Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 14
Clinical burden of illness among patients with severe eosinophilic COPD
Authors Müllerová H , Meeraus WH, Galkin DV , Albers FC , Landis SH
Received 14 November 2018
Accepted for publication 11 March 2019
Published 28 March 2019 Volume 2019:14 Pages 741—755
DOI https://doi.org/10.2147/COPD.S194511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Russell
Hana Müllerová,1 Wilhelmine H Meeraus,1 Dmitry V Galkin,2 Frank C Albers,2 Sarah H Landis1,3
1Respiratory Epidemiology, GSK, Uxbridge, UK; 2Respiratory Medical Franchise, GSK, Uxbridge, NC, USA; 3Patient Centered Outcomes and Value Evidence and Outcomes, GSK, Collegeville, PA, USA
Background: There are currently limited real-world data on the clinical burden of illness in patients with COPD who continue to exacerbate despite receiving triple therapy. The aim of this study was to compare the burden of COPD in patients with and without a phenotype characterized by a high blood eosinophil count and high risk of exacerbations while receiving triple therapy.
Methods: This retrospective cohort study (GSK ID: 207323/PRJ2647) used UK Clinical Practice Research Datalink records linked with Hospital Episode Statistics. Eligible patients had a COPD medical diagnosis code recorded between January 1, 2004 and December 31, 2014, and a blood eosinophil count recorded on/after that date. Patients were followed from index date (first qualifying blood eosinophil count) until December 31, 2015. The study phenotype was defined as ≥2 moderate/≥1 severe acute exacerbation of COPD (AECOPD) in the year prior to the index date, current use of multiple-inhaler triple therapy (MITT), and blood eosinophil count ≥150 cells/μL on the index date. Outcomes measured during follow-up included moderate/severe AECOPDs, severe AECOPDs, all-cause mortality, primary care (GP) clinical consultations, and non-AECOPD-related unscheduled hospitalizations.
Results: Of 46,814 patients eligible for inclusion, 2512 (5.4%) met the definition of the study phenotype. Adjusted rate ratios (95% CI) of moderate/severe AECOPDs and all-cause mortality in patients with the study phenotype versus those without were 2.32 (2.22, 2.43) and 1.26 (1.16, 1.37), respectively. For GP visits and non-AECOPD-related unscheduled hospitalizations, adjusted rate ratios (95% CI), in patients with the study phenotype versus those without, were 1.09 (1.05, 1.12) and 1.31 (1.18, 1.46), respectively.
Conclusion: Patients with COPD and raised blood eosinophil counts who continue to exacerbate despite MITT represent a distinct subgroup who experience substantial clinical burden and account for high healthcare expenditure. There is a need for more effective management and therapeutic options for these patients.
Keywords: acute exacerbations, burden of illness, eosinophils, multiple-inhaler triple therapy
Plain language summary
Why was the study done?
Some patients with COPD have high blood eosinophil counts, which may be associated with severe disease. Such patients experience episodes of frequent symptom worsening, known as exacerbations, despite receiving the recommended treatment (inhaled triple therapy). There are currently limited real-world data in these patients.
What did the researchers do and find out?
This study used de-identified electronic medical records to describe patients with COPD and ≥1 recorded blood eosinophil count. We described the proportion of patients with COPD who had: (1) prescriptions for inhaled triple therapy, (2) blood eosinophil count ≥150 cells/µL, and (3) ≥2 moderate/≥1 severe exacerbation in the previous year. Patients who met these criteria were referred to as having the study phenotype. These patients had an increased rate of exacerbations, GP visits and non-COPD-related unscheduled hospitalizations, and death compared with those who did not have the study phenotype.
What do these results mean?
Patients with COPD who continue to experience exacerbations despite receiving the recommended treatment and have high blood eosinophil levels have a high clinical burden of illness. These patients could benefit from more effective management programs.
