Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Clinical and Ultrasound Evaluation of Skin Quality After Subdermal Injection of Two Non-Crosslinked Hyaluronic Acid-Based Fillers
Authors Bezpalko L, Filipskiy A
Received 17 March 2023
Accepted for publication 18 July 2023
Published 10 August 2023 Volume 2023:16 Pages 2175—2183
DOI https://doi.org/10.2147/CCID.S402409
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Jeffrey Weinberg
Lyudmyla Bezpalko,1 Andriy Filipskiy2
1Plastic and Aesthetic Surgery Department, Clinical Hospital of Lviv Railway, Lviv, Ukraine; 2Radiology Department, Postgraduate Faculty, Danylo Halytsky Lviv National Medical University, Revive Aesthetic Medicine Clinic, Lviv, Ukraine
Correspondence: Lyudmyla Bezpalko, Plastic and Aesthetic Surgery Department, Clinical Hospital of Lviv Railway, Ivana Ohiienka St. 5, Lviv, Lviv Oblast, Lviv, Ukraine, 79000, Tel +380322264308, Fax +380322264308, Email [email protected]
Introduction: Nowadays patients want to get an immediate result from skin rejuvenation techniques without sign of injections and consequent limitations in social life. Therefore, the least traumatic, more effective, and longer lasting treatment approach for skin quality improvement should be favored.
Purpose: Assess skin quality outcomes by clinical examination and self-reporting in patients treated with two non-crosslinked hyaluronic acid (HA) products, injected by cannula. Investigate the skin thickness and the longevity of dermal fillers in soft tissues by ultrasound examination.
Patients and Methods: Fifteen female patients (mean age 41 years) were selected for injection with two non-crosslinked HA products (one for each hemiface and hemi neck). Subdermal injections were performed bilaterally and the retrograde linear fanning technique with a 25G 50 mm cannula from three entry points was used. An ultrasound examination of the skin layers thickness was carried out before the procedure and every 6– 7 days up to three weeks, when patients skin quality improvement was assessed by GAIS (Global Aesthetic Improvement Scale) and patients asked about their satisfaction level.
Results: On the right hemiface, the use of the non-crosslinked HA-product with lidocaine was not associated with pain in the sites of injection. On both face sides, the signs of bruising or edema were minor and not associated with downtime or social life limitation after the procedure. After three weeks, despite both injected products could not be detected by ultrasound technique, signs of skin stimulation and skin layers hydration were still observed: The dermis became thicker on both hemifaces while the epidermis became thinner but showed more pronounced radiance and densification effect on the right hemiface.
Conclusion: Subdermal injections of non-crosslinked HA “skin boosters” could be a good option for minimal traumatic and effective 3-week lasting skin quality improvement.
Keywords: HA, skin booster, lidocaine, tissue longevity, ultrasound scan, skin tone, skin texture
Introduction
Skin damage is induced by extrinsic factors like exposure to ultraviolet (UV) A and B radiation and pollution as well as lifestyle influence (diet, smoking). Such factor effects contribute to the acceleration of the physiological aging process.1–3 The premature skin aging is manifested by skin dryness, decreases in elasticity and firmness, patchy pigmentation, loss of radiance and wrinkling.4
Nowadays beautiful skin is a youth and health hallmark, as well as a reason for social recognition. Therefore, skin beautification and rejuvenation treatments are highly demanded by patients who prefer fast effective, long lasting, and less painful non-surgical aesthetic procedures.5 The most frequently used substance to achieve rapid skin hydration and quality improvement is non--crosslinked hyaluronic acid (HA). It shows hygroscopic properties, multiple stimulating effects both on dermal cellular (eg, fibroblasts) and extracellular components (eg, neocollagenesis mimicking the healing process, elastin production, and expression/release of growth factors) which help restore skin hydration and structure respectively.6,7 The most common injection technique of non-crosslinked HA products (also known as “skin boosters”) consists in multiple boluses by using a needle, which may cause transient bruising and edema requiring a few days of downtime. To prevent any social life limitation and obtain an immediate result, the least traumatic but still effective approach should be favored.
In our study we aimed at describing clinically relevant outcomes on skin quality as well as provide ultrasound assessment of subdermal injections with cannula of the two most used different non-crosslinked HA products (with and without lidocaine) in female patients.
