Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Clinical Characteristics, Glycemic Control, and Microvascular Complications Compared Between Young-Onset Type 1 and Type 2 Diabetes Patients at Siriraj Hospital – A Tertiary Referral Center
Authors Preechasuk L , Tantasuwan S, Likitmaskul S , Santiprabhob J , Lertbannaphong O , Plengvidhya N, Tangjittipokin W , Nitiyanant W , Lertwattanarak R
Received 18 December 2021
Accepted for publication 1 April 2022
Published 2 May 2022 Volume 2022:15 Pages 1375—1387
DOI https://doi.org/10.2147/DMSO.S354787
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ming-Hui Zou
Lukana Preechasuk,1 Suchavadee Tantasuwan,2 Supawadee Likitmaskul,1 Jeerunda Santiprabhob,1,3 Ornsuda Lertbannaphong,1,3 Nattachet Plengvidhya,2,4 Watip Tangjittipokin,4,5 Wannee Nitiyanant,1,2 Raweewan Lertwattanarak1,2
1Siriraj Diabetes Center of Excellence, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 2Division of Endocrinology and Metabolism, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 3Division of Endocrinology and Metabolism, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 4Siriraj Center of Research Excellence for Diabetes and Obesity, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand; 5Department of Immunology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Correspondence: Raweewan Lertwattanarak, Division of Endocrinology and Metabolism, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, 2 Wanglang Road, Bangkoknoi, Bangkok, 10700, Thailand, Tel +66 2-419-7799, Fax +66 2-419-7792, Email [email protected]
Purpose: This study aimed to investigate the clinical characteristics, glycemic control, and microvascular complications compared between young-onset type 1 (T1DM) and type 2 diabetes (T2DM) patients at Siriraj Hospital.
Patients and Methods: We collected demographic, clinical, glycemic control, and microvascular complication data of young-onset (onset < 30 years of age) T1DM and T2DM patients at our center using February 2019-December 2020 data from the Thai Type 1 Diabetes and Diabetes diagnosed Age before 30 years Registry, Care and Network (T1DDAR CN).
Results: Of 396 patients, 76% had T1DM and 24% had T2DM. At diagnosis, T1DM were significantly younger (9.7± 5.4 vs 16.9± 6.4 years, p< 0.001), had a lower body mass index (17.2± 4.1 vs 30.8± 7.9 kg/m2, p< 0.001), higher prevalence of diabetic ketoacidosis (DKA) (66.1% vs 13.7%, p< 0.001), and higher HbA1c level (12.8± 2.6% vs 10.9± 3.1%, p=0.002) compared to T2DM. Regarding glycemic control, the mean HbA1c at registry enrollment did not differ between groups (T1DM 8.3± 1.8% vs T2DM 8.1± 2.2%, p=0.303), but T1DM achieved HbA1c < 7% significantly less than T2DM (19.3% vs 47.8%, p< 0.001). T1DM showed deterioration of glycemic control during 10– 20 years of age, and gradually improved during 20– 30 years of age, whereas patients with T2DM showed progressive worsening of glycemic control over time. Concerning microvascular complications, the prevalence of diabetic retinopathy (10.6% vs 9%, p=0.92) and diabetic neuropathy (3.4% vs 5.5%, p=0.514) between T1DM and T2DM was not significantly different. However, T2DM had a significantly higher prevalence of diabetic nephropathy (T1DM 10.1% vs T2DM 40.2%, p< 0.001) that developed within a significantly shorter duration of diabetes (T1DM 11.0± 6.8 vs T2DM 4.3± 5.1 years, p< 0.001) compared to T1DM.
Conclusion: T1DM had a significantly high prevalence of DKA at presentation, and most T1DM did not achieve the glycemic target, especially during adolescence. T2DM had a significantly higher prevalence of diabetic nephropathy that developed within a shorter duration of diabetes compared to T1DM.