Introduction
High clinical burden of illness in COPD is well recognized. Acute exacerbations of COPD (AECOPDs) are key drivers of morbidity and mortality among patients with COPD.1–3 Patients experiencing frequent exacerbations have significantly worse health-related quality of life compared with those experiencing infrequent exacerbations,4 and each hospitalized AECOPD can lead to a substantial decline in lung function.5 In addition, patients experiencing hospitalized AECOPDs have a higher risk of mortality than those who do not experience this event.6,7
Pharmacological and non-pharmacological therapies for COPD aim to alleviate symptoms and reduce the risk and severity of exacerbations; indeed, studies based on healthcare data have shown that continued COPD exacerbations are the most significant drivers of treatment escalation in the clinic.8–11In recognition of the significant proportion of patients with COPD who continue to experience frequent exacerbations, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) report has extended its recommendations to include treatment escalation strategies.1 Since 2017, GOLD has recommended escalation to triple therapy comprising inhaled corticosteroid (ICS)/long-acting β2-agonist (LABA)/long-acting muscarinic antagonist (LAMA) for patients with COPD who continue to experience persistent symptoms or further exacerbations despite treatment with ICS/LABA or LABA/LAMA dual therapy. However, it has been reported that approximately 30–50% of the patients receiving ICS/LABA/LAMA triple therapy continue to experience frequent or hospitalized exacerbations.12–17
As such, there is a substantial unmet need for more effective therapeutic options for patients who continue to exacerbate despite receiving ICS/LABA/LAMA triple therapy. Recent studies have demonstrated that, like asthma, COPD is a heterogeneous condition comprising several different phenotypes; therefore, patients may benefit from more tailored therapeutic approaches that are based on particular phenotypic or endotypic traits.18 For example, a subset of patients with COPD demonstrate increased eosinophilic airway inflammation during exacerbations.19 Furthermore, peripheral blood eosinophil counts of ≥150 cells/µL have been shown to be associated with an increased risk of AECOPDs.19–21 Importantly, patients with increased blood eosinophil counts have a greater probability of benefit from treatment with ICS13,22–24 and treatments that specifically target eosinophilic inflammation.25
The aims of this study were to describe clinical burden of illness in patients with COPD at high risk of exacerbations while receiving ICS/LABA/LAMA multiple-inhaler triple therapy (MITT) and blood eosinophils ≥150 cells/µL and compare these outcomes in patients with COPD without this phenotype.
Materials and methods
Study design and data source
This was a retrospective cohort study conducted using electronic medical records from the UK Clinical Practice Research Datalink (CPRD). The CPRD database contains anonymized, longitudinal primary care medical records of patients registered with contributing general practitioners.26 Information contained within the database includes patient registration information and demographics, prescriptions issued in primary care, clinical events, immunizations, referrals to specialists and secondary care settings, lifestyle information, and date of death.26 Primary care data from CPRD were linked to secondary healthcare data from Hospital Episode Statistics (HES) in England, producing a linked dataset (hereafter referred to as CPRD-HES) with comprehensive, fully de-identified, patient-level primary and secondary care data. The study was approved by the CPRD Independent Scientific Advisory Committee on January 26, 2017 (protocol identifier: 16_295).
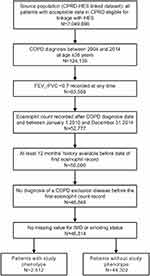
An overview of the study design is provided in Figure S1. Eligible patients were aged ≥35 years with a validated COPD medical diagnosis code27 recorded between January 1, 2004 and December 31, 2014, and a forced expiratory volume in 1 s/forced vital capacity <0.7 at any time. Additionally, patients were required to have a qualifying peripheral blood eosinophil count recorded between January 1, 2010 and December 31, 2014, defined as an eosinophil count record that occurred on or after the date of COPD diagnosis, contained at least one non-missing result, and had ≥12 months of patient clinical history preceding the record. The date of the first qualifying eosinophil count was defined as the patient’s index date. Patients were followed from their index date to the first of: end of study (December 31, 2015); patient death; patient left the general practitioner (GP) practice; or, the GP practice stopped providing data to CPRD.
Patients were excluded from the study if they had <365 days up-to-standard data available in CPRD prior to the index date (earliest qualifying eosinophil count) or a diagnosis of a condition that either severely modifies the natural course of COPD or where COPD could be a secondary diagnosis, such as fibrotic processes (idiopathic, cystic), lung resection, and/or transplantation or lung congenital malformations.
