Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 15
Clinical Assessment and Management in Improving the Quality of Life of HIV/AIDS Patients with Oral Candidiasis: A Case Series
Authors Novianti Y , Sufiawati I
Received 1 September 2023
Accepted for publication 27 October 2023
Published 14 November 2023 Volume 2023:15 Pages 683—696
DOI https://doi.org/10.2147/HIV.S434175
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Olubunmi Akindele Ogunrin
Yessy Novianti,1 Irna Sufiawati2
1Oral Medicine Residency Program, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia; 2Department of Oral Medicine, Faculty of Dentistry, Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Yessy Novianti, Oral Medicine Residency Program, Faculty of Dentistry, Universitas Padjadjaran, Jalan Sekeloa Selatan I, Bandung, 40132, Indonesia, Tel +6281368429045, Email [email protected]
Introduction: Oral candidiasis is the most prevalent opportunistic infection in patients with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS), impacting their quality of life. This report aims to emphasize the importance of clinical assessment and management of HIV/AIDS patients with oral candidiasis to improve their quality of life.
Case: Five male patients, aged between 32 and 71 years, came to the HIV clinic and complained of white plaques in their mouths and painful swallowing. The World Health Organization’s (WHO) clinical staging of all patients was 4. Three patients had not yet received antiretroviral therapy (ART), and their total lymphocyte counts (TLC) of < 1.170 cells/mm3. Two patients had dropped out of ART with CD4 counts were < 40 cells/mm3. The body mass index of two patients was underweight, while the others were normal. The oral hygiene index simplified (OHI-S) of the patients was fair to poor. The quality of life assessment using the oral health impact profile 14 (OHIP-14) questionnaires before therapy showed values from 6– 20. Clinical examination defined the diagnosis as oral candidiasis, exfoliative cheilitis, oral hairy leukoplakia, and a cytomegalovirus-related ulcer.
Case Management: The patients were treated with fluconazole, 0.2% chlorhexidine gluconate mouthwash, 2% miconazole cream, diphenhydramine, and multivitamins. The oral lesions were improved within 14 days to a month of treatment, and OHIP-14 scores were significantly reduced (0– 3).
Conclusion: Clinical assessment is important in managing HIV/AIDS patients with oral candidiasis, which improves the patient’s quality of life. Therefore, routine clinical assessment and management of HIV/AIDS patients are strongly recommended.
Keywords: AIDS, HIV, oral candidiasis, quality of life
Introduction
In the domain of global health, the human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) continue to pose significant challenges, marked by their high mortality rates.1–3 The pervasive nature of these issues means that no nation is exempt from the grip of HIV/AIDS. The World Health Organization (WHO) reports that as of the close of 2021, a staggering 38.4 million people were living with HIV, representing 0.7% of the population between the ages of 15 and 49.4,5 Notably, Indonesia has witnessed an alarming surge in HIV infections, positioning it as a prominent hotspot in Asia with an estimated prevalence of 540,000 people in 2022.6
This global health concern has given rise to a range of oral manifestations closely associated with HIV infection. These include oral candidiasis, oral hairy leukoplakia (OHL), Kaposi’s sarcoma, linear gingival erythema (LGE), necrotizing ulcerative gingivitis (NUG), necrotizing ulcerative periodontitis (NUP), and non-Hodgkin’s lymphoma.7,8 Among these, oral candidiasis, commonly referred to as “candidosis” or its older name “moniliasis”, stands out as the most prevalent opportunistic fungal infection in the oral cavity of immunosuppressed individuals due to HIV/AIDS.1,9,10 Its clinical presentation encompasses various patterns, including pseudomembranous, erythematous, hyperplastic, and angular cheilitis. Another condition, OHL, emerges as a secondary infection linked to the Epstein–Barr virus (EBV), characterized by adherent, uniform, and at times hair-like white patches on the lateral sides of the tongue. In contrast, Kaposi sarcoma manifests as red, blue, or purple macules, papules, or nodules, predominantly developing at the junction of the hard and soft palates. LGE presents as a non-plaque-induced localized or generalized gingivitis with a distinct erythematous band of 2–3 mm along the gingival margin. NUG is identified by gingival necrosis and ulcer formation, which may progress to NUP.11
Oral health plays an integral role in assessing the general health status of people living with AIDS (PLWA).9,10 Notably, oral candidiasis emerges as one of the earliest indicators of HIV infection and a harbinger of the progression to AIDS. Its incidence has risen notably since the onset of the HIV epidemic, with ranging from 25% to 90% of HIV-infected individuals experiencing oral candidiasis.9,12–15 In severe cases, the condition can extend to the pharynx or the lungs, often leading to fatal outcomes.7,16 Its complications may impede eating and drinking, disrupt nutritional intake, cause weight loss, and negatively impact the patient’s general health.12
Within the context of healthcare, the concept of quality of life is a well-established metric, relevant across various aspects of physical and mental well-being, including oral health. One commonly used tool is the Oral Health Impact Profile 14 (OHIP-14).17–22 The 14 questions were grouped into seven functional domains: limitations, physical pain, psychological discomfort, physical disabilities, psychological disabilities, social disabilities, and handicaps, and were analyzed for each domain. The answer options for each item were: hardly ever, very often, fairly often, sometimes, and never/ do not know, and were coded as 4, 3, 2, 1, and 0.23 The total OHIP score was calculated, with a higher score indicating a greater impact on oral health-related quality of life.17 The maximum calculated score is 56, with a good category of 0–18, a moderate category of 19–37, and a poor category of 38–56.18
Clinical assessment of HIV/AIDS patients could prevent fatal complications of oral candidiasis.24 Therefore, a comprehensive clinical assessment must be done early and routinely. This report highlights the importance of clinical assessment and management for HIV/AIDS patients with oral candidiasis to improve their quality of life.
