Back to Journals » Journal of Asthma and Allergy » Volume 16
Clinical Characteristics of Eosinophilic Chronic Rhinosinusitis with Nasal Polyps in Adolescents
Authors Huang CC , Chang PH, Huang YL , Lee TJ, Huang CC, Wu PW
Received 23 September 2023
Accepted for publication 25 October 2023
Published 30 October 2023 Volume 2023:16 Pages 1197—1206
DOI https://doi.org/10.2147/JAA.S437876
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Luis Garcia-Marcos
Chien-Chia Huang,1,2 Po-Hung Chang,1,* Yen-Lin Huang,3– 5 Ta-Jen Lee,1,2,6 Chi-Che Huang,1,2 Pei-Wen Wu1,2,*
1Division of Rhinology, Department of Otolaryngology, Chang Gung Memorial Hospital and Chang Gung University, Taoyuan, Taiwan; 2School of Medicine, Chang Gung University, Taoyuan, Taiwan; 3Department of Anatomic Pathology, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 4School of Medicine, National Tsing-Hua University, Hsinchu, Taiwan; 5Institute of Stem Cell and Translational Cancer Research, Chang Gung Memorial Hospital, Taoyuan, Taiwan; 6Department of Otolaryngology, Xiamen Chang Gung Hospital, Xiamen, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Pei-Wen Wu, Division of Rhinology, Department of Otolaryngology, Chang Gung Memorial Hospital and Chang Gung University, No. 5, Fu-Shin Street, Kweishan, Taoyuan, 333, Taiwan, Tel +886-3-3281200 ext. 8466, Fax +886-3-3979361, Email [email protected]
Introduction: Eosinophilic chronic rhinosinusitis with nasal polyps (CRSwNP) is frequently associated with greater inflammation, poorer prognosis, and a high recurrence rate after sinus surgery.
Objective: This study evaluated the clinical and imaging characteristics of eosinophilic CRSwNP in patients aged 12– 17.
Methods: We retrospectively enrolled 139 patients aged 12– 17 with bilateral CRSwNP. Clinical characteristics, computed tomography (CT) features, tissue eosinophil counts, and eosinophil activity were evaluated.
Results: Twenty-three (16.5%) patients had recurrent nasal polyps that required revision surgery. Patients requiring revision surgery had higher tissue eosinophil infiltration in the sinus mucosa than those not requiring revision surgery. The optimal cut-off value to distinguish the need for revision surgery was a tissue eosinophil count > 21.5/high-power field determined by the receiver operating characteristic curve. The Lund-Mackay and olfactory cleft opacification scores on CT images were significant predictors of tissue eosinophil count in the univariate analysis, and only olfactory opacification scores remained statistically significant in the multivariate analysis.
Conclusion: This study revealed that the CT feature of the olfactory cleft opacification score could be a significant characteristic of eosinophilic CRSwNP in adolescents.
Plain Language Summary: Chronic rhinosinusitis (CRS) is an inflammation of the nasal and sinus mucosa characterized by nasal obstruction, mucopurulent rhinorrhea, facial pain/pressure, and decreased or loss of smell for over 12 weeks.
CRS is classified as CRS without nasal polyps or CRS with nasal polyps (CRSwNP), depending on the presence of nasal polyps. Based on the predominance of tissue inflammatory cell infiltration by eosinophils or neutrophils, CRS can be categorized into eosinophilic and neutrophilic entities. Eosinophilic CRSwNP is clinically characterized by a greater extent of inflammation, more severe clinical symptoms and high recurrence rate after sinus surgery. Thus, early identification of the endotypes in patients with CRS is important for determining prognosis and treatment strategies.
This study retrospectively enrolled 139 patients aged 12– 17 with bilateral CRSwNP. Clinical characteristics, CT features, tissue eosinophil counts, and eosinophil activity were evaluated. The results showed that patients requiring revision surgery had higher tissue eosinophil infiltration in the sinus mucosa than those not requiring revision surgery. The optimal cut-off value to distinguish the need for revision surgery was a tissue eosinophil count > 21.5/high-power field. The Lund-Mackay and olfactory cleft opacification scores on CT images were significant predictors of tissue eosinophil count in the regression analysis.