Definition of the study phenotype
From the pool of eligible patients identified from the CPRD-HES database, a subgroup of patients who had the study phenotype of interest were selected. The study phenotype was defined as having ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, plus current prescriptions for MITT on the index date, plus a peripheral blood eosinophil count of ≥150 cells/µL recorded on the index date. The cut point of ≥150 cells/µL is consistent with that used in other published studies.28–30 A moderate AECOPD was defined based on a validated algorithm using a combination of recorded respiratory symptoms, exacerbation or lower respiratory tract infection diagnoses, and prescriptions for oral corticosteroids and/or antibiotics recorded in CPRD data only.31 A severe AECOPD was defined as a hospitalized exacerbation, identified using HES inpatient data.32 MITT use was defined as overlapping prescriptions of a LAMA combined with an ICS and a LABA, delivered via two or three devices. Patients who did not meet all the above criteria were considered not to have the study phenotype.
Outcomes
Clinical burden of illness was assessed using the following outcomes: rate of moderate/severe AECOPD, rate of severe AECOPD, and rate of all-cause mortality. Health-Care Resource Utilization (HCRU) outcomes included primary (GP) clinical consultations and non-AECOPD-related uncheduled hospitalizations.
Sample size and statistical analysis
Based on an expected sample size of 43,200 with 2.8% (approximately 1200) patients with the study phenotype and an average follow-up of 1 year, the analysis had >90% power to detect rate/hazard ratios of 1.2–2.0 for all outcomes except for severe AECOPD (>90% to detect rate ratios of 1.4 or greater) and all-cause mortality (>90% power to detect hazard ratios of 1.4 or >80% power to detect hazard ratios of 1.8 or greater, depending on baseline mortality).
With the exception of all-cause mortality, rate ratios for all outcomes were estimated using negative binomial regression. For all-cause mortality, hazard ratios were estimated using Cox proportional hazards regression. For all HCRU outcomes, annualized rates were calculated using the subset of patients with ≥12 months of follow-up after their index date. The following variables were included as pre-specified covariates in all models: age; sex; ethnicity; region; social deprivation (as measured by the Index of Multiple Deprivation); month of index date; body mass index (BMI) group; smoking status; number of primary care consultations in the 12 months prior to the index date; prior comorbidity including depression, anxiety, gastroesophageal reflux disease, lung cancer, acute myocardial infarction, congestive heart failure, and stroke. These variables are potential confounders and effect modifiers in the comparison of those patients with and without the study phenotype. Furthermore, patients without the study phenotype would be expected to have a milder course of COPD than those with the study phenotype based on the qualifying criteria. For this reason, variables directly related to COPD clinical severity were not included as covariates in any of the models, to allow for quantification of real differences between those patients with the study phenotype and those without.
Two sensitivity analyses were conducted to assess the strength of the study phenotype definition. In each sensitivity analysis, one defining criterion of the study phenotype was adjusted, while the other two were kept constant. In the first sensitivity analysis, the peripheral blood eosinophil count required on the index date was increased from ≥150 cells/µL to ≥300 cells/µL (study phenotypeS1). This sensitivity analysis was conducted to address the fact that in published literature various thresholds for blood eosinophils have been explored as markers of COPD disease activity and therapeutic response. In a second sensitivity analysis, patients were required to have continuous MITT use for ≥12 months prior to the index date, instead of current MITT use on the index date (study phenotypeS2), to exclude any cases where MITT was initiated shortly prior to the index date.
