Back to Journals » Clinical Audit » Volume 16
Clinical Audit of Non-Selective Beta Blockers Titration in the Management of Portal Hypertension at a National Hospital in Tanzania
Authors Pazi SK, Mwanga AH, Lyuu TA, Ng’wanasayi M, Rwegasha J, Komba EV, Nkandala I
Received 13 March 2023
Accepted for publication 10 July 2023
Published 18 April 2024 Volume 2024:16 Pages 39—44
DOI https://doi.org/10.2147/CA.S412250
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zoka Milan
Swaleh Kitabu Pazi,1 Ally Hamisi Mwanga,2 Tuzo A Lyuu,1 Masolwa Ng’wanasayi,3 John Rwegasha,1 Ewaldo Vitus Komba,4 Igembe Nkandala5
1Department of Internal Medicine, Muhimbili National Hospital, Dar es Salaam, Tanzania; 2Department of Surgery, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; 3Department of Internal Medicine, Aga Khan Hospital, Dar es Salaam, Tanzania; 4Department of Internal Medicine, Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania; 5Department of Internal Medicine, Catholic University of Health and Allied Sciences, Mwanza, Tanzania
Correspondence: Igembe Nkandala, Department of Internal Medicine, Catholic University of Health and Allied Sciences, P.O Box 1464, Mwanza, Tanzania, Tel +255 717 301956, Email [email protected]
Background: Portal hypertension – a major complication of chronic liver disease – is associated with multiple complications that include ascites, varices, and hepatic encephalopathy; these complications can lead to substantial morbidity and mortality. Randomized control trials have demonstrated the efficacy of nonselective beta blockers (NSBB) for preventing primary and secondary gastroesophageal variceal bleeding. These drugs should be titrated to target the resting heart rate of 55– 60 beats per minute and the systolic blood pressure should not decrease < 90 mm Hg. The objective of this study was to perform an audit of the titration of nonselective beta blockers in patients with portal hypertension at the national referral hospital.
Methods: The audit involved all adults aged 18 years and above with portal hypertension and evidence of esophageal varices who were regularly attending gastroenterology and hepatology clinics of Muhimbili National Hospital between January 2019 and December 2019. The patients’ clinical data were extracted from the electronic medical records. Permission to conduct the study was obtained from the hospital management.
Results: Over the audit period, a total of 151 patients with esophageal varices who attended gastroenterology and hepatology clinics were included. The mean age of the patients was 42.4 years and males accounted for 61% of the cohort. About 90% (136/151) of patients attended the clinic more than three times; 92% (139/151) had an unchanged dose of NSBB for at least two consecutive visits and 60.2% (91/151) were admitted in subsequent visits due to variceal bleeding. Critically, the records showed that 100% of patients did not have their pulse rate and blood pressure recorded.
Conclusion: The audit results indicated a low rate of titration of NSBB and poor recording of pulse pressure and blood pressure. It was recommended that NSBB should be appropriately titrated with corresponding precautions taken and the patients’ systolic blood pressures and pulse rates should be recorded.
Keywords: esophageal varices, periportal fibrosis, cirrhosis
Introduction
Portal hypertension (PH) is a clinical syndrome defined by a portal venous pressure gradient exceeding 5 mm Hg; when this pressure gradient is >10 mm Hg, it is called clinically significant portal hypertension (CSPH).1
PH is the most important predictor of variceal bleeding in patients with liver cirrhosis.2,3 The estimated prevalence of esophageal varices in patients with cirrhosis is 50%, and their presence is correlated with the severity of liver disease and the presence of CSPH.4 Bleeding from ruptured varices is a serious medical emergency, especially in patients with poor liver function test (Child-Pugh score C).1,5 The 6-week mortality rate from variceal bleeding ranges from 20% to 35%.6 In a retrospective study in northwestern Tanzania, esophageal varices were present in 51% of adults who underwent endoscopic examination following upper gastrointestinal bleeding;7 in a subsequent study in the same setting, it was found that esophageal varices are the most common cause of bleeding among patients admitted for gastrointestinal bleeding.8
Randomized control trials have demonstrated the efficacy of NSBB in preventing primary and secondary gastroesophageal variceal bleeding.9–12 Therefore, traditional NSBB such as propranolol and nadolol are indicated for primary and secondary prophylaxis of variceal bleeding.5 RCTs have also demonstrated that NSBB titration is the best clinical approach recommended with a target pulse rate above 50−60 beats/minutes and SBP more than 90 mmHg.4,5,13 Regular checking of the blood pressure and pulse rate is critical in patients with portal hypertension in order to determine the efficacy and safety of the NSBB prophylaxis.3,14 The extent to which routine practice of care of patients with portal hypertension at Muhimbili National Hospital complies with 2015 Baveno VI5 guidelines and the 2016 Practice Guidance by the American Association for the Study of Liver Diseases13 is not known. This clinical audit was performed to study the titration of NSBB in patients with portal hypertension and varices who were attending Muhimbili National Hospital.