Keywords: adolescent, chronic rhinosinusitis, computed tomography, eosinophil, nasal polyp
Introduction
Chronic rhinosinusitis (CRS) is an inflammation of the nasal and sinus mucosa characterized by nasal obstruction, mucopurulent rhinorrhea, facial pain/pressure, and decreased or loss of smell for over 12 weeks.1 The diagnosis of CRS requires findings of polyps, purulence, or edema on nasal endoscopy and/or inflammation or mucosal changes within the paranasal sinuses on imaging studies.1
CRS is classified as CRS without nasal polyps or CRS with nasal polyps (CRSwNP), depending on the presence of nasal polyps. Based on the predominance of tissue inflammatory cell infiltration by eosinophils or neutrophils, CRS can be categorized into eosinophilic and neutrophilic entities.2 Eosinophilic CRSwNP is frequently associated with comorbid asthma and/or aspirin intolerance and is clinically characterized by a greater extent of inflammation, more severe endoscopic and computed tomography (CT) scores, more severe clinical symptoms and olfactory dysfunction, poorer prognosis, and high recurrence rate after sinus surgery.3,4 Thus, early identification of the endotypes in patients with CRS is important for determining prognosis and treatment strategies. Histopathological examination is currently the standard method of diagnosing eosinophilic CRSwNP.4,5 However, tissue analysis of polyp biopsies is invasive and time-consuming. Therefore, it is necessary to characterize the clinical and imaging features of eosinophilic CRSwNPs.
The prevalence of CRS in individuals under the age of 18 was reported to be 2%–6%.6 Additionally, CRSwNP is uncommon in children, with an estimated prevalence of 0.1%.7 For pediatric patients with CRS who fail to receive medical therapy, especially in older children or those with nasal polyps, endoscopic sinus surgery (ESS) is considered an effective treatment to remove obstructive nasal polyps and reestablish sinus ventilation and drainage.8,9 However, the requirement for revision surgery due to disease recurrence has been reported to be 9%–13%,10,11 especially for those with severe tissue eosinophilic infiltration.12
Adolescence, age 12–17, is characterized by important behavioral and physiological changes.13 The adolescent population is equally affected by CRS; however, they are more suitable for ESS because of the relatively developed sinus structure and good cooperation with postoperative care compared with those aged < 12 years.9,14 Thus, this study evaluated the clinical and imaging characteristics of eosinophilic CRSwNP in patients aged 12–17 by investigating CT features and tissue eosinophilic infiltration requiring revision surgery. These findings may be beneficial for identifying those recalcitrant to current therapeutic modalities and optimizing treatment outcomes in the future.
Materials and Methods
Patients
Searching from histopathology database and manual chart reviews identified adolescents aged 12–17 who underwent ESS for bilateral CRSwNP at Chang Gung Memorial Hospital between 2004 and 2017. All sinus tissues removed during surgery would be sent for histopathologic analysis in our institute. Thus, all patients who underwent ESS would be included. CRSwNP was diagnosed according to the EPOS 2020 definition.1 Patients who experienced two or more symptoms, one of which was nasal blockage/obstruction/congestion, nasal discharge (anterior/posterior nasal drip), facial pain/pressure, or cough for more than 12 weeks. The presence of nasal polyps was confirmed using endoscopic and histopathological findings. We excluded those with a concomitant diagnosis of cystic fibrosis, primary ciliary dyskinesia, immunologic complications, or benign or malignant sinonasal neoplasms. Patients without preoperative CT data and those with a postoperative follow-up period of < 12 months were excluded from the analysis. The ESS targeted the abnormalities observed on the CT images during the primary surgery mostly including maxillary sinus antrostomy and ethmoidectomy, and more extensive revision surgery with involvement of frontal or sphenoid sinuses was performed for recurrent CRSwNP. The surgeries were performed by seven experienced rhinologists at a tertiary referral medical center. No difference in the recurrent rate was observed among the surgeons.
The clinical characteristics were collected, including patients’ laboratory results and CT features. The requirement for informed consent was waived because of the retrospective nature and anonymity of the data used in the study. This study was approved by the Institutional Review Board of Chang Gung Medical Foundation (IRB number: 202201253B0). All study procedures were performed in accordance with the relevant guidelines and regulations, and the Declaration of Helsinki.
Tissue Eosinophil Quantification
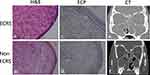
Sinus mucosa embedded in paraffin was obtained. A slice of the standard 5-µm section was stained with hematoxylin and eosin. The number of eosinophils in each tissue section was counted at three epithelial area with most severe inflammatory cell infiltration in ×400 magnification (high-power field, HPF) microscopic fields by a technician blinded to the clinical information (Figure 1a and b).