Materials and Methods
We conducted a prospective non-consecutive case series on 15 female patients, from 38 to 44 years of age (mean 41 years), during our private clinical practice in the Department of Plastic and Aesthetic Surgery in Clinical Hospital of Lviv Railway and Revive Clinic (Lviv, Ukraine). An IRB or ethics committee approval has not been asked because by local Ukrainian law, rules, and regulations, this was not mandatory for conducting our study (ie, case series with Ukraine Health Authorities certified products for dermal injections in the approved indications). Nevertheless, all patients provided written informed consent prior to any study procedure and were injected by the same physician. In addition, all patients signed a consent form to publish their anonymized and cumulative data in a scientific journal with one of them also providing the consent for her ultrasound images and face pictures to be published. Patients presenting with skin dryness, loss of skin firmness, dyschromia, “tired-looking” skin, and signs of mild to moderate photoaging were eligible for the study. Patients with contra-indications to an HA injection procedure as per the devices’ information for use were excluded.
Two products formulated with non-crosslinked HA with or without lidocaine were used. The right hemiface and hemineck of each patient were injected with 15 mg/mL of non-crosslinked high molecular weight (HMW) HA with lidocaine hydrochloride 0.3% by mass, in a supplemented phosphate buffer (Redensity 1®, Teoxane, Geneva, Switzerland).8 The left face and neck sides were treated with a buffered physiological solution of 32 mg/mL of HMW and low molecular weight (LMW) non-crosslinked HA (Profhilo ®, IBSA Farmaceutici, Lodi, Italy).9 All injections were subdermal and performed by retrograde linear fanning technique with a 25 G, 50 mm cannula from three entry points on each face side (zygomatic arch, 1 cm medial to the tragus, and middle horizontal neck line anterior to sternocleidomastoid muscle). In total 2 mL of each product were injected: 1.5 mL per hemiface (0.15 mL per thread) and 0.5 mL per hemi neck (0,1 mL per thread).
A standardized ultrasound examination was carried out in 2 points of the face and 1 point of the neck (Figure 1) before the procedure, immediately after the injections (not shown), and every 6–7 days until 3 weeks in both side (left and right) as shown in Figures 2 and 3A–D. The ultrasound machine (GE Logiq P9 with a linear transducer L3-12 with frequency 12 MHz on dermis preset in classic B-mode) was used to quantify several parameters of the epidermis, dermis, and hypodermis (ie, margins, borders, thickness, structure, and echogenicity).
 |
Figure 1 The points of ultrasound examination. |
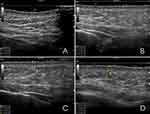 |
Figure 2 Ultrasound qualification of dermal changes of the left hemiface: (A) Before the injection procedure, (B) After 1 week, (C) After 2 weeks, (D) After 3 weeks. |
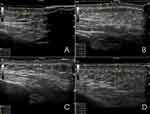 |
Figure 3 Ultrasound qualification of dermal changes of the right hemiface: (A) Before the procedure, (B) After 1 week, (C) after 2 weeks, (D) after 3 weeks. |
In addition, patients were asked about postprocedural period (erythema, bruising, pain, skin irregularities) and after three weeks about their overall satisfaction level and global skin quality improvement (Global Aesthetic Improvement Scale, GAIS).10
Results
Performance Outcomes Assessment
Immediately after the injection treatment, we assessed by ultrasound the signs of dermis hydration, together with the presence of injected product. We evaluated the level of hydration in a qualitative and indirect way, according to the different echogenicity of the tissues. Potential signs of higher hydration rate were confirmed by clinical examination.
In addition, we measured the thickness of the skin layers in the three pre-defined face and neck points on both sides. The results are reported in Table 1 and 2 as Gray scale B mode appearance of dermal layers.
 |
Table 2 Mean Thickness of Dermal Layers Before and After Injections of Non-Crosslinked HA (±0.1 Mm) in the Two Face and Neck Sides |
One week after the treatment, in the same pre-selected points, we recorded a decreased echogenicity and local thickening of the dermis with hyperechoic tiny particles of HA. On the other hand, the epidermis became thinner, and the outline became less clear. In the superficial layer of the hypodermis, the echogenicity decreased locally. There were no significant differences between the two sides in the dynamics of those indicators (Figures 2B and 3B).
After two weeks, we reported signs of hydration of both epidermis and dermis which were more visible on the right side. The dermis became thicker on both sides with a local decrease in echogenicity on the left and homogenous decrease on the right. The epidermis looked thinner without clear outline on both sides. At the same time, the HA-based product could no longer be detected on the right side while tiny particles of HA were visible on the left one (Figures 2C and 3C).
After three weeks, the HA was not detectable under the skin on both face and neck side, but we could still observe signs of skin stimulation and hydration. In all the three points on the left side of the face we observed a local and homogenous decrease in echogenicity of dermis. The hypodermis looked like before the procedure (Figure 2D). On the contrary, on the right side, the dermis was still thick, and thicker compared to the left side with a local decrease in echogenicity, but the hypodermis looked like before the procedure (Figure 3D).