Methodology
Study Design
Hospital based clinical audit.
Study Site
The study was conducted at Muhimbili National Hospital.
Study Population
The study included all adults (aged 18 years and above) with portal hypertension with medium-to-large varices or prior history of variceal bleeding who were attending the gastroenterology and the hepatology clinics at Muhimbili National Hospital. Audit population comprised patients who had been followed up for a period of 12 months between January 2019 and December 2019. There were no exclusion criteria.
Data Collection Methods
Patients with portal hypertension and esophageal varices were identified from the gastroenterology and the hepatology clinics database. Patients were enrolled in the study after meeting the inclusion criteria. A standard checklist was used to obtain the patient’s information with regard to the following information: age, gender, insurance status, date of initial diagnosis, systolic blood pressure, pulse rate, dose of propranolol prescribed and duration of follow-up.
Data Entry Analysis
Data were checked for completeness and consistency, and errors or discrepancies found were promptly corrected. Microsoft Excel was used to enter and analyze the data. The data were expressed as medians and interquartile ranges or means ± standard deviations. Categorical data are expressed as percentages.
Ethical Consideration
Permission to conduct the clinical audit was obtained from the management of Muhimbili National Hospital. Ethical clearance was not required because patients were not randomized into different groups, the study protocol did not require a change in treatment or patient care from usual practice, and data was collected retrospectively. All collected patient information was kept strictly confidential with strict adherence to the Declaration of Helsinki.
Results
Over the audit period, a total of 151 patients attended both gastroenterology and hepatology clinics were included, the mean age of the patients in the cohort was 42.4 years, and males accounted for 61% of the cohort. The clinical characteristics are shown in Table 1. About 90% (136/151) of patients had more than 3 visits (Figure 1). Among these, 92% (139/151) had an unchanged dose of NSBB for at least two consecutive visits (Figure 2). About 60% (91/151) of patients were subsequently admitted due to variceal bleeding (Figure 2). None of the patients had their pulse rate or blood pressure electronically recorded.
 |
Table 1 Clinical Characteristics of Audit Participants |
 |
Figure 1 Percentage of subjects whose dose was titrated (N=151). |
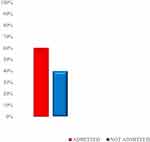 |
Figure 2 Percentage of subjects admitted for variceal bleeding during the audit period (N=151). |
Discussion
NSBBs have to be appropriately titrated in order to achieve favorable outcomes in the prevention of variceal bleeding and its complications.4,5,15 However, this clinical audit revealed the majority of patients who were diagnosed with portal hypertension and had been initiated on NSBBs had unchanged doses of the NSBBs in the subsequent visits. One possible reason for this observation is that the attending clinicians did not assess for adequacy and safety of the dose by checking the pulse rate and the blood pressure. Another possible reason is intolerance of patients to the NSBBs; however, we think this is unlikely given the high rate of unchanged dose. Another possibility is that the patients were already on the optimal dose; we think this is also unlikely as these were patients who had initiated NSBBs within a short period of the time of the audit and therefore unlikely to have started straight away on the optimal target dose without the requirement for dose titration. Therefore, the most likely explanation is the first one.
In subsequent visits, these patients had a high rate of admission due to variceal bleeding. This is most likely an outcome of the unchanged dose of NSBB in the patient’s preceding clinic visits, further demonstrating the importance of NSBBs titration in the prevention of variceal bleeding.