Results
Patient population
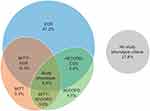
Of the 7.05 million patients included within the CPRD-HES dataset at the time of analysis, 46,814 were eligible for inclusion in this study, and 2512 (5.4%) met the definition of the study phenotype (Figure S2). Overall, 10,225 patients (21.8%) did not meet any of the study phenotype criteria (ie, did not have ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, a current prescription for MITT on the index date, or a peripheral blood eosinophil count of ≥150 cells/µL). A total of 11,269 patients (24.1%) were currently treated with MITT on their index date, 9937 patients (21.2%) experienced ≥2 moderate or ≥1 severe exacerbations in the 12 months prior to their index date, and 30,583 patients (65.3%) had blood eosinophils ≥150 cells/µL on their index date. Notably, of the 11,269 patients with current MITT use on the index date, 3920 (34.8%) also experienced frequent exacerbations in the prior year. The proportions of patients who fulfilled each combination of the three study phenotype criteria are shown in Figure 1.
Age and gender distributions were similar across the patients with and without the study phenotype (Table 1). Overall, patients with the study phenotype experienced more severe COPD based on a distribution of GOLD 2017 groups and their components but had similar levels of comorbidities and smoking status as patients without the study phenotype (Table 1).
 | Table 1 Baseline demographic and clinical characteristics of patients with and without the study phenotype |
Clinical burden of illness outcomes
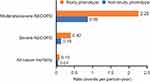
Mean follow-up time was similar for patients with and without the study phenotype criteria (2.3 years vs 2.5 years, respectively). The rates of all clinical burden of illness outcomes were higher in patients with the study phenotype, versus those without. The crude rate of moderate/severe AECOPDs in patients with the study phenotype was 2.29 events per person-year, compared with 0.89 events per person-year in patients without the study phenotype. Similarly, crude rates of severe AECOPDs and all-cause mortality were higher among patients with the study phenotype, compared with those without (0.42 vs 0.15 events per person-year and 0.10 vs 0.07 events per person-year, respectively; Figure 2).
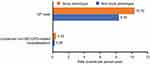
After adjusting for potential confounders, patients with the study phenotype were found to have an increased rate ratio (95% CI) of 2.32 (2.22, 2.43) for moderate/severe AECOPDs, and 2.34 (2.15, 2.55) for severe AECOPDs, compared with patients without the study phenotype (Figure 3A). Additionally, patients with the study phenotype had an adjusted hazard ratio (95% CI) for all-cause mortality of 1.26 (1.16, 1.37), compared with patients without the study phenotype (Figure 3A).
HCRU outcomes
For HCRU analysis, the study population was limited to 36,780 patients who had at least 12 months of follow-up data after their index date. Of these, 660 had the study phenotype and 36,120 did not. Compared with the primary study population, no meaningful differences in the characteristics of this subpopulation were observed. Crude rates of GP visits and unplanned non-AECOPD-related hospitalizations were higher in patients with the study phenotype compared with patients without the study phenotype (10.32 vs 8.35 events per person-year, and 0.42 vs 0.26 events per person-year, respectively; Figure S3). In addition, adjusted rates of both HCRU outcomes were significantly higher among patients with the study phenotype versus those without, with a 9% increased rate of GP visits and a 31% increased rate of non-AECOPD-related unscheduled hospitalizations (Figure S4A).
Sensitivity analysis
When the blood eosinophil count threshold used to define the study phenotype was increased from ≥150 cells/µL to ≥300 cells/µL (study phenotypeS1), the number of patients who met the definition of the study phenotype decreased to 1271 (2.7%). Baseline characteristics such as age and gender were similar in both the primary study phenotype and study phenotypeS1 populations. The adjusted rate ratios and hazard ratios (95% CI) of moderate/severe AECOPDs, severe AECOPDs and all-cause mortality in patients with vs without the study phenotypeS1 were 2.30 (2.15, 2.45), 2.25 (2.01, 2.53), and 1.21 (1.07, 1.36), respectively (Figure 3B). This increased disease burden in those patients with the study phenotype is consistent with the primary analysis.
When the MITT criterion used to define the study phenotype was changed from current MITT use on the index date to continuous MITT use for ≥12 months preceding the index date (study phenotypeS2), the size of the population that fulfilled all three study phenotype criteria decreased to 851 patients (1.8%). Again, baseline characteristics were broadly similar between the primary study phenotype population and the study phenotypeS2 population, with the exception that the latter had a slightly higher proportion of patients in GOLD group D within 24 months prior to the index date (61.9% vs 67.8%). Overall, patients meeting the definition of study phenotypeS2 experienced marginally higher rates of AECOPDs and all-cause mortality (within 10% relative difference) compared with patients meeting the primary study phenotype definition (Figure 3C).