Safety Assessment
During the procedure all patients experienced pain only on the left hemiface and hemi neck sites of injections, where the HA without lidocaine hydrochloride was used.
Immediately after the procedure, we observed more lifting and volumizing effects on the left side of the face and neck which were anyway associated with more visible post-traumatic signs such as erythema-like. Thirteen patients did not have any bruising or edema limiting social life immediately after the procedure. One patient had bruising on the left hemiface and hemi neck along the threading lines and another one had small ecchymosis on the left and right sides at the cannula entry points.
All the patients reported light pain on the left side for 2–3 days post-procedure.
Patients’ Self-Assessment of the Esthetic Improvement (GAIS)
Three weeks after the injection of HA-based products, all patients reported a sustained improvement of their skin hydration, firmness, elasticity, and skin complexion on both sides but more radiance, better texture, and uniform skin tone on the right side. All of them had a very high satisfaction level, much improved skin appearance according to GAIS (Table 3 and Figure 4), and willingness to repeat the procedure as well as recommend it.
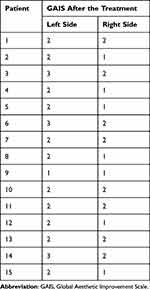 |
Table 3 GAIS Results 3 Weeks After the Treatment |
 |
Figure 4 40-years old patient pictures, taken: (A) Before the injection procedure, (B) Immediately after, (C) After 3 weeks. |
Discussion
Decreasing epidermal HA content, increasing avidity of dermal HA associated with tissue structures and HA extractability loss, progressive cross-linking of collagen and the steady loss of collagen extractability are the predominant histochemical changes observed in aging skin.11,12 The synthesis of epidermal HA is influenced by the underlying dermis and it is independent from the synthesis of dermal HA.13 HA is an essential component of the extracellular matrix (ECM) responsible for binding and retaining water molecules. Therefore, its deficiency results in loss of skin hydration and manifests in skin dryness, atrophy, loss of elasticity and thinning. In addition to the physiological skin aging process, repeated exposure to UV radiation is the main responsible for premature skin aging in about 80% of cases.14 In the dermis and epidermis, HA is co-localized with CD44 cell receptors. CD44-HA interactions have been reported to mediate the binding of Langerhans cells to HA in the matrix surrounding keratinocytes by their CD44-rich surfaces, as they migrate through the epidermis.15
It is proven that the aging process also leads to an overall alteration and insufficiency in other main components of the ECM such as collagen and elastin, which are produced mainly by fibroblasts. Therefore, skin quality lost with aging can be improved by restoring the HA content in skin layers and stimulating fibroblasts to produce new “young” components of ECM.
In our study, we described the changes in skin layers assessed by ultrasound examination after subdermal injections of two non-crosslinked HA-based products. Normal B mode gray scale of ultrasound is a picture that shows the combination of different shades of gray. If the tissue is more hydrophilic and has a high fluid content, its ultrasound image has a darker shade, and the liquid component gets totally black (with some tiny particles inside). When the tissue is stiff with low amount of fluid, it appears brighter, or even white with post-echo-shadow in the case of a bone margin.16 The HA molecule can trap about 100-fold of its weight of water.11 Thus, subdermal injections of a very hygroscopic molecule led to ultrasound signs of skin layers rehydration such as increased thickness of dermis and decreased echogenicity.
The higher the concentration of HA is, the bigger volume of fluid is trapped. Nevertheless, during the procedure all patients experienced more pain on the left side of injections that could be explained by the highest concentration of HA (twice higher than the product injected in the right side), strongest stretching of the tissues and absence of lidocaine in the product.
As expected, immediately after injections we reported more lifting and volumizing effects on the left side; however, this effect disappeared after 2 weeks, because of breakdown of non-crosslinked HA by endogenic hyaluronidases.17 In fact, we recorded a complete degradation of HA, and initial dehydration of epidermis, which on ultrasound scanning images appeared as decreased thickness, without increased echogenicity. The changes in the epidermis are influenced by several factors including skin type, at home skin care and other external aggressive factors, such as makeup or UV. Therefore, epidermis hydration changes observed by ultrasound should be interpreted with caution and were not considered as a key indicator.