Case
Case 1
A 32-year-old male patient was referred to the HIV clinic Dr. Hasan Sadikin Hospital Bandung, complained of pain when eating and difficulty opening his mouth a week ago. He feels uncomfortable with white plaques in the oral cavity and can only consume liquid diets. Twelve years ago, he was diagnosed with HIV stage IV. He has received antiviral therapy, such as tenofovir, lamivudine, and dolutegravir (TLD), but has been off for 1.5 years. His weight is 56 kg, and his height is 168 cm. His quality of life was measured using the OHIP-14, with a score of 18 (good).
Extraoral examination revealed pruritic papular eruptions on his face, anemic conjunctiva, non-icteric sclera, and dry exfoliative lips. The corners of the left and right lips have painful, reddish fissures. The submandibular lymph nodes are palpable and painless. Intraoral examination revealed thick yellowish-white plaques on the labial mucosa, buccal mucosa, palate, dorsal tongue, ventral tongue, floor of the mouth, and upper anterior gingiva that could be scraped, leaving areas of erythema and pain. Painless white plaques with vertical striations on the lateral borders of the tongue could not be scraped off (Figure 1). Plaque and calculus were present in all regions, with an OHI-S score of 3.2 (poor).
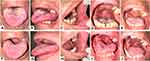
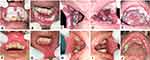 |
Figure 1 Intraoral appearance of the patient in Case 1 (A–E) There were “cottage cheese-like” lesions involving the entire oral cavity on the first visit (F–J) After two weeks, the lesions healed. |
The results of laboratory tests showed a decrease in hemoglobin, hematocrit, erythrocytes, neutrophils, and mean corpuscular hemoglobin concentration (MCHC), indicating anemia. An increase in leukocytes and white blood cells shows the presence of infection. The anti-HIV immunoserology test was reactive, and the CD4 count was below 40 cells/mm3. The laboratory test results are detailed in Table 1.
 |
Table 1 Serological Examination Result |
The clinical diagnosis of this patient was acute pseudomembranous candidiasis, angular cheilitis, and oral hairy leukoplakia. He was cured with fluconazole for two weeks, 0.2% chlorhexidine gluconate, 2% miconazole cream, and multivitamins. The lesions healed after two weeks. His weight increased to 60 kg, and the OHIP-14 score after oral treatment is 0 (good).
Case 2
A 48-year-old man was referred to the oral medicine specialist at Dr. Hasan Sadikin Hospital with chief complaints of white spots on the tongue and red patches on the palate two weeks ago. A week ago, he went to the HIV clinic complaining of itching all over his body and nails and received fluconazole and multivitamins. He was diagnosed with HIV stage IV two years ago. He had been off the antiviral drug (TLD) for 1.5 months. His weight and height are 55 kg and 171 cm, respectively. His OHIP-14 score is 6 (good).
Extraoral examination found very pale conjunctiva, non-icteric sclera, and fissures on the bilateral corners of the lips. Intraoral examination found reddish patches on the palate, labial mucosa, buccal mucosa, and dorsal tongue. In addition, there were scrapable white spots, leaving areas of erythema on the dorsal and lateral sides of the tongue (Figure 2). Plaque and calculus were observed in all regions with an OHI-S score of 3.0 (moderate).
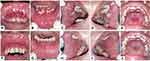
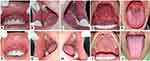 |
Figure 2 Clinical features of the patient in Case 2 (A–E) On the first visit, white spots on the dorsal tongue and erythematous areas (F–J) The lesions have improved after one month. |
The blood tests were performed, as shown in Table 1. The blood tests showed decreased hemoglobin, hematocrit, erythrocytes, leukocytes, neutrophil segments, and mean corpuscular hemoglobin concentration (MCHC), indicating inflammation. The anti-HIV immunoserology test was reactive, and the CD4 count was 39.3 cells/mm3.
Based on his medical history, clinical examination, and laboratory examination, he was diagnosed with angular cheilitis, pseudomembranous candidiasis, and acute atrophic candidiasis. The oral treatment consisted of continuing fluconazole and multivitamins and adding 2% miconazole cream and 0.12% chlorhexidine digluconate. The lesions healed on the second visit; 1 month later, his weight increased to 67 kg, and his OHIP-14 score became 0 (good).
Case 3
A 71-year-old man came to the oral medicine specialist complaining of swallowing pain, canker sores since one month ago, and white plaques on the tongue since two weeks ago, preceded by a fever. He went to the general practitioner to overcome the complaints. The white plaques have decreased, but painful swallowing and canker sores remain. He only consumes a liquid diet and drinks less than 1 liter of water daily. He was diagnosed with HIV stage IV just three days ago. He has not yet received ART but plans to get TLD soon from the HIV clinic. The anthropometric examination showed a body weight of 48 kg and a height of 165 cm. At his first visit, the OHIP-14 score was 20 (moderate).