Immunohistochemistry (IHC) for Eosinophilic Cationic Protein (ECP) Expression
The most distinctive feature of eosinophils is their large secondary granules, which consist of a major basic protein core surrounded by a matrix of eosinophil peroxidase, eosinophil protein X/eosinophil-derived neurotoxins, and ECP.15 ECP is a well-described and established marker of tissue eosinophilia and eosinophil activation and has been used to evaluate eosinophil activity.16–18
To evaluate ECP expression, paraffin-embedded sections of sinus mucosal specimens from sinus surgery were dewaxed in xylene and rinsed in absolute alcohol. Then the sections were microwaved for 8 min in 1 mM ethylenediaminetetraacetic acid (pH 8.0) for antigen retrieval and incubated in 3% H2O2 for 10 min to block endogenous peroxidase activity. Sections were incubated with an antibody against ECP (1:300) (Bioss Antibodies, MA, USA) (Genetex, CA, USA) at 4°C for 18 h. The slides were then rinsed three times with phosphate-buffered saline before incubation with the secondary antibody and further processed for IHC using the IHC Select HRP Detection Set (Merck KGaA, Darmstadt, Germany). The slides were stained with (DAB; Dako, CA, USA) and counterstained with hematoxylin. IHC images were captured using an Olympus BX50 microscope with an Olympus E5 camera (Olympus, TOKYO, JAPAN) (Figure 1c and d). Immunostaining was quantified using the ImageJ Fiji Software (version 1.2; WS Rasband, National Institute of Health, Bethesda, MD, USA), as described previously.19 Areas with positive staining in the three regions of interest (ROIs; intact mucosa with the most severe inflammatory cell infiltration) of each sample were identified in the captured images by setting a threshold value and were computed according to the software instructions. The percentages of positively stained areas were calculated. The mean intensity of each ROI was calculated as the optical density of all the areas for each ROI.
Features on Sinonasal CT
Two experienced rhinologists independently reviewed the sinonasal CT images of each patient. The Lund-Mackay scoring system was used to quantify CRS severity based on CT imaging findings.12,20 The frontal, anterior ethmoidal, posterior ethmoidal, maxillary, and sphenoid sinuses and ostiomeatal complex were assigned a score of 0 (no abnormalities), 1 (partial opacification), or 2 (complete opacification). The ostiomeatal complex was assigned a score of 0 (not obstructed) or 2 (obstructed). The total bilateral score was 24.
Based on the Lund-Mackay scoring results, the ethmoid/maxillary (EM) ratio was calculated by dividing the sum of the CT scores of the bilateral ethmoid sinuses by those of the bilateral maxillary sinuses.21 A higher ratio indicates a higher disease proportion in the ethmoid sinus area.
The severity of opacification in the olfactory cleft on CT images was rated on a scale of 0–3, indicating clear (score 0), less than half (score 1), more than half (score 2), and total (score 3) opacification on each side of the olfactory cleft (Figure 1e and f).12
Statistical Analyses
The data were demonstrated as mean ± standard deviation and analyzed statistically with GraphPad Prism 5 (GraphPad Prism Software, Inc., San Diego, Calif, USA) and SPSS version 27.0 (IBM, Armonk, NY, USA). Categorical variables were compared using the χ2 test or Fisher’s exact test, as appropriate. Continuous variables were analyzed using the Mann–Whitney U-test to compare the two groups. Receiver operating characteristic (ROC) curves were generated to analyze the cut-off values for tissue eosinophil counts to predict the need for revision surgery. Revision-free survival curves of patients with CRSwNP were drawn and compared using the log-rank (Mantel-Cox) and Gehan–Breslow–Wilcoxon tests. Univariate and multivariate linear regression analyses assessed the association between tissue eosinophil counts and variables. Statistical significance was set at p < 0.05.
Results
Clinical Characteristics of the Study Population
The demographic data of the 139 adolescents with bilateral CRSwNP, including 85 males and 54 females, are shown in Table 1. The average patient age was 15.3 ± 1.8 years. Twenty-three (16.5%) patients underwent revision surgery.
 |
Table 1 Clinical Characteristics of Participants |
Evaluation of Tissue Eosinophilia
The average tissue eosinophil counts in each HPF was 26.9 ± 29.8 for the total cases, 43.7 ± 39.9 in patients with revision surgery, and 23.0 ± 26.2 in patients without revision surgery. Eosinophil infiltration in the sinus mucosa of patients requiring revision surgery was significantly more severe than in those without revision surgery (Figure 2a).
ROC curves were generated, and the area under the curve (AUC) was calculated to evaluate the sensitivity and specificity of the tissue eosinophil count in predicting the probability of requiring revision surgery in our cohort (Figure 2b). The ROC curve of tissue eosinophil count had AUC significantly greater than 0.5 (AUC = 0.665, p = 0.013). The optimal cut-off value was tissue eosinophil count > 21.5/HPF (sensitivity, 56.6%; specificity, 65.5%).
Thus, specimens with a tissue eosinophil count > 21.5/HPF were defined as eosinophilic CRSwNP, and immunostaining revealed a higher level of ECP expression in the mucosal tissues of eosinophilic CRSwNP compared to non-eosinophilic CRSwNP (Figure 1c and d).