The relative rates of all HCRU outcomes were similar when using the primary study phenotype, study phenotypeS1ʹ and study phenotypeS2 definitions (Figure S4).
Discussion
This study investigated the clinical burden of illness among patients with severe eosinophilic COPD, defined here as patients who had ≥2 moderate or ≥1 severe AECOPD in the 12 months prior to the index date, and were receiving MITT and had a peripheral blood eosinophil count ≥150 cells/µL on the index date. This is one of the first studies to characterize this patient population and report the clinical burden of illness among patients with this COPD phenotype, based on information from a real-world database.
From a large cohort of patients (N=46,814), 5.4% fulfilled all three criteria used to define the study phenotype. The rates of all outcomes were substantially higher in the study phenotype population than in patients without the study phenotype; however, patient demographics, lifestyle characteristics, and comorbidities were similar between the two patient populations. Patients with the study phenotype also had a more than two-fold increase in the rate of moderate/severe AECOPDs and a higher hazard ratio for all-cause mortality, compared with patients who did not meet all three study phenotype criteria. Sensitivity analyses in which either the eosinophil threshold required on the index date was increased to ≥300 cells/µL or the MITT criterion was altered to require ≥12 months continuous MITT use prior to the index date, produced similar results to the primary analysis. A higher rate of study outcomes in the population with the study phenotype was expected and driven by the definition of these traits, namely history of exacerbations. A recent study also reported that patients with COPD who had an eosinophil count >150 cells/µL had higher all-cause and COPD-related healthcare costs than those with an eosinophil count <150 cells/µL.33 Taken together, these results highlight the high clinical burden of illness among patients with this phenotype and suggest that these individuals have a more severe disease course than other patients with COPD who do not meet the study phenotype criteria. There is need for a consistently identifiable and treatable phenotypic trait that will allow physicians to identify patients at increased risk of frequent COPD exacerbations and poor outcomes and provide appropriate therapeutic interventions.
In the present study, no substantial differences were observed in demographic and lifestyle characteristics between patients with and without the study phenotype. This is consistent with a prior case–control study, in which no significant differences were observed in demographic characteristics between patients with and without frequent COPD exacerbations.34 In contrast, other studies characterizing the COPD frequent exacerbator phenotype have reported that female gender, lower BMI, and severe airflow limitation are associated with more frequent COPD exacerbations.35–37 Notably, several studies have previously reported that a history of exacerbations is a significant predictor of future exacerbations.35,37–39 Moreover, elevated eosinophil counts have also been associated with an increased risk of COPD exacerbations in a primary care database analysis,35 and in two post hoc analyses of clinical trials in COPD.23,24 Based on the criteria used in our study, we would expect those patients with the study phenotype to have an increased clinical burden of illness compared with those without, and our results show that this is indeed the case. Based on this and other studies, it seems likely that a history of prior exacerbations plus elevated peripheral blood eosinophil counts could serve as a strong indicator of increased risk for future exacerbations.
The present study had several strengths that increase the clinical relevance of the results. First, the CPRD reflects a representative sample of patients with recognized disease and complete medical care records provided by the UK National Health Service.26 As such, the results of this study have high generalizability within the UK. Second, validated algorithms with high sensitivity and positive predictive values were used to identify patients with COPD and AECOPDs.27,31 Lastly, we conducted sensitivity analyses employing either a more stringent blood eosinophil count threshold or a stricter definition of MITT use. Both sensitivity analyses produced similar results to the primary analysis, indicating that patients with blood eosinophil counts ≥150 cells/µL have similar burden of disease to those with counts ≥300 cells/µL and that patients were generally receiving long-term MITT. Overall, the consistency in the results for the primary analysis population and sensitivity analysis populations demonstrates durability of the phenotype described.