The extraoral examination observed pale conjunctiva, non-icteric sclera, and exfoliative lips. The intraoral examination observed yellowish-white plaques that could be rubbed off with gauze, leaving erythematous zones and pain on the dorsal tongue. There was a painful single ulcer on the left buccal mucosa with a yellowish-white base surrounded by a halo erythematous, well-defined, round, about 2 mm in diameter. We also found an irregular single ulcer, about 4 cm in size, with a yellow base surrounded by erythema on the palatoglossal arch, and painful (Figure 3). Plaque and calculus were present in all regions, with an OHI-S score of 4.3 (poor).
Serological examination showed decreased hemoglobin, hematocrit, erythrocytes, neutrophil bands, lymphocytes, and basophils, indicating anemia. It also showed a reactive anti-HIV test, a reactive syphilis rapid test, and a reactive anti-CMV IgG. His total lymphocyte count (TLC) was 1210 cells/mm3. The results of the serological examination are shown in Table 1. Based on clinical and serological tests, he was diagnosed with acute pseudomembranous candidiasis, cytomegalovirus-associated ulceration, and exfoliative cheilitis. He was treated with fluconazole for two weeks, 0.2% chlorhexidine gluconate, diphenhydramine, petroleum jelly, and multivitamins. The oral lesions wholly resolved after four weeks, his weight reached 50 kg, and the OHIP-14 score decreased to 3 (good) after the treatment.
Case 4
A 32-year-old male patient was referred by the HIV clinic Dr. Hasan Sadikin Bandung to the oral medicine specialist complained of painful swallowing and ulcers on the gingiva and ventral tongue a week ago. He was diagnosed with HIV stage IV and lung tuberculosis (TB) after being hospitalized a month ago. He is 160 cm tall, weighs 47 kg, and has never had ART. The OHIP-14 score is 20 (moderate).
Extraoral examination revealed anemic conjunctiva, non-icteric sclera, and dry and exfoliative lips. Left cervical lymph nodes are palpable but not painful; multiple nodules have a hard consistency; size varies from 2–5 cm. Intraoral examination revealed white pseudomembranous on the labial mucosa, buccal mucosa, dorsal tongue, ventral tongue, the floor of the mouth, palate, and uvula. It was scrapable and left an erythematous base during removal. There was a painful single ulcer with an irregular, yellowish base and diffuse border on the right lateral tongue and left buccal mucosa (Figure 4). Plaque and calculus were present in all regions, with an OHI-S score of 3.1 (poor).
Blood test results showed decreased hemoglobin, hematocrit, erythrocytes, leukocytes, platelets, basophils, and mean corpuscular hemoglobin concentration (MCHC), indicating inflammation. Confirmatory tests performed for HIV were positive, and the total lymphocyte count was 1340 cells/mm3. A complete blood test yielded results, as seen in Table 1. The clinical diagnosis was acute pseudomembranous candidiasis, suspected tuberculous ulcers on the right lateral tongue and left buccal mucosa, and exfoliative cheilitis. He was prescribed fluconazole for two weeks, 0.2% chlorhexidine gluconate, and multivitamins. The lesions recovered after four weeks. His weight increased to 52 kg, and his OHIP-14 score is 0 (good) after treatment.
Case 5
A 38-year-old man complaining about white spots in his mouth for three weeks was referred to the Hasan Sadikin Hospital’s oral medicine specialist. He has felt uncomfortable, has had nausea while eating, a numb jaw, and swelling in the right neck since four days ago. He was diagnosed with HIV stage IV three weeks ago and has not received ART. He smoked 1–2 packs daily and quit three weeks ago. His OHIP-14 score was 18 (good), he weighed 51 kg, and he was 155 cm tall.
Extraoral examination found non-anemic conjunctiva and exfoliative dry lips. The palpable right submandibular and right cervical lymph nodes were painless single nodules of firm consistency, 1.5–2 cm in diameter, and fixed. Intraoral examination found white plaques on the labial mucosa, buccal mucosa, dorsal tongue, ventral tongue, and floor of the mouth that could be scraped and left erythematous areas and pain. There were yellowish-white plaques that could be scraped off, an erythematous area on the median palate, and pain. There were also painless white corrugated plaques, non-scrapable, with diffuse borders on the lateral tongue (Figure 5). Plaque and calculus were present in all regions with an OHI-S score of 2.8 (medium). Deep profunda caries occur at tooth 48.
Laboratory test results showed decreased hematocrit, leukocytes, segmental neutrophils, and total basophils. The anti-HIV test was reactive, and the total lymphocyte count was 1170 cells/mm3. Complete laboratory test results can be seen in Table 1. Based on clinical and laboratory examination, he was diagnosed with oral candidiasis, exfoliative cheilitis, oral hairy leukoplakia, and irreversible pulpitis tooth 48. His medication was fluconazole for two weeks, chlorhexidine gluconate 0.2%, petroleum jelly, and multivitamins. The lesions healed after four weeks. His weight increased to 57 kg, and the OHIP-14 score is 0 (good) after treatment.