Moreover, this audit showed that none of these patients with portal hypertension had their blood pressure or pulse rate recorded electronically during their follow-up. Many guidelines recommend pulse rate and blood pressure recording because titration of NSBBs is dependent on the values of two parameters.4,5,11 This means that the degree of attainment of the optimal dose of NSBBs could not be determined.
The audit results were presented in the gastroenterology and hepatology unit of the hospital and it was recommended that NSBBs should be appropriately titrated with corresponding precautions taken and the patients’ systolic blood pressures and pulse rates should be recorded. A subsequent audit was also recommended to monitor the change in the clinical practice.
Acknowledgments
We would like to thank the staff at the department of information technology of Muhimbili National Hospital for assisting with access to the data.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Brunner F, Berzigotti A, Bosch J. Prevention and treatment of variceal haemorrhage in 2017. Liver Int off J Int Assoc Study Liver. 2017;37(1):104–115. doi:10.1111/liv.13277
2. Mauro E, Gadano A. What’s new in portal hypertension? Liver Int off J Int Assoc Study Liver. 2020;40(1):122–127. doi:10.1111/liv.14366
3. Bhardwaj A, Kedarisetty CK, Vashishtha C, et al. Carvedilol delays the progression of small oesophageal varices in patients with cirrhosis: a randomised placebo-controlled trial. Gut. 2017;66(10):1838–1843. doi:10.1136/gutjnl-2016-311735
4. Garcia-Tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65(1):310–335. doi:10.1002/hep.28906
5. Kamath PS, Mookerjee RP. Individualized care for portal hypertension: not quite yet. J Hepatol. 2015;63(3):543–545. doi:10.1016/j.jhep.2015.05.022
6. Takuma Y, Nouso K, Morimoto Y, et al. Measurement of spleen stiffness by acoustic radiation force impulse imaging identifies cirrhotic patients with esophageal varices. Gastroenterology. 2013;144(1):92–101.e2. doi:10.1053/j.gastro.2012.09.049
7. Jaka H, Koy M, Liwa A, et al. A fibreoptic endoscopic study of upper gastrointestinal bleeding at Bugando Medical Centre in northwestern Tanzania: a retrospective review of 240 cases. BMC Res Notes. 2012;5:1–9. doi:10.1186/1756-0500-5-200
8. Chofle AA, Jaka H, Koy M, et al. Oesophageal varices, schistosomiasis, and mortality among patients admitted with haematemesis in Mwanza, Tanzania: a prospective cohort study. BMC Infect Dis. 2014;14(1):1–10. doi:10.1186/1471-2334-14-303
9. Rodrigues SG, Mendoza YP, Bosch J. Beta-blockers in cirrhosis: evidence-based indications and limitations. JHEP Rep. 2020;2(1):1–17. doi:10.1016/j.jhepr.2019.12.001
10. Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2019;393(10181):1597–1608. doi:10.1016/S0140-6736(18)31875-0
11. Simonetto DA, Liu M, Kamath PS. Portal hypertension and related complications: diagnosis and management. Mayo Clin Proc. 2019;94(4):714–726. doi:10.1016/j.mayocp.2018.12.020
12. Téllez L, Ibáñez-Samaniego L, Pérez Del Villar C, et al. Non-selective beta-blockers impair global circulatory homeostasis and renal function in cirrhotic patients with refractory ascites. J Hepatol. 2020;73(6):1404–1414. doi:10.1016/j.jhep.2020.05.011
13. Garcia-tsao G, Abraldes JG, Berzigotti A, Bosch J. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American association for the study of liver diseases A. Purpose and Scope. 2017;65(1):310–335. doi:10.1002/hep.28906
14. Mahassadi AK, Bathaix FY, Assi C, et al. Usefulness of noninvasive predictors of oesophageal varices in black African cirrhotic patients in Côte d’Ivoire (West Africa). Gastroenterol Res Pract. 2012;2012. doi:10.1155/2012/216390
15. Seo YS. Prevention and management of gastroesophageal varices. Clin Mol Hepatol. 2018;24(1):20–42. doi:10.3350/cmh.2017.0064
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