Despite these strengths, there were several limitations inherent to the study design. First, as all patients were required to have at least one blood eosinophil count recorded, many patients within the CPRD-HES database were not eligible for inclusion in this study. However, a recent report demonstrated that the presence of an eosinophil count record in the CPRD database is not associated with any significant trait among patients with COPD.29 Second, a single blood eosinophil record was used to determine level of eosinophils. This was a pragmatic decision, given blood eosinophil counts are infrequently recorded with long gaps between records, and supported by good stability in this primary care population as reported previously.30 Moreover, blood eosinophil count records may not have been representative of steady-state counts, as they may have been recorded at the time of acute infection or AECOPD, or during use of antibiotics, ICS, or SCS. As such, patients may have been misclassified as having elevated blood eosinophil counts, or vice versa, although the impact of this limitation should have been minimized by the large cohort size and the fact that primary care data were analyzed (which are mostly recorded during routine care). Third, as ascertainment of patients with COPD, AECOPDs, and MITT use was based on pre-specified coding lists and algorithms, there was potential for misclassification of these events. This issue was mitigated by using previously validated code lists and strategies reviewed by a clinician. Finally, although CPRD contains near-complete records of primary care prescribing, information on dispensing, adherence, and secondary care prescribing is lacking, which may have resulted in misclassification of MITT use.
Conclusion
This study has described a severe eosinophilic phenotype that is present in a small proportion of patients with COPD and have a high risk of adverse disease outcomes. Our findings demonstrate that these patients represent a substantial clinical burden and continue to frequently exacerbate or experience hospital admissions despite receiving ICS/LABA/LAMA triple therapy. As such, these patients may benefit from treatment with additional interventions that specifically target their phenotypic traits.
Ethics approval and informed consent
CPRD’s processes have been reviewed by the Confidentiality Advisory Group (CAG) and approved by the Health Research Authority (HRA) and Secretary of State to process patient identifiable information without consent under Regulation 5 of the Health Service (Control of Patient Information) Regulations 2002. This effectively removes the obligation to obtain patient consent for the use of confidential patient information for conducting purely observational research using CPRD databases and associated linked datasets. The study was approved by the CPRD Independent Scientific Advisory Committee on January 26, 2017 (protocol identifier: 16_295).
Data availability
GSK makes available anonymized individual participant data and associated documents from interventional clinical studies which evaluate medicines, upon approval of proposals submitted to
Acknowledgments
This study was funded by GSK (GSK ID: 207323; PRJ2647). The authors would like to thank Victoria Benson and Joe Maskell for their contributions to data analyses for this study. Editorial support (in the form of writing assistance, including development of the initial draft from the study report based on author input, assembling tables and figures, collating authors comments, grammatical editing and referencing) was provided by Natasha Dean, MSc, at Fishawack Indicia Ltd, UK, and was funded by GSK. This study was funded by GSK (GSK ID number: 207323; PRJ2647).
Author contributions
All authors contributed to the conception and design of the study, and analysis and interpretation of the data. All authors were involved in critically revising the manuscript, approved the final version to be published, and agree to be accountable for the accuracy and integrity of the work.
Disclosure
All authors are employees of and hold stocks/shares in GSK. Dr Hana Müllerová reports non-financial support from GSK during the conduct of the study, was an employee of GSK, and owns shares and stock options of GSK. Dr Wilhelmine Meeraus reports non-financial support from GSK during the conduct of the study, is an employee of GSK. Dr Dmitry Galkin reports not-financial support from GSK during the conduct of the study, is an employee of GSK, owns GSK stock and shares and is a GSK employee. Dr Frank Albers reports non-financial support from GSK during the conduct of the study and is an emplyee of GSK. Dr Sarah Landis reports non-financial support from GSK during the conduct of the study.
References
1. From the global strategy for the diagnosis, management, and prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). 2018. Available from:
2. Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1418–1422. doi:10.1164/ajrccm.157.5.9709032
3. Soler-Cataluna JJ, Martinez-Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi:10.1136/thx.2005.040527
4. Mullerova H, Gelhorn H, Wilson H, et al. St George’s respiratory questionnaire score predicts outcomes in patients with COPD: analysis of individual patient data in the COPD biomarkers qualification consortium database. Chronic Obstr Pulm Dis. 2017;4(2):141–149.