All five patients in this report carried HIV/AIDS and manifested various oral candidiasis clinical characteristics. The differences between each characteristic, clinical assessment, clinical features, diagnosis, prognosis, therapy, and follow-up condition are succinctly explained in Table 2.
 |
Table 2 A Brief Comparison of Five Cases |
Discussion
Oral candidiasis is still a crucial health problem related to quality of life in HIV/AIDS patients that can lead to morbidity and mortality.25 The characteristics of oral candidiasis in HIV/AIDS patients are that it is more severe, more resistant to antifungal therapy, and more challenging to cure. HIV/AIDS-positive patients are more vulnerable to developing oral candidiasis than HIV-negative patients. It should be due to impaired barrier protection of the oral mucosa, microbiota dysbiosis, decreased salivary flow rate, and suppression of CD4+ T cells.2,26,27
The skin and mucosal membranes represent the main portal of entry for opportunistic pathogens, leading to life-threatening systemic infection in the immunocompromised host.28,29 Mucosal epithelial cells as a barrier are essential in maintaining homeostasis and protecting hosts against injuries, including from microbes.3,29–33 Impaired barrier protection mechanisms in HIV/AIDS patients may facilitate an opportunistic infection.34
HIV plays a role in oral epithelial cell barrier damage by inducing mitogen-activated protein kinase (MAPK). HIV-1 envelope glycoprotein gp120 binds to the galactosylceramide (GalCer) and chemokine receptors CCR5 and CXCR4, inducing MAPK activation. MAPK activates NF-kB signals to upregulate matrix metalloproteinase-9 (MMP-9) expression. The overexpression of MMP-9 is associated with disruption of tight and adherens junctions. On the other hand, in normal oral mucosal epithelium, MMP-9 expression is negative or weak.12,34–36 This is one of the underlying reasons fungal infections quickly occur in HIV patients.
Microbial translocation and microbiota dysbiosis are two significant microbial system alterations in HIV infection.37,38 Candida albicans may infect HIV patients through their ability to interact with the microbiota, induce dysbiosis, and evade immune system control.1,36 The predominant species causing oral candidiasis in HIV-infected individuals is Candida albicans. However, various other Candida species (Candida tropicalis, Candida parapsilosis, Candida dubliniensis, Candida famata, Candida krusei, and Candida glabrata) have also contributed.1,12,13,25,30,36,39–44 Candida dubliniensis was found relatively more often in HIV-infected individuals.45
Oral candidiasis in HIV infection has a variety of clinical patterns (pseudomembranous, erythematous, hyperplastic, and angular cheilitis), as seen in all the cases.1,46,47 In case 2, three clinical patterns of oral candidiasis, such as pseudomembranous, erythematous, and angular cheilitis, may appear in one individual. It may indicate severe immune suppression, treatment failure, and a more advanced stage of HIV disease.12 Pseudomembranous candidiasis, or thrush, is the most familiar clinical form of white plaque oral candidal infection in HIV/AIDS patients, as seen in cases 1–5.1,48,49 Erythematous candidiasis was found in patients in cases 2 and 5, with a clinical appearance of red patches or velvet-textured plaques.
Angular cheilitis (perlèche) is characterized by erythema, fissures, scaling, and crusting at the corners of the mouth, as seen in patient cases 1 and 2. It may occur as an isolated finding or as a component of chronic multifocal candidiasis. Microbiologic studies suggest that the etiology of this condition is Candida albicans and Staphylococcus aureus.41 One of the studies found that angular cheilitis and papular pruritic eruptions were external predictors of a CD4 cell count of fewer than 250 cells/mm3, which strengthens and parallels the second case.50
Oral lesions are often clearly visible, and several can be accurately diagnosed based on clinical features alone.16 All oral lesions found in HIV-positive patients may also occur in patients with other diseases associated with immunosuppression.41 In most cases, The presumptive diagnosis of intraoral candidiasis is made based on the typical clinical features and disease history. The presumptive diagnosis is strengthened if the patient responds to an empirical trial of antifungal therapy.7,8,11,51 Identification and possible resolution of local or systemic causative factors are essential for treating candidal infection. In cases where difficulty diagnosing oral candidiasis requires further investigations.13 In this case report, the oral candidiasis diagnosis is based only on clinical features.