Revision-free survival curves of patients with CRSwNP were drawn and compared between patients with tissue eosinophil counts > and < 21.5/HPF using the log-rank (Mantel-Cox) test and the Gehan-Breslow-Wilcoxon test (p = 0.004, Figure 2c).
CT Features
Comparisons of clinical characteristics between patients with tissue eosinophil counts > and < 21.5/HPF were performed and are shown in Table 1. Patients with a tissue eosinophil count of > 21.5/HPF had significantly higher olfactory cleft opacification scores than those with < 21.5/HPF. However, the two study groups had no significant difference in the total Lund-Mackay score or E/M ratio.
Regression Analysis
Associations between variables and tissue eosinophil counts were examined using linear regression analysis (Table 2). In the univariate analysis, the Lund-Mackay and olfactory cleft opacification scores were significant predictors of tissue eosinophil counts. Only the olfactory cleft opacification scores in the multivariate analysis remained statistically significant. In the analysis, olfactory cleft opacification scores were correlated with tissue eosinophil counts using Spearman correlation coefficient (p = 0.001) (Figure 2d).
 |
Table 2 Regression Analyses for the Associated Factors of Tissue Eosinophil Counts > 21.5/HPF |
Discussion
ESS is an effective treatment for adolescents with CRSwNP in whom medical therapy fails to remove obstructive nasal polyps and re-establish sinus ventilation and drainage.8,9 Compared with those aged < 12 years, the adolescent population is equally affected by CRS; however, they are more suitable for ESS because of the relatively developed sinus structure and good cooperation with postoperative care.11 However, eosinophilic CRSwNP is frequently associated with more severe sinonasal inflammation, greater potential for poorer surgical outcomes, and greater need for revision surgery.3,4 Therefore, it is important to identify patients with eosinophilic CRSwNP during management.
In this study, we first determined the optimal cut-off values of tissue eosinophil count (> 21.5/HPF) to predict the probability of requiring revision surgery in our cohort. Furthermore, the revision-free survival curve of patients with CRSwNP showed that patients with a tissue eosinophil count > 21.5/HPF were significantly more vulnerable to revision surgery than those with a tissue eosinophil count < 21.5/HPF. Thus, specimens with a tissue eosinophil count > 21.5/HPF were defined as eosinophilic CRSwNP, and immunostaining revealed a higher level of ECP expression in the mucosal tissues of eosinophilic CRSwNP compared to those of non-eosinophilic CRSwNP. We found a close association between tissue eosinophil count and olfactory cleft opacification score in regression and correlation analyses. These results indicate that eosinophilic CRSwNP has a greater potential for poor surgical outcomes and the need for revision surgery in adolescents. A tissue eosinophil count > 21.5/HPF is an optimal cut-off value to distinguish between eosinophilic and non-eosinophilic CRSwNP in this cohort based on the need for revision surgery. CT features of the olfactory cleft opacification score may significantly predict eosinophilic CRSwNP. Patients with eosinophilic CRSwNP should receive more intense postoperative adjuvant therapy and closer follow-up, which may benefit future therapeutic regimens such as type 2 biologics.
The classification of CRS phenotypes is based on observed clinical features; however, CRS endotypes are categorized based on immunological biomarkers involved in the pathophysiology of the disease.22 Tissue eosinophil count is one of the most widely used biomarkers for CRS endotyping, which allows for the precise treatment of CRS based on the degree of tissue eosinophilic infiltration and can predict the risk of recurrence after sinus surgery.23–25 Some studies have used serum eosinophil counts as a predictor of tissue eosinophil counts;26 however, serum eosinophil counts can also increase owing to parasitic infections, allergies, autoimmune diseases, or adverse drug reactions.27 Therefore, it is important to identify the endotypes of patients with CRS based on their clinical and imaging characteristics to determine the prognosis and treatment strategies. In this study, there was no correlation between serum and tissue eosinophil counts in our study cohort. However, the Lund-Mackay and olfactory cleft opacification scores were significant predictors of tissue eosinophil count in the univariate regression analysis, and only the olfactory cleft opacification scores remained statistically significant in the multivariate analysis. The severity of olfactory cleft opacification may be a marker of eosinophilic CRSwNP in adolescents.
