can also be evaluated qualitatively using a scale such as the Visual Analog Scale. There is a method in which two ophthalmologists determine the presence or absence of periocular edema. However, this study was retrospective, and the presence or absence of pain could only be evaluated based on the patient’s complaint, and periocular edema may be attributed to the subjective opinion of one ophthalmologist.
The fourth limitation is that the number of cases was small. Orbital inflammation was classified into four types, which were fewer than in previous reports. In addition, since we classified cases by the results of MRI, 3 cases span combination types. If we classify each combination of types, the sample size of each group will be small, which may make it harder to detect general trends. We compared IOP between the diffuse type and the dacryoadenitis type and found a significant elevation of IOP in the diffuse type. If the number of cases in the diffuse type increases, a significant difference may be detected between the diffuse-type and the other types. It needs to consider increasing the number of cases in future studies.
Conclusion
In conclusion, we examined the clinical characteristics of idiopathic orbital inflammation and changes in IOP before and after treatment. Our results showed that the diffuse type of idiopathic orbital inflammation is prone to develop high IOP. Patients with high IOP exhibit many symptoms, such as restriction of extraocular movement and ocular pain.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
1. Eshraghi B, Sonbolestan SA, Abtahi MA, et al. Clinical characteristics, histopathology, and treatment outcomes in adult and pediatric patients with nonspecific orbital inflammation. J Curr Ophthalmol. 2019;31(3):327–334. doi:10.1016/j.joco.2019.03.004
2. Khochtali S, Zayani M, Ksiaa I, et al. Idiopathic orbital inflammatory syndrome: report of 24 cases. J Fr Ophthalmol. 2018;41(4):333–342. doi:10.1016/j.jfo.2017.09.013
3. Halimi E, Rosenberg R, Wavreille O, et al. Clinical features and management of acute myositis in idiopathic orbital inflammation. J Fr Ophthalmol. 2013;36(7):567–574. doi:10.1016/j.jfo.2012.09.012
4. Swamy BN, McCluskey P, Nemet A, et al. Idiopathic orbital inflammatory syndrome: clinical features and treatment outcomes. Br J Ophthalmol. 2007;91(12):1667–1670. doi:10.1136/bjo.2007.124156
5. Gavard-Perret A, Lagier J, Delmas J, et al. Rationale for a diagnostic approach in non-Graves’ orbital inflammation–Report of 61 patients. J Fr Ophthalmol. 2015;38(10):912–923. doi:10.1016/j.jfo.2015.04.017
6. Reggie S, Neimkin M, Holds J. Intralesional corticosteroid injections as treatment for non-infectious orbital inflammation. Orbit. 2018;37(1):41–47. doi:10.1080/01676830.2017.1353110
7. El Nasser A, Mohammad A. Local steroid injection for management of different types of acute idiopathic orbital inflammation: an 8-year study. Ophthalmic Plast Reconstr Surg. 2013;29(9):286–289. doi:10.1097/IOP.0b013e318293750c
8. Suhler EB, Lim LL, Beardsley RM, et al. Rituximab therapy for refractory orbital inflammation: results of a Phase 1/2, dose-ranging, randomized clinical trial. JAMA Ophalmol. 2014;132(5):572–578. doi:10.1001/jamaophthalmol.2013.8179
9. Mokhtech M, Nurkic S, Morris CG, et al. Radiotherapy for orbital pseudotumor: the University of Florida experience. Cancer Investig. 2018;36(6):330–337. doi:10.1080/07357907.2018.1489550
10. Yeşiltaş YS, Gündüz AK. Idiopathic orbital inflammation: review of literature and new advances. Middle East Afr J Ophthalmol. 2018;25(2):71–80. doi:10.4103/meajo.MEAJO_44_18
11. Nassr MA, Morris CL, Netland PA, et al. Intraocular pressure change in orbital disease. Surv Ophthalmol. 2009;54(5):519–544.
12. Bernardino CR, Davidson RS, Maus M, et al. Angle-closure glaucoma in association with orbital pseudotumor. Ophthalmology. 2001;108(9):1603–1609. doi:10.1016/S0161-6420(01)00700-X
13. Kurtz S, Moisseiev J, Gutman I, et al. Orbital pseudotumor presenting as acute glaucoma with choroidal and retinal detachment. Ger J Ophthalmol. 1993;2(1):61–62.
14. Násser LS, Liendo da Costa VL, Taniguchi MP, et al. Angle-closure glaucoma secondary to nonspecific orbital inflammatory: case report. Arq Bras Oftalmol. 2007;70(6):1029–1033. doi:10.1590/S0004-27492007000600028
15. Zborowski-Gutman L, Gutman I, Chen V, et al. Acute angle closure glaucoma precipitated by orbital pseudotumour. Br J Ophthalmol. 1988;72(2):142–144.
16. Stanciu M, Popa FL, Totoian IG, et al. Orbital pseudotumor can mimic graves’ ophthalmopathy. Acta Endocrinol. 2016;12(3):344–348.
17. Nishikawa Y, Oku H, Tonari M, et al. C-reactive protein may be useful to differentiate idiopathic orbital inflammation and orbital cellulitis in cases with acute eyelid erythema and edema. Clin Ophthalmol. 2018;12:1149–1153. doi:10.2147/OPTH.S164306
18. Hibino M, Kondo T. Dacryoadenitis with ptosis and diplopia as the initial presentation of granulomatosis with polyangiitis. Intern Med. 2017;56(19):2649–2653. doi:10.2169/internalmedicine.8761-16
19. Teke TA, Kaman A, Metin O, et al. Idiopathic orbital inflammation in a child mimicking orbital cellulitis. Clin Pediatr. 2016;55(9):877–879. doi:10.1177/0009922815611643
20. Brunelle C, Bally S, Provencal J, et al. Orbital inflammatory pseudotumor secondary to sarcoidosis. J Fr Ophthalmol. 2016;39(7):179–181. doi:10.1016/j.jfo.2015.07.017
21. Kobak S, Topaloglu A, Öncel G, et al. Sarcoidosis presenting as orbital pseudotumor. Reumatismo. 2015;67(2):78–81. doi:10.4081/reumatismo.2015.832
22. Kozaki A, Inoue R, Komoto N, et al. Proptosis in dysthyroid ophthalmopathy: a case series of 10,931 Japanese cases. Optom Vis Sci. 2010;87(3):200–204. doi:10.1097/OPX.0b013e3181ce5702
23. Chaudhry IA, Al-Obaisi S, Al-Sheikh O, et al. Unilateral optic neuritis, scleritis and exudative retinal detachment due to recurrent orbital pseudotumor. Saudi J Ophthalmol. 2012;26(4):449–451. doi:10.1016/j.sjopt.2012.09.003
24. Chaudhry IA, Shamsi FA, Arat YO, et al. Orbital pseudotumor: distinct diagnostic features and management. Middle East Afr J Ophthalmol. 2008;15(1):17–27. doi:10.4103/0974-9233.53370
25. Bang SP, Lee CE, Kim YC. Comparison of intraocular pressure as measured by three different non-contact tonometers and Goldmann applanation tonometer for non-glaucomatous subjects. BMC Ophthalmol. 2017;17(1):199. doi:10.1186/s12886-017-0593-1
26. Sasan M, Safizadeh M, Mazloumi M, et al. Evaluation of corneal biomechanical properties in patients with thyroid eye disease using ocular response analyzer. J Glaucoma. 2016;25(3):269–273. doi:10.1097/IJG.0000000000000254
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