Clinical assessment is an integral part of the diagnostic sequence.48 The importance of clinical assessment is to establish baseline data about the patient’s general health when diagnosed with HIV and before starting ART, identify opportunistic infections that need treatment, identify any other chronic conditions that may develop while a patient is on ART, and monitor the effectiveness of therapy. An accurate clinical assessment may contribute to an appropriate patient care plan and improve the patient’s quality of life. Clinical assessment variables for HIV patients include age, gender, weight, height, BMI, duration of HIV diagnosis, WHO clinical stage, antiretroviral drugs (ART), CD4 count, viral load copies, TLC, OHI-S score, and OHIP-14 score.52
Clinical assessment based on gender found that all HIV/AIDS patients with oral candidiasis in cases 1–5 were male. Oral candidiasis can affect both men and women. The highest percentage of HIV-infected patients with oral candidiasis are males compared to females. In some studies, it was observed that males predominate, with a male-to-female ratio of 1.8:1.53 More men have HIV/AIDS because of the large number of men who engage in risky sexual and injecting drug use than women, who are more likely to get it from their sexual partners.9
Clinical assessment based on age found that one patient in Case 3 was 70 years old or elderly; the others were aged 32–48 years old. Patient cases 1 and 2 had been diagnosed with HIV for a long time (2–12 years ago), while cases 3, 4, and 5 were newly diagnosed with HIV (3 days–1 month ago) and had not yet received ART. Oral candidiasis is most commonly affected in the age group of 20–45 years. The time range since a person diagnosed with HIV develops AIDS can be between 5 and 10 years. The longer the duration of being diagnosed with HIV, the higher the risk of developing oral candidiasis.53,54
Clinical assessment based on WHO clinical stages of HIV/AIDS found that five patients with oral candidiasis were at stage 4. The WHO clinical stages of HIV range from stage 1 (asymptomatic) to stage 4 (AIDS). The clinical stage of HIV/AIDS patients relates to oral candidiasis infection.47 In HIV infection, oral candidiasis may occur in stage 3 and oropharyngeal candidiasis in stage 4. Oropharyngeal candidiasis is one of the earliest manifestations of HIV-induced immunodeficiency and usually affects most people with advanced HIV infection who are not treated. The proportion of HIV/AIDS patients with oral candidiasis based on clinical stage was highest in patients with end-stage (stage 4).9
Clinical assessment based on antiretroviral drugs (ART) found that two patients had dropped out of ART for 1.5 years and 1.5 months, respectively. In comparison, three patients have never received ART. Oral candidiasis is significantly correlated with ART use and immune suppression.12 The prevalence of oral candidiasis may increase in HIV patients who have not received ART or have stopped taking ART. The use of ART has been reported to significantly reduce the prevalence of oral lesions and opportunistic illnesses associated with HIV and mortality.55
Clinical assessment based on CD4+ count found that two patients had CD4+ counts below 40 cells/mm3, while three patients had not had their CD4+ count tested but had their total lymphocyte count (TLC) tested with TLC values of 1210, 1340, and 1170, respectively. Based on the literature, it was stated that there is a fair correlation between CD4 counts of 200 cells/µL and total lymphocyte counts of 1200 cells/µL. WHO guidelines for TLC acknowledge that TLC is a valuable marker of disease progression.53 Several studies have shown a significant correlation between CD4+ T lymphocytes and Candida colonization. The depletion of the CD4+ T lymphocyte cell population is a pathognomonic event in the occurrence of HIV infection and is an underlying cause of the higher incidence of oral candidiasis.47 Immune system suppression is seen in a persistent decrease in CD4+ lymphocytes in the blood. The CD4+ cell count assesses an infected individual’s ability to defend against opportunistic infections. An average CD4+ cell count is above 700 cells/mm3; initial immune suppression is indicated by a CD4+ cell count below 500 cells/mm3; and severe immune suppression is confirmed by a CD4+ cell count below 200 cells/mm3.56 The lower the CD4+ cell count, the more vulnerable the individual is to bacterial, viral, parasitic, and fungal infections.7,41
Clinical assessment based on nutrition used body mass index (BMI) as the parameter and found that two patients before oral therapy were underweight, and three patients were normal. Five patients gained weight after oral treatment, but according to BMI, four patients were normal, and one was still underweight. There is a correlation between infections and malnutrition. The disease causes a decline in food intake, a reduction in negligible intestinal nutrient absorption, and a rise in the catabolism of nutrients necessary for tissue repair. Malnutrition can alter a patient’s immune system and increase their risk of infection, particularly enteral conditions, due to diminished intestinal mucosal barrier protection. Low food intake can occur due to oral candidiasis. The patients complained of difficulty and pain in swallowing, and on physical examination, there were signs of oral candidiasis. Oral candidiasis can cause pharyngeal involvement, which can cause dysphagia and respiratory distress.14,20 Nutritional problems may further affect their quality of life.55
Clinical assessment based on the oral hygiene status of patients, in this case, the report revealed a range score of 2.8–4.3 (fair to poor). We used the OHI-S, which provides inferences on the oral cleanliness of individuals in a quantitative manner. The index has two components: oral debris and oral calculus, factors considered in oral cleanliness.19,57 The overall mean OHI-S score was higher among the HIV patients. The higher the OHI-S score, the poorer the oral hygiene status and the greater the risk of oral candidiasis in immunocompromised patients such as HIV/AIDS. Patients with more severe AIDS manifestations complained of a poorer quality of oral symptoms, functional limitations, and emotional and social well-being related to their oral health.57
Based on hematologic tests, abnormalities were found in all HIV/AIDS patients (cases 1–5). A complete blood count is standard and routinely performed when monitoring HIV/AIDS patients. Poor hematologic status is a risk factor for oral candidiasis in HIV/AIDS patients.58,59 Decreased hemoglobin indicates an increase in the progression of HIV infection closer to AIDS. Assessment of hemoglobin and hematocrit is also essential to evaluate anemia because it is most often found in people with HIV/AIDS.27 This is related to anemia in four HIV/AIDS patients (cases 1–4). Thus, correcting the hematological abnormalities and appropriate oral lesion management can improve HIV/AIDS patients’ quality of life.58
The management of oral candidiasis could have a positive impact on HIV infection progression and prognosis.60 Oral candidiasis may be treated with antifungal medication either topically or systemically. Treatment should be maintained for 7–14 days. The response to treatment is often good.48 In HIV/AIDS patients, oral candidiasis may indicate progression of immunodeficiency and thus warrant prompt referral for the institution of effective combined antiretroviral therapy. In addition, the initiation or optimization of combined antiretroviral therapy is essential for addressing underlying HIV infection and reducing the risk of recurrence of candidiasis.1 All patients in cases 1–5 were given oral fluconazole for their oral candidiasis.