Keywords: clinical characteristics, glycemic control, microvascular complications, young-onset type 1 and type 2 diabetes patients, Siriraj Hospital, Thailand
Introduction
Diabetes is a major health problem that causes a substantial healthcare burden globally. The Global Burden of Disease study reported a global prevalence of diabetes and disability-adjusted life-years associated with diabetes of 476.0 million and 67.9 million in 2017, and those values are projected to increase to 570.9 million and 79.3 million by 2025 – both respectively.1 The number of children and young adults with diabetes is also increasing annually. The incidence of type 1 diabetes (T1DM) increases by 2–3% per year, and the greatest increase is observed in children aged younger than 15 years.2 The prevalence of young-onset type 2 diabetes (T2DM), which is usually defined as diabetes onset at an age less than 40 years, is also increasing in most regions of the world – especially in Southeast Asia and the Western Pacific.3 In Thailand, the incidence of T1DM among children (age range: 0–15 years) increased from 0.2 per 100,000/year in 1984 to 1.65 per 100,000/year in 1995.4 The 6th Thai National Health Examination Survey reported an increased prevalence of all types of diabetes among adolescents and young adults (age range: 15–29 years) from 0.6% in 2009 to 1.7% in 2020, as well as an increased prevalence of obesity (body mass index [BMI] ≥25 kg/m2) from 19.5% in 2009 to 32.7% in 2020.5,6
Despite an overlap age of onset between young-onset T1DM and T2DM, some clinical characteristics can be used to differentiate between these two types of diabetes. The majority of young people with T2DM are overweight or obese with the presence of signs of insulin resistance or family history of T2DM. They might be asymptomatic or have mild hyperglycemic symptoms and infrequently present with ketoacidosis. They usually have comorbidities such as hypertension, dyslipidemia and polycystic ovarian syndrome.3,7 For glycemic control, International Society for Pediatric and Adolescent Diabetes (ISPAD) 2018 recommends HbA1c <7% for children and adolescent with T1DM who can access comprehensive care, HbA1c <7.5% for those who cannot access comprehensive care or have hypoglycemic unawareness,8 and HbA1c <7% as a treatment goal of youth with T2DM.9 The vast majority of children and adolescent of both types cannot meet glycemic target.8,10,11 Countries with well-funded and strong health care support system trended to have better glycemic control.8 Chronic diabetes complications cause significant morbidities and decrease quality of life of patients with diabetes. Some data suggested that young-onset T2DM had a higher burden of diabetes complications compared to patient with T1DM.12
The Thai Type 1 Diabetes and Diabetes diagnosed Age before 30 years Registry, Care and Network (T1DDAR CN) was established in 2014. T1DDAR CN is a collaboration among the Diabetes Association of Thailand, the Thai Society for Pediatric Endocrinology, and the Endocrine Society of Thailand. The aim of T1DDAR CN is to improve quality of care and to collect long-term data of patients with T1DM, and of patients diagnosed with any type of diabetes before age 30 years. A multicenter retrospective study that used T1DDAR CN data to evaluate glycemic control and complications in T1DM patients was published in 2021.13 In February 2019, a multicenter 5-year prospective study of Type 1 Diabetes and Diabetes diagnosed Age before 30 years Registry (T1DDAR) was started. The Faculty of Medicine Siriraj Hospital, Mahidol University is the largest tertiary referral center in the T1DDAR CN, and it is the administrative center for the T1DDAR study. The aim of this study was to investigate the clinical characteristics, glycemic control, and microvascular complications compared between young-onset type 1 and type 2 diabetes patients at Siriraj Hospital – a tertiary referral center. Patients with other specific types of diabetes will be analyzed in further studies.
Materials and Methods
Patients with T1DM in all age groups and patients with diabetes diagnosed at age less than 30 years who attended the Pediatric Diabetes Clinic or the Medicine Diabetes Clinic at the Faculty of Medicine Siriraj Hospital, Mahidol University were enrolled in a 5-year prospective T1DDAR study from February 2019. An electronic case record form was developed using Research Electronic Data Capture (REDCap; Vanderbilt University, Nashville, TN, USA), which is a web-based program. REDCap is hosted by the Research Institute for Health Sciences of Chiang Mai University, Chiang Mai, Thailand. Demographic data, socioeconomic data, clinical data at presentation, latest glycemic control and treatment, and diabetes complications were recorded in the baseline dataset. Glycemic control, diabetes treatment, and diabetes complications were recorded annually in the follow-up dataset. We abstracted and analyzed data from the baseline dataset of patients with T1DM or T2DM diagnosed before 30 years of age who were enrolled in the T1DDAR study during February 2019 to December 2020 at the Faculty of Medicine Siriraj Hospital. The study protocol was approved by the Central Research Ethics Committee of Thailand (approval no. COA-CREC003/2019) and the Siriraj Institutional Review Board (SIRB). The study complied with the Declaration of Helsinki. Participants aged ≥18 years provided written informed consent to participate. Patients aged <18 years gave verbal assent, and a parent or legal guardian gave written informed consent to participate.