Previous studies on adult CRS have demonstrated that nasal symptom scores and decreased olfactory function are higher in patients with eosinophilic CRS than in non-eosinophilic CRS.28 The Japanese Epidemiological Survey of Refractory Eosinophilic CRS (JESREC) study reported that tissue eosinophilia (≥ 70/HPF) was associated with decreased olfactory function.29 Compared to patients with maxillary sinus‐dominant cases, those with the dominant disease in ethmoid cells on CT images were significantly refractory to surgery.21 Furthermore, eosinophilia in the superior turbinate is highly associated with olfactory decline in patients with CRS.30 As a result, Eosinophilic CRS tends to involve the central part of the sinonasal area, such as the olfactory clefts and ethmoid sinuses and is associated with olfactory dysfunction.21,30 Therefore, the severity of olfactory cleft opacification may be a marker of eosinophilic CRSwNP.
The Lund-Mackay staging system was developed to quantify the extent of sinus inflammation.20 A higher Lund-Mackay score was associated with an increased risk of nasal polyps and multiple sinus involvement. Because eosinophilic CRSwNP is frequently associated with a greater extent of inflammation, the severity of tissue eosinophilic infiltration was correlated with CT scores in this study, similar to the results of studies on adult CRS.28
The JESREC study reported that CRS patients with a CT shadow displaying the ethmoid ≥ maxillary sinus had a significantly higher occurrence of eosinophilia (tissue eosinophil ≥ 70/HPF) and were refractory to treatment.29 Meng et al reported that the E/M ratio with a cut-off point of > 2.59 is a useful predictor for distinguishing adult eosinophilic CRSwNP from non-eosinophilic CRSwNP.21 Type 2 inflammation tends to involve the central part of the sinonasal area, including the nasal septum, olfactory cleft, middle turbinates, and ethmoid cells.31,32 The mechanism responsible for the predominance of ethmoid sinus inflammation in patients with type 2 CRS remains unclear. One possible explanation is that there are regional differences in the expression patterns of type 2 eosinophilic inflammatory molecules in the nasal cavity.33,34 However, the results of the current study showed that the ethmoid/maxillary sinus ratio was not associated with the tissue eosinophil count in adolescent CRSwNP. One possible reason for this may be the relatively greater severity of the disease in our cohort (averaged Lund-Mackay score: 19.0 ± 3.8). In paediatric CRSwNP, surgical intervention is usually reserved for those who have severe polyposis and/or are refractory to treatment. As the severity of sinus inflammation increases, the extent involvement of the sinuses increases, and the difference between ethmoid and maxillary sinus involvement may decrease.
Research on the immunopathological characteristics of pediatric CRSwNP is limited because of its low prevalence or inaccessibility of tissue samples.35–38 Chan et al found that tissue eosinophilic infiltration in CRS was more obvious in adults and older children than in younger children.35 Berger et al reported diminished tissue eosinophilia in pediatric CRS compared to adult CRS.36 Coffinet et al demonstrated that lymphocytes were more prevalent than eosinophils in young children with CRS.37 However, Jiang et al revealed that tissue eosinophils were more abundant in the younger CRSwNP group than in the older CRSwNP or CRSsNP groups and showed that Chinese pediatric CRSwNP is eosinophilic with mixed type 2/type 3 inflammation.2 Brown et al recently reported that most (57.8%) pediatric CRS cases have a lymphocyte-predominant inflammatory background; in contrast, most (66.5%) adult CRS have a lymphoplasmacytic-predominant inflammatory background.38 Differences in the presence of polyps, age, disease severity, and environmental factors may contribute to different mechanisms and immunopathological features of CRSwNP in pediatric populations.8 The pediatric population comprises all participants under 18. Patients with CRS in different age groups may present with different immunopathological characteristics, such as inflammatory patterns and disease severity. Adolescents aged 12–17 are in a period characterized by important changes in behavioral and physiological development.13 The adolescent population is equally affected by CRS. However, they are more suitable for ESS because of the relatively developed sinus structure and good cooperation with postoperative care compared to those aged < 12 years.9,14 In addition, this study evaluated the clinical characteristics of eosinophilic CRS with nasal polyps in patients aged 12–17 years by investigating tissue eosinophilic infiltration, the requirement for revision surgery, and CT features. The results showed that adolescent CRSwNP seemed to be more similar to that in the adult population. These results may be beneficial for the identification of patients recalcitrant to current therapeutic modalities and provide optimization of treatment outcomes in the future, such as novel type 2 biologics.39
Currently, three biologics have been approved as add-on therapies for adult CRSwNP recalcitrant to the standard care of CRS, including intranasal corticosteroids and endoscopic sinus surgery.40,41 Dupilumab is a monoclonal antibody that targets interleukin (IL)-4 receptor alpha, which shares receptor subunits with IL-4 and IL-13 and plays a major role in type 2 inflammatory responses. Omalizumab is a monoclonal antibody that targets IgE and is approved by the FDA for treating CRSwNP in 2020. Mepolizumab is a monoclonal antibody that binds to and inactivates IL-5, inhibiting eosinophilic inflammation. The European Forum for Research and Education in Allergy and Airway Diseases has proposed five criteria before initiating biological therapy in patients with CRSwNP.42,43 These criteria include evidence of type 2 inflammation, the need for systemic corticosteroids in the past 2 years, significant quality-of-life impairment, loss of smell, and a diagnosis of comorbid asthma. These recommendations suggest biologic therapy in patients with prior sinus surgery if they meet three criteria. Patients who have not undergone sinus surgery must meet four of these criteria to be eligible for biological treatment. However, there are no approved biologics for treating pediatric or adolescent CRSwNPs. Approximately 13–20% of pediatric CRSwNP require revision surgery; however, ESS has shown promising results in improving sinonasal symptoms and quality of life.11,12 Some patients, especially those with severe eosinophilic CRSwNP, experience early recurrence or require multiple surgeries. These patients could benefit from novel type 2 biological therapies. Thus, future studies on the use of biologics in pediatric and adolescent patients with recalcitrant CRSwNP are necessary. This study characterized the clinical and imaging features of eosinophilic CRSwNP in an adolescent population and helped clinicians identify those that are difficult to treat.