Clinical assessment based on the quality of life used the OHIP-14 as the parameter and found that two patients before oral therapy were moderate with a score of 20. Three patients were good, with a range score of 6–18. After oral treatment, five patients improved their quality of life, but according to the OHIP-14 score, four patients were 0 (good), and one patient was 3 (good). Oral candidiasis interferes negatively with the quality of life (QoL) of people living with HIV/AIDS.18–20,61,62
Conclusion
Oral candidiasis remains the most common oral lesion in HIV/AIDS patients with low CD4 count or TLC values, poor hematological state, not under ART, malnutrition, and poor oral hygiene. Therefore, comprehensive clinical assessment and management are essential for a higher quality of life.
Consent Statements
All patients provided informed consent for the publication of their case details and any accompanying images. The institution has also granted its consent for the publication of this report.
Acknowledgments
The authors extend their sincere gratitude to the patients for granting their consent. The authors would also like to acknowledge the support staff in the Teratai Clinic, Dr. Hasan Sadikin Hospital Bandung, and the Oral Medicine Department, Faculty of Dentistry, Universitas Padjadjaran.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Neville BW, Damm DD, Allen CM, et al. Color Atlas of Oral and Maxillofacial Diseases. Elsevier Inc; 2019.
2. Caputo V, Libera M, Sisti S, Giuliani B, Diotti RA, Criscuolo E. The initial interplay between HIV and mucosal innate immunity. Front Immunol. 2023;14:1–18. doi:10.3389/fimmu.2023.1104423
3. Moyes DL, Richardson JP, Naglik JR. Candida albicans-epithelial interactions and pathogenicity mechanisms: scratching the surface. Virulence. 2015;6(4):338–346. doi:10.1080/21505594.2015.1012981
4. World Health Organization. Summary of the Global HIV Epidemic. Available from: https://www.who.int/data/gho/data/themes/hiv-aids.
5. Cornea A, Lata I, Simu M, Rosca EC. Assessment and diagnosis of HIV-associated dementia. Viruses. 2023;15(2):1–23. doi:10.3390/v15020378
6. World Health Organization (WHO). Indonesia HIV Country Profile 2022; 2022. Available from: https://cfs.hivci.org/index.html.
7. Rajendran R. Shafer’s Textbook of Oral Pathology. Elsevier Inc.; 2016.
8. Lomelí-Martínez SM, González-Hernández LA, Ruiz-Anaya A, et al. Oral manifestations associated with HIV/AIDS Patients. Medicina. 2022;58(9). doi:10.3390/medicina58091214
9. Putranti A, Asmarawati TP, Rachman BE, Hadi U. Oral candidiasis as clinical manifestation of HIV/AIDS infection in Airlangga University hospital patients. IOP Conf Ser Earth Environ Sci. 2018;125(1):012063. doi:10.1088/1755-1315/125/1/012063
10. Aitken-Saavedra J, Dos Santos BC, Uchoa Vasconcellos AC, Flores DP, Maturana-Ramirez A. Oral lesions diagnosis associated with HIV. Report of 3 clinical cases. Rev Estomatol Hered. 2021;31(2):140–145. doi:10.20453/reh.v31i2.3975
11. Farah C, Balasubramaniam R, Mccullough MJ. Contemporary Oral Medicine. Springer Nature; 2019; doi:10.1007/978-3-319-72303-7
12. Mensana MP, Ernawati DS, Nugraha AP, et al. Oral candidiasis profile of the Indonesian HIV-infected pediatric patients at UPIPI Dr. Soetomo General Hospital, Surabaya, Indonesia. HIV AIDS Rev. 2018;17(4):272–277. doi:10.5114/hivar.2018.80259
13. Keser G, Namdar Pekiner F. Treatment approach for oral candidiasis: two case reports. Clin Exp Heal Sci. 2021;11:371–374. doi:10.33808/clinexphealthsci.862928
14. Rahardjo TM, Sumarlie BO, Kho EK, et al. Severe dehydration due to low intake in patients with oral candidiasis in malignancy: a case report. Med Clin Updat. 2023;2(1):62–65. doi:10.58376/mcu.v2i1.36
15. Taverne-Ghadwal L, Kuhns M, Buhl T, et al. Epidemiology and prevalence of oral candidiasis in HIV patients from chad in the post-HAART Era. Front Microbiol. 2022;13. doi:10.3389/fmicb.2022.844069
16. Ongole R. Textbook of Oral Medicine, Oral Diagnosis and Oral Radiology.
17. Ratnawidya W, Rahmayanti F, Soegiyanto AI, Mandasari M, Wardhany II. Indonesian short version of the oral health impact profile (OHIP-14). J Int Dent MedRes. 2018;11(3):1065–1071.