Diabetes was diagnosed according to American Diabetes Association diagnostic criteria.14 T1DM was diagnosed in patients having a positive result from pancreatic autoantibody testing. For patients without pancreatic autoantibody testing or a negative result, type of diabetes was determined based on clinical presentation. T1DM was diagnosed in patients who were not obese, not having sign of insulin resistance, and/or presenting with diabetic ketoacidosis (DKA). T2DM was diagnosed in patients who had a negative result from pancreatic autoantibody testing, were obese, or had signs of insulin resistance. Clinical presentations were stratified by age at onset into 1 of 3 groups, including age <10 years, age 10 to <20 years, and age 20 to <30 years. Glycemic control was stratified by age at registry into 1 of the following 3 groups: age <10 years, aged 10 to <20 years, and aged ≥20 years. Glycemic control was classified as good glycemic control (glycated hemoglobin [HbA1c] <7%), un-optimal control glycemic control (HbA1c 7 to <9%), and poor glycemic control (HbA1c ≥9%). Obesity was diagnosed in patients aged >18 years if they had a BMI of ≥25 kg/m2. Obesity was diagnosed in patients aged <18 years if they had weight for height ≥140%. Hypertension was diagnosed if patients had systolic BP and/or diastolic BP ≥95th percentile for gender, age, and height or were treated with antihypertensive medication. Hypercholesterolemia was diagnosed if low-density lipoprotein cholesterol was >100 mg/dL or if patients were receiving lipid lowering medication.13,15
Diabetic retinopathy was defined as the presence of any severity of diabetes retinopathy, macular edema, vitreous hemorrhage, or tractional retinal detachment. Diabetic nephropathy was diagnosed when the patient had persistent albuminuria >30 mg/g creatinine. Diabetic neuropathy was diagnosed by monofilament examination, loss of reflex, or loss of vibratory sensation.
Statistical Analysis
All statistical analyses were performed using SPSS Statistics v. 20 (SPSS, Inc., Chicago, IL, USA). For normally distributed continuous variables, the data are presented as mean plus or minus standard deviation. For non-normally distributed continuous variables, the data are presented as median and interquartile range. For categorical data, the data are presented as number and percentage. For comparisons between young-onset T1DM and T2DM, Student’s t-test and Mann–Whitney U-test were used for normally distributed and non-normally distributed continuous variables, respectively. Chi-square test or Fisher’s exact test was used to compare categorical variables. Binary logistic regression analysis was used to adjust factors associated with diabetic retinopathy and diabetic nephropathy. A p-value of <0.05 was considered statistically significant.
Results
Clinical Characteristics Compared Between Young-Onset T1DM and T2DM
Of the 396 patients that were enrolled, 76% had T1DM and 24% had T2DM (Figure 1). The median (interquartile range, IQR) duration of disease was 6.7 (3.5, 12.1) years for T1DM, and 5.2 (1.6, 9.4) years for T2DM. Patients with T1DM had younger age at onset of diabetes (9.7±5.4 vs 16.9±6.4 years, p<0.001) and lower BMI at diagnosis (17.2±4.1 vs 30.8±7.9 kg/m2, p<0.001). Most of T1DM (66.1%) and 13.7% of T2DM patients had DKA at presentation. In the T1DM group, the prevalence of DKA at presentation was significantly decreased from 73.9% in the age <10 years group to 31.6% in the age 20–30 years group. Patients with T1DM had higher random plasma glucose (25.3±8.5 vs 14.4±6.0 mmol/L, p=0.001) and higher HbA1c at diagnosis (12.8±2.6 vs 10.9±3.1%, p=0.002) compared to T2DM. Patients with T1DM had a lower prevalence of family history of diabetes, hypercholesterolemia, and hypertension than patients with T2DM. The sociodemographic and clinical characteristics of young-onset T1DM and T2DM patients are shown in Table 1.
 |
Table 1 Sociodemographic and Clinical Characteristics Compared Between Young-Onset Type 1 Diabetes Mellitus (T1DM) and Type 2 Diabetes Mellitus (T2DM) Patients |
 |
Figure 1 Type of young-onset diabetes compared between genders among all included patients and stratified by age group. |
Pancreatic Antibodies in Patients with T1DM
Among the 301 patients with T1DM, pancreatic autoantibodies were tested in 238 patients (79.1%). The number of patients who were tested for one, two, and three antibodies was 17 (5.6%), 192 (63.8%), and 29 (9.6%), respectively. Anti-GAD, Anti-IA2, and Anti-ZnT8 were tested in 237 (78.7%), 222 (73.8%), and 29 (9.6%) patients, respectively. Anti-GAD, Anti-IA2, and Anti-ZnT8 were detected in 166, 147, and 13 patients with T1DM, respectively (Figure 2). All patients found to be positive for Anti-ZnT8 also had a positive result for Anti-GAD and/or Anti-IA2. Two hundred and twenty-one patients were tested for both Anti-GAD and Anti-IA2, and 109 (48.9%) patients had a positive result for both antibodies. Forty-two (18.8%) patients had a positive result for only Anti-GAD, and 37 (16.6%) patients had positive result for only Anti-IA2. There were 35 (15.7%) patients who had a negative result for both Anti-GAD and Anti-IA2, and 4 of those 35 patients were tested for Anti-ZnT8 and also had a negative result.