5. Dransfield MT, Kunisaki KM, Strand MJ, et al. Acute exacerbations and lung function loss in smokers with and without chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;195(3):324–330. doi:10.1164/rccm.201701-0150WS
6. Mullerova H, Maselli DJ, Locantore N, et al. Hospitalized exacerbations of COPD: risk factors and outcomes in the ECLIPSE cohort. Chest. 2015;147(4):999–1007. doi:10.1378/chest.14-0655
7. Serra-Picamal X, Roman R, Escarrabill J, et al. Hospitalizations due to exacerbations of COPD: A big data perspective. Respir Med. 2018. doi:10.1016/j.rmed.2018.01.008
8. Hurst JR, Dilleen M, Morris K, Hills S, Emir B, Jones R. Factors influencing treatment escalation from long-acting muscarinic antagonist monotherapy to triple therapy in patients with COPD: a retrospective THIN-database analysis. Int J Chron Obstruct Pulmon Dis. 2018;13:781–792. doi:10.2147/COPD.S153655
9. Wurst KE, Punekar YS, Shukla A. Treatment evolution after COPD diagnosis in the UK primary care setting. PLoS One. 2014;9(9):e105296. doi:10.1371/journal.pone.0105296
10. Jones SE, Barker RE, Nolan CM, Patel S, Maddocks M, Man WDC. Pulmonary rehabilitation in patients with an acute exacerbation of chronic obstructive pulmonary disease. J Thorac Dis. 2018;10(Suppl 12):S1390–S1399. doi:10.21037/jtd.2018.03.18
11. Washko GR, Fan VS, Ramsey SD, et al. The effect of lung volume reduction surgery on chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;177(2):164–169. doi:10.1164/rccm.200708-1194OC
12. Frith PA, Thompson PJ, Ratnavadivel R, et al. Glycopyrronium once-daily significantly improves lung function and health status when combined with salmeterol/fluticasone in patients with COPD: the GLISTEN study, a randomised controlled trial. Thorax. 2015;70(6):519–527. doi:10.1136/thoraxjnl-2014-206670
13. Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680. doi:10.1056/NEJMc1711583
14. Siler TM, Kerwin E, Singletary K, Brooks J, Efficacy CA. Safety of umeclidinium added to fluticasone propionate/salmeterol in patients with COPD: results of two randomized, double-blind studies. Copd. 2016;13(1):1–10. doi:10.3109/15412555.2015.1084613
15. Siler TM, Kerwin E, Sousa AR, Donald A, Ali R, Church A. Efficacy and safety of umeclidinium added to fluticasone furoate/vilanterol in chronic obstructive pulmonary disease: results of two randomized studies. Respir Med. 2015;109(9):1155–1163. doi:10.1016/j.rmed.2015.06.006
16. Sousa AR, Riley JH, Church A, Zhu CQ, Punekar YS, Fahy WA. The effect of umeclidinium added to inhaled corticosteroid/long-acting beta2-agonist in patients with symptomatic COPD: a randomised, double-blind, parallel-group study. NPJ Prim Care Respir Med. 2016;26:16031. doi:10.1038/npjpcrm.2016.31
17. Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet (London, England). 2017;389(10082):1919–1929. doi:10.1016/S0140-6736(17)30188-5
18. Agusti A, Bel E, Thomas M, et al. Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J. 2016;47(2):410–419. doi:10.1183/13993003.01359-2015