18. Jucá MACL, Silva LBD, Silva IAPD, Queiroga DEUD, Carvalho AMALD, Ferreira SMS. Impacts of health of users with HIV/AIDS in a specialized service. Rev Bras Enferm. 2019;72(6):1571–1579. doi:10.1590/0034-7167-2018-0649
19. Shrestha A, Rimal J, Poudyal N. CD4 count and oral health related quality of life of HIV-infected individuals at a tertiary healthcare center in Dharan – a cross-sectional study. J Coll Med Sci. 2017;13(4):392–396. doi:10.3126/jcmsn.v13i4.17808
20. Yengopal V, Naidoo S. Do oral lesions associated with HIV affect quality of life?. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2008;106(1):66–73. doi:10.1016/j.tripleo.2007.12.024
21. Sarangapany J, Batterham M, Begley K, et al. Application of oral health impact profile-14 on an HIV-1 infected population living in Sydney Method; 2009:2–3.
22. Muralidharan S, Mahendrakar S, Talekar A, et al. Oral health-related quality of life in HIV: a systematic review. J Contemp Dent Pract. 2020;21(5):585–592. doi:10.5005/JP-JOURNALS-10024-2833
23. Arbune M, Earar K, Brinduse LA. Assessment of dental status and oral health impact in HIV Romanian young adults. Mater Plast. 2016;53(1):3
24. Bhattarai D, Modak A, Suri D. Candidal perforation of the hard palate in an HIV-infected child. BMJ Case Rep. 2019;12(12):1–2. doi:10.1136/bcr-2019-233034
25. Maheshwari M, Kaur R, Chadha S. Candida species prevalence profile in HIV seropositive patients from a Major Tertiary Care Hospital in New Delhi, India. J Pathog. 2016;2016:1–8. doi:10.1155/2016/6204804
26. Lauritano D, Moreo G, Oberti L, et al. Oral manifestations in HIV-positive children: a systematic review. Pathogens. 2020;9(2):1–15. doi:10.3390/pathogens9020088
27. de Tilly AN, Tharmalingam S. Review of treatments for oropharyngeal Fungal infections in HIV/AIDS patients. Microbiol Res. 2022;13(2):219–234. doi:10.3390/microbiolres13020019
28. De Repentigny L, Lewandowski D, Jolicoeur P, et al. Immunopathogenesis of oropharyngeal candidiasis in human immunodeficiency virus infection. Clin Microbiol Rev. 2004;17(4):729–759. doi:10.1128/CMR.17.4.729-759.2004
29. Verma A, Gaffen SL, Swidergall M. Innate immunity to mucosal candida infections. J Fungi. 2017;3(4):1–15. doi:10.3390/jof3040060
30. Horváth M, Nagy G, Zsindely N, et al. Oral epithelial cells distinguish between candida species with high or low pathogenic potential through microRNA regulation. mSystems. 2021;6(3). doi:10.1128/msystems.00163-21
31. Groeger S, Meyle J, Diehl K, Bora SA, Cantorna MT. Oral mucosal epithelial cells. Front Immunol. 2019;10:1–22. doi:10.3389/fimmu.2019.00208
32. Vila T, Sultan AS, Montelongo-Jauregui D, Jabra-Rizk MA. Oral candidiasis: a disease of opportunity. J Fungi. 2020;6(1):1–28. doi:10.3390/jof6010015
33. Nikou SA, Kichik N, Brown R, et al. Candida albicans interactions with mucosal surfaces during health and disease. Pathogens. 2019;8(2):1–23. doi:10.3390/pathogens8020053
34. Sufiawati I, Tugizov SM. HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. J Gen Virol. 2018;99(7):937–947. doi:10.1099/jgv.0.001075
35. Sufiawati I, Tugizov SM. HIV-associated disruption of tight and adherens junctions of oral epithelial cells facilitates HSV-1 infection and spread. PLoS One. 2014;9(2). doi:10.1371/journal.pone.0088803
36. Zaongo SD, Ouyang J, Isnard S, et al. Candida albicans can foster gut dysbiosis and systemic inflammation during HIV infection. Gut Microbes. 2023;15(1). doi:10.1080/19490976.2023.2167171
37. Nazli A, Chan O, Dobson-Belaire WN, et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 2010;6(4):1–20. doi:10.1371/journal.ppat.1000852
38. Zevin AS, McKinnon L, Burgener A, Klatt NR. Microbial translocation and microbiome dsybiosis in HIV- associated immune activation. Curr Opin HIV AIDS. 2016;11(2):182–190. doi:10.1053/j.gastro.2016.08.014.CagY
39. Goulart LS, Souza WW, Vieira CA, et al. Oral colonization by Candida species in HIV-positive patients: association and antifungal susceptibility study. Einstein. 2018;16(3):eAO4224. doi:10.1590/S1679-45082018AO4224
40. Ribeiro Ribeiro AL, De Alencar Menezes TO, De Melo Alves-Junior S, De Menezes SAF, Marques-Da-Silva SH, Rosário Vallinoto AC. Oral carriage of Candida species in HIV-infected patients during highly active antiretroviral therapy (HAART) in Belém, Brazil. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;120(1):29–33. doi:10.1016/j.oooo.2015.03.008