Glycemic Control and Diabetes Treatment at Registry
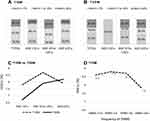
The most recent HbA1c level was not significantly different between T1DM and T2DM patients (8.3±1.8 vs 8.1±2.2, p=0.303). Good glycemic control (HbA1c <7%) was significantly less often achieved in the T1DM group compared to the T2DM group (19.3% vs 47.8%, p<0.001). The proportions of patients having good, un-optimal control, and poor glycemic control are shown in Figure 3A and B. Patients with T1DM and T2DM had different glycemic patterns across age groups, as shown in Figure 3C. In the T1DM group, the mean HbA1c increased from 7.8±1.1% in the age <10 years group to 8.7±1.9% in the age 10–20 years group, and then decreased to 7.9±1.6% in the age ≥20 years group. In the T2DM group, HbA1c gradually increased from 6.7% in the age <10 years group to 7.9±2.4% in the age 10–20 years group, and to 8.2±2.1% in the age ≥20 years group.
Regarding T1DM treatment, 77.7% of patients used intensive insulin therapy (76.4% used basal bolus insulin regimen, and 1.3% used continuous subcutaneous insulin infusion), and 22.3% used conventional insulin regimen. For T2DM treatment, 43.2% of patients used insulin and oral anti-diabetes drugs, and 38.9% used only oral anti-diabetes drugs. The mean frequency of self-monitoring of blood glucose (SMBG) in T1DM and T2DM patients was 2.9±1.3 and 0.8±1.3 times per day, respectively. In the T1DM group, patients who performed SMBG ≥4 times per day had a significantly lower HbA1c (7.8±1.4%) than patients who performed SMBG ≤1 times per day (8.6±1.8%, p=0.020), as shown in Figure 3D. There was no significant difference in HbA1c level among T2DM patients who performed SMBG a different number of times per day. Glycemic control and management at the time of registration in the T1DDAR CN compared between young-onset T1DM and T2DM are shown in Table 2.
Microvascular Complications of Diabetes at Registry
Significantly fewer T1DM patients received diabetic retinopathy (DR) screening (81.4% vs 93.7%, p=0.002) and diabetic neuropathy screening compared to the number of T2DM screened (39.5% vs 57.9%, p=0.002). There was no significant difference in the rate of diabetic nephropathy (DN) screening between groups. The prevalence of DR (10.6% vs 9%, p=0.92) and diabetic neuropathy (3.4% vs 5.5%, p=0.514) between T1DM and T2DM was not significantly different. However, patients with T2DM had a significantly higher prevalence of DN compared to patients with T1DM (10.1% vs 40.2%, p<0.001). T2DM patients with DN had a significantly shorter duration of diabetes, but a similar most recent HbA1c level compared to T1DM. The prevalence of diabetes complications is shown in Table 3.
Univariate analysis of factors associated with DR and DN compared between young-onset T1DM and T2DM patients is shown in Table 4. Binary logistic regression analysis demonstrated that factors associated with DR in T1DM were older age at diagnosis (odds ratio: 1.13 per year, 95% confidence interval: 1.04–1.22; p=0.003) and longer diabetes duration (odds ratio: 1.19 per year, 95% confidence interval: 1.10–1.28; p<0.001); factor associated with DR in T2DM was longer diabetes duration (odds ratio: 1.30 per year, 95% confidence interval: 1.10–1.54; p=0.002). Factors associated with DN in T1DM were higher latest HbA1c level (odds ratio: 1.31 per 1%, 95% confidence interval: 1.03–1.67; p=0.026) and having hypertension (odds ratio: 5.58, 95% confidence interval: 1.25–24.90; p=0.024); factor associated with DN in T2DM was having dyslipidemia (odds ratio: 3.31, 95% confidence interval: 1.01–10.94; p=0.049). Hypertension had a trend of association with DN in T2DM (odds ratio: 2.69, 95% confidence interval: 0.92–7.88; p=0.071). Factor associated with diabetes neuropathy were not analyzed due to low number of events.