This study had some limitations. First, a small portion of missing data and loss to follow-up (seven cases) were inevitable complicating factors due to the retrospective study design. For instance, allergies and asthma were confirmed by manually reviewing patients’ medical records. The lack of clinical information and incomplete surveys may have contributed to this underestimation. To diminish these concerns, we enrolled 139 patients who underwent detailed clinical and histopathological evaluations. Second, only adolescents with CRSwNP who underwent sinus surgery were recruited because of the retrieval of cases from the pathology database. Therefore, patients who did not undergo sinus surgery were excluded. This may have contributed to selection bias. Third, a normal control was difficult to enroll due to the retrospective study design. Instead, we made comparison between patients with tissue eosinophil count < and > 21.5/HPF to characterize the clinical features of eosinophilic CRSwNP. Fourth, subjective evaluations such as clinical symptoms and smell function could not be included in the study because of the lack of comprehensive questionnaires. Future prospective studies that include subjective evaluations are necessary to identify additional clinical characteristics of eosinophilic CRSwNP in adolescents.
Conclusions
This study determined the optimal cut-off values of tissue eosinophil count (> 21.5/HPF) for determining eosinophilic CRSwNP and predicting the probability of requiring revision surgery in the adolescent population. In addition, a close association between tissue eosinophil count and the olfactory cleft opacification score was found, indicating that the CT feature of the olfactory cleft opacification score could be a significant characteristic of eosinophilic CRSwNP in adolescents. This could help clinicians identify patients with eosinophilic CRSwNP who are potentially difficult to treat and provide more intensive postoperative adjunct therapy and follow-up.
Abbreviations
CRS, Chronic rhinosinusitis; CRSwNP, CRS with nasal polyps; CT, computed tomography; CRS, chronic rhinosinusitis; CT, computed tomography; ESS, endoscopic sinus surgery; HPF, high-power field; IHC, immunohistochemistry; ECP, eosinophilic cationic protein; ROI, regions of interest; EM ratio, ethmoid/maxillary ratio; ROC, receiver operating characteristic; AUC, area under the curve; JESREC, the Japanese Epidemiological Survey of Refractory Eosinophilic CRS study; IL, interleukin.
Terms and Definitions
Chronic rhinosinusitis: an inflammation of the nasal and sinus mucosa for more than 12 weeks.
Nasal polyp: Description of benign inflammatory and hyperplastic outgrowths of the sinonasal mucosa.
Eosinophilia: Description of an unusual infiltration of high number of eosinophils in tissue.
Adolescent: Description of someone between the ages of 12 and 17.
Endoscopic sinus surgery: Description of a procedure to remove blockages and treat other problems in the sinuses using an endoscope.
Funding
The authors received research grants from the Chang Gung Memorial Hospital (CMRPG2K0161) and Taiwan National Science and Technology Council (110-2314-B-182 -063 -; 111-2314-B-182 -067 -;111-2635-B-182A-008 -). The funder had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Fokkens WJ, Lund VJ, Hopkins C, et al. European position paper on rhinosinusitis and nasal polyps 2020. Rhinology. 2020;58(1):1–464. doi:10.4193/Rhin20.401
2. Jiang L, Zeng Y, Huang Z, et al. Immunopathologic characteristics of Chinese pediatric patients with chronic rhinosinusitis. World Allergy Organ J. 2021;14(12):100616. doi:10.1016/j.waojou.2021.100616
3. Cho SH, Hamilos DL, Han DH, Laidlaw TM. Phenotypes of chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2020;8(5):1505–1511. doi:10.1016/j.jaip.2019.12.021
4. Hauser LJ, Chandra RK, Li P, Turner JH. Role of tissue eosinophils in chronic rhinosinusitis-associated olfactory loss. Int Forum Allergy Rhinol. 2017;7(10):957. doi:10.1002/alr.21994
5. Jin J, Guo B, Zhang W, et al. Predictive value of eosinophil cationic protein in nasal secretions in eosinophilic chronic rhinosinusitis. Laryngoscope. 2023. doi:10.1002/lary.30692
6. Adams PF, Hendershot GE, Marano MA; Statistics CfDCaPNCfH. Current estimates from the national health interview survey, 1996. Vital Health Stat. 1999;10(200):1–203.