41. Hirata CHW. Oral manifestations in AIDS. Braz J Otorhinolaryngol. 2015;81(2):120–123. doi:10.1016/j.bjorl.2014.12.001
42. Hamza OJM, Matee MIN, Moshi MJ, et al. Species distribution and in vitro antifungal susceptibility of oral yeast isolates from Tanzanian HIV-infected patients with primary and recurrent oropharyngeal candidiasis. BMC Microbiol. 2008;8:1–9. doi:10.1186/1471-2180-8-135
43. Egierska DM, Perszke M, Grzelewski A, et al. Oral diseases in patients infected with HIV. J Educ Heal Sport. 2022;12(8):556–571. doi:10.12775/JEHS.2022.12.08.059
44. Peña DER, Innocentini LMAR, Saraiva MCP, Lourenço AG, Motta ACF. Oral candidiasis prevalence in human immunodeficiency virus-1 and pulmonary tuberculosis coinfection: a systematic review and meta-analysis. Microb Pathog. 2021;150:1–6. doi:10.1016/j.micpath.2020.104720
45. Patil S, Majumdar B, Sarode SC, Sarode GS, Awan KH. Oropharyngeal candidosis in HIV-infected patients-an update. Front Microbiol. 2018;9:1–9. doi:10.3389/fmicb.2018.00980
46. Frimpong P, Amponsah EK, Abebrese J, Kim SM, Kim SM. Oral manifestations and their correlation to baseline CD4 count of HIV/AIDS patients in Ghana. J Korean Assoc Oral Maxillofac Surg. 2017;4:29–36. doi:10.5125/jkaoms.2017.43.1.29
47. Shekatkar M, Kheur S, Gupta AA, et al. Oral candidiasis in human immunodeficiency virus-infected patients under highly active antiretroviral therapy. Disease-a-Month. 2021;67(9):101169. doi:10.1016/j.disamonth.2021.101169
48. Ghom AG, Ghom SA. Textbook of Oral Medicine.
49. Sachdeva S. Oral thrush in an infant: a case report with treatment modalities. Pediatr Dent Care. 2016;1:1–3. doi:10.4172/2573-444X.1000106
50. Mowla MR, Manchur MA, Islam AQMS, Maurer T. Cutaneous manifestations of HIV/AIDS in the Era of highly active antiretroviral therapy: evidence from Bangladesh. Int J Dermatology Venereol. 2022;5(1):8–14. doi:10.1097/JD9.0000000000000205
51. Glick M, Greenberg M, Lockhart P, et al. Burket’s Oral Medicine. Vol. 4.
52. Stephen BD, Babatunde AA, Nicaise N, Fati MI, Patrick SD, Ahmad TA. Immunological and clinical assessment of adult HIV patients following switch to second-line antiretroviral regimen in a large HIV program in North-central Nigeria. J AIDS HIV Res. 2017;9(5):106–116. doi:10.5897/jahr2017.0416
53. Chinnappan JA, Lakshmipriya N, Umadevi U, et al. Distribution of candida species with antifungal susceptibility isolated from oral thrush in people living with HIV/AIDS and its correlation with CD4 count. Curr Res HIV AIDS. 2020;04(01):2–5. doi:10.29011/2575-7105.100121
54. Shah Jigna S, Tarsariya Vivek M, Barot Kaushik S, et al. Oral health related quality of life in HIV patients Shah. J Adv Med Dent Sci Res. 2021;9(6):159–164.
55. Umeizudike KA, Osagbemiro BB, Daramola OO, Adeyemo TA. Oral health related quality of life among HIV positive patients attending two HIV outpatient clinics in Nigeria-a cross sectional study. Afr Health Sci. 2021;21(2):566–575. doi:10.4314/ahs.v21i2.11
56. Silverman S. Essentials of Oral Medicine. BC Decker Inc; 2002.
57. Jain P, Sontakke P, Anup N, Sikka M, Biswas G, Shravani G. Oral health-related quality of life among HIV patients at antiretroviral therapy center government hospital, Jaipur. J Indian Assoc Public Heal Dent. 2015;13(2):148. doi:10.4103/2319-5932.159051
58. Sufiawati I, Sasanti H. Herpes labialis and oral candidiasis in HIV-infected intravenous drug users with poor hematologic status. Padjadjaran J Dent. 2008;20(3):179–185. doi:10.24198/pjd.vol20no3.14125
59. Sufiawati I, Sasanti H, Djauzi S. Correlation between saliva IgA level and T cell CD4+ in HIV/AIDS patients. Padjadjaran J Dent. 2007;19(2):67–72. doi:10.24198/pjd.vol19no2.14169
60. Indrastiti RK, Wardhany II, Soegyanto AI. Oral manifestations of HIV: can they be an indicator of disease severity? (A systematic review). Oral Dis. 2020;26(S1):133–136. doi:10.1111/odi.13394
61. Bajomo AS, Ayo-Yusuf OA, Rudolph MJ, Tsotsi NM. Impact of oral lesions among South African adults with HIV/AIDS on oral health-related quality of life. J Dent Sci. 2013;8(4):412–417. doi:10.1016/j.jds.2013.04.011
62. Mohamed N, Saddki N, Yusoff A, Mat Jelani A. Association among oral symptoms, oral health-related quality of life, and health-related quality of life in a sample of adults living with HIV/AIDS in Malaysia. BMC Oral Health. 2017;17(1):1–11. doi:10.1186/s12903-017-0409-y
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.