Discussion
The results of this study demonstrate differences in clinical presentation, glycemic control, and microvascular complications between young-onset T1DM and young-onset T2DM. The mean age at diagnosis of patients with T1DM and T2DM in our study was 9.7±5.4 year and 16.9±6.4 years, respectively, which is close to the peak incidence in young-onset T1DM and T2DM from previous studies.7,16–18 The peak incidence of T1DM diagnosis was observed in children aged 10–14 years,16,17 and the peak age at presentation of young-onset T2DM was during mid to late puberty.7,18 In the present study, 66.1% of T1DM patients had DKA as first presentation. Children aged <10 years had the highest proportion (73.9%) of DKA at first presentation, with a decrease to 58.8% in patients aged 10 to <20 years, and a further reduction to 31.6% in patients aged 20 to 30 years. A higher incidence of DKA at presentation in younger children was observed in many countries.19–21 A systematic literature review22 of T1DM in children and adolescents during April 2011 to May 2016 found a wide range of DKA incidence as first presentation from 14.7% (Denmark, human development index [HDI]: 0.902) to 79.8% (Saudi Arabia, HDI: 0.826). The authors of that review reported a higher latitude and higher human development index (HDI) to be significantly associated with a lower DKA rate at presentation. Those same authors also hypothesized that countries with a high prevalence of T1DM were more effective at detecting the symptoms of DKA and new-onset T1DM, which resulted in a lower DKA rate at first presentation.22 Therefore, the high DKA rate at first presentation in our study might be explained by Thailand’s HDI of 0.777 (United Nations Human Development Report 2020),23 and the fact that Thailand has a comparatively lower prevalence of T1DM (1.65 per 100,000/year in 1991–1995),4 which resulted in a lower level of awareness of T1DM symptoms in children and adolescents. The T2DM patients in our study had typical clinical presentation of T2DM in children and adolescents,7,18 including obesity (mean BMI: 30.8±7.9 kg/m2), sign of insulin resistance (acanthosis nigricans, 82.8%), family history of DM (80%), and low rate of DKA (13.7%) at presentation. Reinehr18 reported that 5–25% of non-Caucasian children and adolescents presented with DKA at diagnosis of T2DM.
The detection rate of Anti-GAD and Anti-IA2 in our study was comparable with the detection rate in recent-onset T1DM patients from a study conducted in the United Kingdom.24 Half of the patients in our study had a positive result for both Anti-GAD and Anti-IA2, whereas some patients (approximately 16–20%) had a single positive result for Anti-GAD or Anti-IA2. Therefore, we recommend measuring both Anti-GAD and Anti-IA2 in routine clinical practice. Approximately 15% of patients had a negative result for both Anti-GAD and Anti-IA2, and this proportion is similar to the previously reported 10–15% rate of autoantibody negative T1DM.25 Previous study showed that Anti-ZnT8 improves the sensitivity of T1DM diagnosis because Anti-ZnT8 was found in 26% of T1DM subjects classified as autoantibody-negative on the basis of existing markers, including Anti-GAD, Anti-IA2, antibodies to insulin, and islet cytoplasmic autoantibodies.26 However, the measurement rate of Anti-ZnT8 in our study was low (9.6%) because Anti-ZnT8 testing was only recently introduced at our center (December 2018). Therefore, the clinical implication of Anti-ZnT8 in our population requires further study.
There was no significant difference in mean HbA1c between patients with T1DM and patients with T2DM in our study. However, a significantly larger percentage of T2DM patients achieved good glycemic control compared to patients with T1DM. The mean HbA1c of T1DM patients in our study (8.3±1.8%) was lower than that in the Thai T1DM national registry (9.35±2.41%).13 The better glycemic control of T1DM patients at our center compared to national registry data might be explained by a higher percentage of intensive insulin therapy (77.7% vs 57.0%) and a higher frequency of SMBG (3.0±1.1 vs 2.06±1.41 times daily). In addition, all T1DM patients at our center were treated by pediatric or adult endocrinologists, and they received intensive diabetes self-management education (DSME) from a multidisciplinary team. The ISPAD 201827 and ADA 202128 guidelines both recommend intensive insulin therapy for patients with T1DM since basal bolus insulin regimen has the best potential for imitating the physiological human insulin profile. The T1DM Exchange clinic registry in the United States found a higher number of SMBG measurements per day to be strongly associated with a lower HbA1c level in all age groups.29 The DPV-Wiss database from Germany and Austria revealed that one additional SMBG per day was significantly associated with better metabolic control with a drop in HbA1c of 0.2%.30 Frequent SMBG improves HbA1c due to an improved ability to quickly correct out-of-target range glucose values by determining bolus insulin, and by decreasing the risk of overcorrection of hypoglycemia due to early detection of low glucose value before the onset of symptomatic hypoglycemia.8
The pattern of deterioration of glycemic control in patients with T1DM during adolescence followed by gradual improvement in adulthood was also observed in previous studies from the United States and Europe.31–33 Reported factors that may influence this deterioration include increased insulin resistance due to endocrine change, erratic meal and exercise pattern, missing insulin doses, transition from pediatric to adult diabetes clinic, decreased parental support, and psychological illness.34,35 In contrast, glycemic control in patients with T2DM in our study worsened as they got older and had a longer duration of diabetes. One possibility that may explain this worsening of glycemic control is rapid decline in β cell function.3 In young-onset T2DM, the initial deterioration of β cell function impairs first-phase nutrient-induced insulin secretion, which is the same as in late-onset T2DM. However, young-onset T2DM showed early decline in second-phase nutrient-induced insulin secretion, and accelerated loss of β cell function compared to late onset T2DM.3