19. Saha S, Brightling CE. Eosinophilic airway inflammation in COPD. Int J Chron Obstruct Pulmon Dis. 2006;1(1):39–47.
20. Bafadhel M, McKenna S, Terry S, et al. Acute exacerbations of chronic obstructive pulmonary disease: identification of biologic clusters and their biomarkers. Am J Respir Crit Care Med. 2011;184(6):662–671. doi:10.1164/rccm.201104-0597OC
21. Landis SH, Wurst K, Le HV, Bonar K, Punekar YS. Can assessment of disease burden prior to changes in initial COPD maintenance treatment provide insight into remaining unmet needs? A Retrospective database study in UK primary care. Copd. 2017;14(1):80–85. doi:10.1080/15412555.2016.1240159
22. Barnes NC, Sharma R, Lettis S, Calverley PM. Blood eosinophils as a marker of response to inhaled corticosteroids in COPD. Eur Respir J. 2016;47(5):1374–1382. doi:10.1183/13993003.01370-2015
23. Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Resp Med. 2015;3(6):435–442. doi:10.1016/S2213-2600(15)00106-X
24. Siddiqui SH, Guasconi A, Vestbo J, et al. Blood eosinophils: a biomarker of response to extrafine beclomethasone/formoterol in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(4):523–525. doi:10.1164/rccm.201502-0235LE
25. Pavord ID, Chanez P, Criner GJ, et al. Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med. 2017;377(17):1613–1629. doi:10.1056/NEJMoa1708208
26. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol. 2015;44(3):827–836. doi:10.1093/ije/dyv069
27. Quint JK, Mullerova H, DiSantostefano RL, et al. Validation of chronic obstructive pulmonary disease recording in the Clinical Practice Research Datalink (CPRD-GOLD). BMJ Open. 2014;4(7):e005540. doi:10.1136/bmjopen-2014-005540
28. Bafadhel M, McCormick M, Saha S, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83(1):36–44. doi:10.1159/000330667
29. Landis S, Suruki R, Maskell J, Bonar K, Hilton E, Demographic CC. Clinical characteristics of COPD patients at different blood eosinophil levels in the UK clinical practice research datalink. Copd. 2018;15(2):177–184. doi:10.1080/15412555.2018.1441275
30. Landis SH, Suruki R, Hilton E, Compton C, Galwey NW. Stability of blood eosinophil count in patients with COPD in the UK clinical practice research datalink. Copd. 2017;14(4):382–388. doi:10.1080/15412555.2017.1313827
31. Rothnie KJ, Mullerova H, Hurst JR, et al. Validation of the recording of acute exacerbations of COPD in UK primary care electronic healthcare records. PLoS One. 2016;11(3):e0151357. doi:10.1371/journal.pone.0151357
32. Rothnie KJ, Mullerova H, Thomas SL, et al. Recording of hospitalizations for acute exacerbations of COPD in UK electronic health care records. Clin Epidemiol. 2016;8:771–782. doi:10.2147/CLEP.S89480
33. Ortega H, Llanos JP, Lafeuille MH, et al. Burden of disease associated with a COPD eosinophilic phenotype. Int J Chron Obstruct Pulmon Dis. 2018;13:2425–2433. doi:10.2147/COPD.S170995
34. Wan ES, DeMeo DL, Hersh CP, et al. Clinical predictors of frequent exacerbations in subjects with severe chronic obstructive pulmonary disease (COPD). Respir Med. 2011;105(4):588–594. doi:10.1016/j.rmed.2011.03.020
35. Kerkhof M, Freeman D, Jones R, Chisholm A, Price DB,
36. McGarvey L, Lee AJ, Roberts J, Gruffydd-Jones K, McKnight E, Haughney J. Characterisation of the frequent exacerbator phenotype in COPD patients in a large UK primary care population. Respir Med. 2015;109(2):228–237. doi:10.1016/j.rmed.2014.12.006
37. Mullerova H, Shukla A, Hawkins A, Quint J. Risk factors for acute exacerbations of COPD in a primary care population: a retrospective observational cohort study. BMJ Open. 2014;4(12):e006171. doi:10.1136/bmjopen-2014-006171
38. Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi:10.1056/NEJMoa1011205
39. Zeiger RS, Tran TN, Butler RK, et al. Relationship of blood eosinophil count to exacerbations in chronic obstructive pulmonary disease. J Allergy Clin Immunol Pract. 2018;6(3):944–954.e945. doi:10.1016/j.jaip.2017.10.004
Supplementary materials
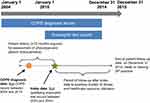 | Figure S1 Overview of study design. Abbreviation: GP, general practitioner. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.