7. Settipane GA. Epidemiology of nasal polyps. Allergy Asthma Proc. 1996;17(5):231–236. doi:10.2500/108854196778662246
8. Heath J, Hartzell L, Putt C, Kennedy JL. Chronic rhinosinusitis in children: pathophysiology, evaluation, and medical management. Curr Allergy Asthma Rep. 2018;18(7):37. doi:10.1007/s11882-018-0792-8
9. Torretta S, Guastella C, Ibba T, Gaffuri M, Pignataro L. Surgical treatment of paediatric chronic rhinosinusitis. J Clin Med. 2019;8(5):684. doi:10.3390/jcm8050684
10. Ramadan HH. Revision endoscopic sinus surgery in children: surgical causes of failure. Laryngoscope. 2009;119(6):1214–1247. doi:10.1002/lary.20230
11. Wu PW, Huang CC, Yang SW, et al. Endoscopic sinus surgery for pediatric patients: prognostic factors related to revision surgery. Laryngoscope. 2020;130(4):1051–1055. doi:10.1002/lary.28106
12. Wu PW, Chiu CH, Huang YL, et al. Tissue eosinophilia and computed tomography features in paediatric chronic rhinosinusitis with nasal polyps requiring revision surgery. Rhinology. 2023;61(4):348–357. doi:10.4193/Rhin22.435
13. Patton GC, Coffey C, Cappa C, et al. Health of the world’s adolescents: a synthesis of internationally comparable data. Lancet. 2012;379(9826):1665–1675. doi:10.1016/S0140-6736(12)60203-7
14. Hamilos DL. Pediatric chronic rhinosinusitis. Am J Rhinol Allergy. 2015;29:414–420. doi:10.2500/ajra.2015.29.4238
15. Marcucci F, Sensi LG, Migali E, et al. Eosinophil cationic protein and specific IgE in serum and nasal mucosa of patients with grass-pollen-allergic rhinitis and asthma. Allergy. 2001;56(3):231–236. doi:10.1034/j.1398-9995.2001.056003231.x
16. Kim KS, Won HR, Park CY, et al. Analyzing serum eosinophil cationic protein in the clinical assessment of chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27(3):e75–e80. doi:10.2500/ajra.2013.27.3901
17. Sun DI, Joo YH, Auo HJ, Kang JM. Clinical significance of eosinophilic cationic protein levels in nasal secretions of patients with nasal polyposis. Eur Arch Otorhinolaryngol. 2009;266(7):981–986. doi:10.1007/s00405-008-0872-9
18. Groger M, Bernt A, Wolf M, et al. Eosinophils and mast cells: a comparison of nasal mucosa histology and cytology to markers in nasal discharge in patients with chronic sino-nasal diseases. Eur Arch Otorhinolaryngol. 2013;270(10):2667–2676. doi:10.1007/s00405-013-2395-2
19. Crowe AR, Yue W. Semi-quantitative determination of protein expression using immunohistochemistry staining and analysis: an integrated protocol. Bio Protoc. 2019;9(24):e3465. doi:10.21769/BioProtoc.3465
20. Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117(3):S35–S40. doi:10.1016/S0194-5998(97)70005-6
21. Meng Y, Lou H, Wang C, Zhang L. Predictive significance of computed tomography in eosinophilic chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2016;6(8):812–819. doi:10.1002/alr.21749
22. Grayson JW, Hopkins C, Mori E, Senior B, Harvey RJ. Contemporary classification of chronic rhinosinusitis beyond polyps vs no polyps: a review. JAMA Otolaryngol Head Neck Surg. 2020;146(9):831–838. doi:10.1001/jamaoto.2020.1453
23. Vlaminck S, Acke F, Prokopakis E, et al. Surgery in nasal polyp patients: outcome after a minimum observation of 10 years. Am J Rhinol Allergy. 2021;35(4):449–457. doi:10.1177/1945892420961964
24. Tomassen P, Vandeplas G, Van Zele T, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol. 2016;137(5):1449–1456.e4. doi:10.1016/j.jaci.2015.12.1324
25. Stevens WW, Peters AT, Tan BK, et al. Associations between inflammatory endotypes and clinical presentations in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2019;7(8):2812–2820.e3. doi:10.1016/j.jaip.2019.05.009