Previous studies reported a significantly higher prevalence of microvascular complications in patients with young-onset T2DM compared to patients with T1DM.3,12,36–38 However, our study showed a similar prevalence of diabetic retinopathy (DR) between groups with a slightly shorter duration of diabetes in patients with T2DM compared to patients with T1DM. Studies comparing the prevalence of DR between patients with young-onset T2DM and T1DM reported mixed results. Data from India showed a similar prevalence of any DR between groups (T1DM 53.3% vs T2DM 52.7%).39 Data from the United Kingdom also showed a similar prevalence of significant DR between groups (T1DM 21.6% vs T2DM 20.8%, p>0.05).36 However, after adjusting for diabetes duration, the T2DM cohort had a significantly higher prevalence of significant DR than T1DM after 10-years duration. Data from the SEARCH study (USA) demonstrated a higher age-adjusted prevalence of DR in patients with young-onset T2DM (T1DM 5.6% vs T2DM 9.1%, p=0.02).40
Interestingly, the prevalence of diabetic nephropathy (DN) in our study was significantly higher in patients with young-onset T2DM than in patients with T1DM despite T2DM having a shorter duration of diabetes. The SEARCH study showed a significantly higher age-adjusted prevalence of DN in patients with young-onset T2DM (T1DM 5.8% vs T2DM 19.9%, p<0.01).40 A study from Australia also found a significantly higher prevalence of microalbuminuria in young-onset T2DM compared to T1DM (T1DM 6% vs T2DM 28%, p<0.0001).41 The TODAY clinical trial, a cohort of newly diagnosed (onset <2 years) adolescent T2DM (aged 10–17 years), showed rapid progression of microalbuminuria from 6.3% at baseline to 16.6% at the end of follow-up (3.9 years).42 Data from Manitoba, Canada revealed that young-onset T2DM had a four-fold increased risk of renal failure compared to young-onset T1DM, and a 23-fold increased risk of renal failure compared to youth without DM.38 Pubertal growth factors and hormonal changes during puberty might influence nephropathy progression.43 While longer diabetes duration, older age, puberty, elevated glucose level, elevated blood pressure, dyslipidemia, and obesity were reported to be risk factors for diabetes complications in children and adolescents with diabetes,15 we found that longer diabetes duration was associated with DR in both T1DM and T2DM, and older age was associated with DR only in T1DM. Latest higher HbA1c and hypertension were associated with DN in T1DM, and dyslipidemia was associated with DN in T2DM. The reasons why glycemic control, which is a significant risk factor of all diabetes complications,44 was only associated with DN in T1DM might be explained by the cross-sectional data collection of our study. Only HbA1c levels within the latest year were available, data of previous glycemic control or glycemic pattern were lacking. Furthermore, the number of microvascular events in our study was low that might limit a power to identify the associated risk factors for microvascular complications. However, because young-onset T1DM and T2DM both have increased risk of atherosclerotic cardiovascular disease, modification of cardiovascular risk factors, including glucose level, blood pressure, cholesterol level and body weight, should be encouraged.
Limitations
Our study has some mentionable limitations. First, data at diagnosis in some patients were not available because some patients were referred from other hospitals. Second, pancreatic autoantibodies were not tested in all patients because the decision regarding pancreatic autoantibody testing was made at the discretion of each physician. Third, microvascular complications were not screened in all patients, and diabetic nephropathy was defined by albuminuria only.
Conclusion
Patients with young-onset T1DM had a significantly higher prevalence of DKA at presentation, and most T1DM did not achieve glycemic target, especially during adolescence. Young-onset T2DM patients had a significantly higher prevalence of diabetic nephropathy that developed within a significantly shorter duration of diabetes compared to young-onset T1DM. Therefore, strategies to increase awareness of T1DM, to improve glycemic control, and to slow the progression of diabetes complications in young-onset diabetes should be promoted.
Acknowledgments
The authors gratefully acknowledge the Thai Type 1 Diabetes and Diabetes diagnosed Age before 30 years Registry, Care and Network (T1DDAR CN) for data management; Chanoknan Luksameejaroenchai, Surisa Arlai, and Parichart Netsakulnee for data collection at the Faculty of Medicine Siriraj Hospital; and Khemajira Karaketklang and Julaporn Pooliam for assistance with statistical analysis.
Funding
This study was supported by a grant from the Health Systems Research Institute (grant number 63-111), Nonthaburi, Thailand.
Disclosure
All authors declare no personal or professional conflicts of interest, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report.
References
1. Lin X, Xu Y, Pan X, et al. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Sci Rep. 2020;10(1):14790. doi:10.1038/s41598-020-71908-9
2. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. Lancet. 2018;391(10138):2449–2462. doi:10.1016/S0140-6736(18)31320-5
3. Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6(1):69–80. doi:10.1016/S2213-8587(17)30186-9
4. Tuchinda C, Likitmaskul S, Unachak K, Panamonta O, Patarakijavanich N, Chetthakul T. The epidemiology of type 1 diabetes in Thai children. J Med Assoc Thai. 2002;85(6):648–652.
5. Aekplakorn W. The 4th Thai National Health Examination Survey 2008–2009. Nonthaburi: The graphic publisher; 2009.
6. Aekplakorn W. The 6th Thai National Health Examination Survey 2019–2020. Nonthaburi: The graphic publisher; 2020.
7. Reinehr T. Type 2 diabetes mellitus in children and adolescents. World J Diabetes. 2013;4(6):270–281. doi:10.4239/wjd.v4.i6.270
8. DiMeglio LA, Acerini CL, Codner E, et al. ISPAD clinical practice consensus guidelines 2018: glycemic control targets and glucose monitoring for children, adolescents, and young adults with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):105–114. doi:10.1111/pedi.12737
9. Zeitler P, Arslanian S, Fu J, et al. ISPAD clinical practice consensus guidelines 2018: type 2 diabetes mellitus in youth. Pediatr Diabetes. 2018;19(Suppl 27):28–46. doi:10.1111/pedi.12719
10. Barr MM, Aslibekyan S, Ashraf AP. Glycemic control and lipid outcomes in children and adolescents with type 2 diabetes. PLoS One. 2019;14(7):e0219144–e0219144. doi:10.1371/journal.pone.0219144
11. Weinstock RS, Braffett BH, Songer TJ. Health care coverage and glycemic control in young adults with youth-onset type 2 diabetes: results from the TODAY2 study. Diabetes Care. 2020;43(10):2469–2477. doi:10.2337/dc20-0760
12. Dabelea D, Stafford JM, Mayer-Davis EJ, et al. Association of type 1 diabetes vs type 2 diabetes diagnosed during childhood and adolescence with complications during teenage years and young adulthood. JAMA. 2017;317(8):825–835. doi:10.1001/jama.2017.0686
13. Dejkhamron P, Santiprabhob J, Likitmaskul S, et al. Type 1 diabetes management and outcomes: a multicenter study in Thailand. J Diabetes Investig. 2021;12(4):516–526. doi:10.1111/jdi.13390
14. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Supplement 1):S13–S28. doi:10.2337/dc19-S002
15. Donaghue KC, Marcovecchio ML, Wadwa RP, et al. ISPAD clinical practice consensus guidelines 2018: microvascular and macrovascular complications in children and adolescents. Pediatr Diabetes. 2018;19(S27):262–274. doi:10.1111/pedi.12742
16. Rogers MAM, Kim C, Banerjee T, Lee JM. Fluctuations in the incidence of type 1 diabetes in the United States from 2001 to 2015: a longitudinal study. BMC Med. 2017;15(1):199. doi:10.1186/s12916-017-0958-6
17. Forga Llenas L, Goñi Iriarte MJ, Cambra Contin K, Ibáñez Beroiz B, Chueca Guendulain M, Berrade Zubiri S. Incidence and temporal trends of childhood type 1 diabetes between 1975 and 2012 in Navarre (Spain). Gac Sanit. 2015;29(1):51–54. doi:10.1016/j.gaceta.2014.06.010
18. Reinehr T. Clinical presentation of type 2 diabetes mellitus in children and adolescents. Int J Obes. 2005;29(2):S105–S110. doi:10.1038/sj.ijo.0803065
19. Choleau C, Maitre J, Filipovic Pierucci A, et al. Ketoacidosis at diagnosis of type 1 diabetes in French children and adolescents. Diabetes Metab. 2014;40(2):137–142. doi:10.1016/j.diabet.2013.11.001
20. Oyarzabal Irigoyen M, García Cuartero B, Barrio Castellanos R, et al. Ketoacidosis at onset of type 1 diabetes mellitus in pediatric age in Spain and review of the literature. Pediatr Endocrinol Rev. 2012;9(3):669–671.
21. Kao KT, Islam N, Fox DA, Amed S. Incidence trends of diabetic ketoacidosis in children and adolescents with type 1 diabetes in British Columbia, Canada. J Pediatr. 2020;221:165–173.e162. doi:10.1016/j.jpeds.2020.02.069
22. Große J, Hornstein H, Manuwald U, Kugler J, Glauche I, Rothe U. Incidence of diabetic ketoacidosis of new-onset type 1 diabetes in children and adolescents in different countries correlates with Human Development Index (HDI): an updated systematic review, meta-analysis, and meta-regression. Horm Metab Res. 2018;50(3):209–222. doi:10.1055/s-0044-102090
23. Miščević NJCJoP. United nations development programme, human development report 2020. The next frontier human development and the anthropocene. Croat J Philos. 2021;21(61):231–235.