26. Zuo K, Guo J, Chen F, et al. Clinical characteristics and surrogate markers of eosinophilic chronic rhinosinusitis in southern China. Eur Arch Otorhinolaryngol. 2014;271(9):2461–2468. doi:10.1007/s00405-014-2910-0
27. Roufosse F, Weller PF. Practical approach to the patient with hypereosinophilia. J Allergy Clin Immunol. 2010;126(1):39–44. doi:10.1016/j.jaci.2010.04.011
28. Kim DH, Kim SW, Basurrah MA, Hwang SH. Clinical and laboratory features of various criteria of eosinophilic chronic rhinosinusitis: a systematic review and meta-analysis. Clin Exp Otorhinolaryngol. 2022;15(3):230–246. doi:10.21053/ceo.2022.00052
29. Tokunaga T, Sakashita M, Haruna T, et al. Novel scoring system and algorithm for classifying chronic rhinosinusitis: the JESREC Study. Allergy. 2015;70(8):995–1003. doi:10.1111/all.12644
30. Wu D, Li Y, Bleier BS, Wei Y. Superior turbinate eosinophilia predicts olfactory decline in patients with chronic rhinosinusitis. Ann Allergy Asthma Immunol. 2020;125(3):304–310.e1. doi:10.1016/j.anai.2020.04.027
31. Wu PW, Lin YL, Lee YS, Chiu CH, Lee TJ, Huang CC. Predictors of surgical intervention for pediatric acute rhinosinusitis with periorbital infection. J Clin Med. 2022;11(13):3831. doi:10.3390/jcm11133831
32. Bai J, Huang JH, Price CPE, et al. Prognostic factors for polyp recurrence in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2022;150(2):352–361.e7. doi:10.1016/j.jaci.2022.02.029
33. Szucs E, Ravandi S, Goossens A, Beel M, Clement PA. Eosinophilia in the ethmoid mucosa and its relationship to the severity of inflammation in chronic rhinosinusitis. Am J Rhinol. 2002;16(3):131–134. doi:10.1177/194589240201600301
34. Seshadri S, Lin DC, Rosati M, et al. Reduced expression of antimicrobial PLUNC proteins in nasal polyp tissues of patients with chronic rhinosinusitis. Allergy. 2012;67(7):920–928. doi:10.1111/j.1398-9995.2012.02848.x
35. Chan KH, Abzug MJ, Coffinet L, Simoes EAF, Cool C, Liu AH. Chronic rhinosinusitis in young children differs from adults: a histopathology study. J Pediatr. 2004;144(2):206–212. doi:10.1016/j.jpeds.2003.11.009
36. Berger G, Kogan T, Paker M, Berger-Achituv S, Ebner Y. Pediatric chronic rhinosinusitis histopathology: differences and similarities with the adult form. Otolaryngol Head Neck Surg. 2011;144(1):85–90. doi:10.1177/0194599810390443
37. Coffinet L, Chan KH, Abzug MJ, Simões EAF, Cool C, Liu AH. Immunopathology of chronic rhinosinusitis in young children. J Pediatr. 2009;154(5):754–758. doi:10.1016/j.jpeds.2008.11.035
38. Brown HJ, Khalife S, Ganesan V, et al. Histopathologic differences between adult and pediatric patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2023;13(1):25–30. doi:10.1002/alr.23037
39. Kim C, Han J, Wu T, et al. Role of biologics in chronic rhinosinusitis with nasal polyposis: state of the art review. Otolaryngol Head Neck Surg. 2021;164(1):57–66. doi:10.1177/0194599820939964
40. Wautlet A, Bachert C, Desrosiers M, Hellings PW, Peters AT. The management of Chronic Rhinosinusitis With Nasal Polyps (CRSwNP) with biologics. J Allergy Clin Immunol Pract. 2023;12:S2213.
41. Orlandi RR, Kingdom TT, Smith TL, et al. International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol. 2021;11(3):213–739. doi:10.1002/alr.22741
42. Fokkens WJ, Lund V, Bachert C, et al. EUFOREA consensus on biologics for CRSwNP with or without asthma. Allergy. 2019;74(12):2312–2319. doi:10.1111/all.13875
43. Hellings PW, Fokkens WJ, Orlandi R, et al. The EUFOREA pocket guide for chronic rhinosinusitis. Rhinology. 2023;61(1):85–89. doi:10.4193/Rhin22.344
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.