24. Richardson CC, Dromey JA, McLaughlin KA, et al. High frequency of autoantibodies in patients with long duration type 1 diabetes. Diabetologia. 2013;56(11):2538–2540. doi:10.1007/s00125-013-3017-7
25. Patel SK, Ma CS, Fourlanos S, Greenfield JR. Autoantibody-negative type 1 diabetes: a neglected subtype. Trends Endocrinol Metab. 2021;32(5):295–305. doi:10.1016/j.tem.2021.02.001
26. Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci. 2007;104(43):17040–17045. doi:10.1073/pnas.0705894104
27. Danne T, Phillip M, Buckingham BA, et al. ISPAD clinical practice consensus guidelines 2018: insulin treatment in children and adolescents with diabetes. Pediatr Diabetes. 2018;19(Suppl 27):115–135. doi:10.1111/pedi.12718
28. American Diabetes Association. 13. children and adolescents: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Supplement 1):S180–S199. doi:10.2337/dc21-S013
29. Miller KM, Beck RW, Bergenstal RM, et al. Evidence of a strong association between frequency of self-monitoring of blood glucose and hemoglobin A1c levels in T1D exchange clinic registry participants. Diabetes Care. 2013;36(7):2009–2014. doi:10.2337/dc12-1770
30. Ziegler R, Heidtmann B, Hilgard D, Hofer S, Rosenbauer J, Holl R. Frequency of SMBG correlates with HbA1c and acute complications in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2011;12(1):11–17. doi:10.1111/j.1399-5448.2010.00650.x
31. Carlsen S, Skrivarhaug T, Thue G, et al. Glycemic control and complications in patients with type 1 diabetes - A registry-based longitudinal study of adolescents and young adults. Pediatr Diabetes. 2017;18(3):188–195. doi:10.1111/pedi.12372
32. Foster NC, Beck RW, Miller KM, et al. State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther. 2019;21(2):66–72. doi:10.1089/dia.2018.0384
33. Gerstl EM, Rabl W, Rosenbauer J, et al. Metabolic control as reflected by HbA1c in children, adolescents and young adults with type-1 diabetes mellitus: combined longitudinal analysis including 27,035 patients from 207 centers in Germany and Austria during the last decade. Eur J Pediatr. 2008;167(4):447–453. doi:10.1007/s00431-007-0586-9
34. Khandelwal S, Sengar GS, Sharma M, Choudhary S, Nagaraj N. Psychosocial illness in children with type 1 diabetes mellitus: prevalence, pattern and risk factors. J Clin Diagnostic Res. 2016;10(9):SC05–SC08. doi:10.7860/JCDR/2016/21666.8549
35. Cameron FJ, Garvey K, Hood KK, Acerini CL, Codner E. ISPAD clinical practice consensus guidelines 2018: diabetes in adolescence. Pediatr Diabetes. 2018;19(Suppl 27):250–261. doi:10.1111/pedi.12702
36. Song SH. Significant retinopathy in young-onset type 2 vs. type 1 diabetes: a clinical observation. Int J Clin Pract. 2016;70(10):853–860. doi:10.1111/ijcp.12789
37. Jaiswal M, Lauer A, Martin CL, et al. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for diabetes in youth follow-up cohort: a pilot study. Diabetes Care. 2013;36(12):3903–3908. doi:10.2337/dc13-1213
38. Dart AB, Sellers EA, Martens PJ, Rigatto C, Brownell MD, Dean HJ. High burden of kidney disease in youth-onset type 2 diabetes. Diabetes Care. 2012;35(6):1265–1271. doi:10.2337/dc11-2312
39. Rajalakshmi R, Amutha A, Ranjani H, et al. Prevalence and risk factors for diabetic retinopathy in Asian Indians with young onset type 1 and type 2 diabetes. J Diabetes Complications. 2014;28(3):291–297. doi:10.1016/j.jdiacomp.2013.12.008
40. Mayer-Davis EJ, Davis C, Saadine J, et al. Diabetic retinopathy in the SEARCH for diabetes in youth cohort: a pilot study. Diabet Med. 2012;29(9):1148–1152. doi:10.1111/j.1464-5491.2012.03591.x
41. Eppens MC, Craig ME, Cusumano J, et al. Prevalence of diabetes complications in adolescents with type 2 compared with type 1 diabetes. Diabetes Care. 2006;29(6):1300–1306. doi:10.2337/dc05-2470
42. L Pyle. Rapid rise in hypertension and nephropathy in youth with type 2 diabetes: the TODAY clinical trial. Diabetes Care. 2013;36(6):1735–1741. doi:10.2337/dc12-2420
43. Solis-Herrera C, Triplitt CL, Lynch JL. Nephropathy in youth and young adults with type 2 diabetes. Curr Diab Rep. 2014;14(2):456. doi:10.1007/s11892-013-0456-y
44. Nathan DM. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: overview. Diabetes Care. 2014;37(1):9–16. doi:10.2337/dc13-2112
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.