At the same time, we could still observe the dermis thickening even after the product degradation. The concentration of an HA-based product is not the only feature to explain the tissue thickness (which depends on the molecular weight and other HA intrinsic features) and the associated clinical effect once the product is degraded. Such phenomenon could be explained by multiple biological effects of HA, such as indirect stimulation of collagenogenesis, elastogenesis, wound healing, angiogenesis, and proliferation of fibroblast growth factors which persist after HA degradation in the tissues.17,18 Wang et al showed that the fibroblasts shape become more stretched with active phenotype because of HA fillers. It was also shown that existing collagen fibers are stretched, and this induces mechanical tension of surrounding fibroblasts which leads to increased production of Types 1 and 3 collagens. They also found a statistically significant increase in procollagen, tissue inhibitors of metalloproteinase (TIMP-1) and gene expression of procollagen 1 and 3, 1 month after injection.19
In some investigations, it was shown that HMW HA (HMWHA) displays anti-inflammatory properties, whereas LMW HA (LMWHA) could cause inflammation through macrophage activation, and stimulation of cytokines production. Other benefits related to HMWHA include antioxidant effects by activation pathways involved in the regulation of cellular redox balance.20 Additionally, numerous studies have shown that HA signaling plays an important role in angiogenesis regulation, mainly by influencing endothelial cells behavior.21,22 Moreover, an evident increase in the expression of various proliferation and growth factors (eg, ki67, transforming growth factor β [TGF- β], and endothelial growth factor [EGF]) confirms the HA biostimulator effect.22 Therefore, subdermal injection of non-crosslinked HA, mainly HMWHA including other anti-inflammatory and antioxidant elements in the final composition, could stimulate the reorganization of ECM which is proven by increasing of the skin thickness, clinical observations, and patients’ subjective opinion. There were no significant differences in the skin layers’ thickness depending on different concentrations of HA in products that were used. Hence, the ideal concentration of HA should still be found.
Another interesting hypothesis is that subdermal HA might contribute to the positive observed clinical outcomes by the stimulation of adipogenesis of subcutaneous fat affected with aging.23,24
Thereby, subdermal injection of non-cross linked HA products could be a preventive approach to facial skin quality impairment during aging process with sustained effects after the products degradations; other non-crosslinked products might be an interesting alternative. The mechanisms involved still deserve further investigation in larger controlled blind-evaluator clinical trials.
Conclusion
Subdermal injection of non-crosslinked HA products with a cannula seems to be effective in skin rejuvenation and overall, less traumatic than multiple bolus injections with a needle. Non-crosslinked HA products remain detectable under the skin for 2–3 weeks depending on HA concentration and rheology. This was associated with visible skin improvement and rehydration, with some product specificity. The rejuvenating effects confirmed by patient self-assessment and satisfaction level were still visible after full disappearance of HA at 3 week-time, probably due to the biostimulator effects of HA on the ECM components of the dermis and potentially to stimulation of adipogenesis. The HMW HA with lidocaine had the advantage to be associated with less pain at the injection site and some better clinical outcomes after 3 weeks, while the one without lidocaine, LMW and higher concentration was associated with more pain and had the temporary advantage to give more lifting effect immediately after the procedure, probably due to the concentration itself and some immediate inflammatory effects. Despite this, the product was fully degraded after 3 weeks with no advantage compared to the less concentrated HA.
The described injection technique of non-crosslinked HA products could get an important place in rejuvenation protocols as confirmed by a recent study.25 Further investigation in randomized controlled trials with validated scales and/or more sophisticated ultrasound imagery (eg, sonoelastography for quantitative assessment of the soft tissue density) is needed to evaluate their efficacy/performance, mechanisms and safety profile when injected with a cannula.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Gu Y, Han J, Jiang C, Zhang Y. Biomarkers, oxidative stress, and autophagy in skin aging. Ageing Res Rev. 2020;59:101036.
2. Tobin DJ. Introduction to skin aging. J Tissue Viability. 2017;26(1):37–46.
3. Wong QYA. Chew FT Defining skin aging and its risk factors: a systematic review and meta-analysis. Sci Rep. 2021;11:22075.
4. Zhang S, Dunan E. Fighting against skin aging: the way from bench to bedside. Cell Transplant. 2018;27(5):729–738.
5. Facial Rejuvenation Market size was estimated to be US$ 19 billion in 2019: insight org/10.1021/cr050247k SLICE September 22, 2020. Available from: https://www.globenewswire.com/newsrelease/2020/09/22/2097541/0/en/FacialRejuvenation-Market-size-was-estimated-to-be-US-19-billion-in-2019insightSLICE.html.
6. Fanian F, Jeudy A, Lihoreau T, et al. In-vivo injection of oligonutrients and high molecular weight hyaluronic acid - results of a randomized controlled trial. Aesthetic Med. 2017;3(4):23–34.
7. Stellavato A, La Noce M, Corsuto L, et al. Hybrid Complexes of High and Low Molecular Weight Hyaluronans Highly Enhance HASCs Differentiation: implication for Facial Bioremodelling. Cell Physiol Biochem. 2017;44(3):1078–1092.
8. TEOSYAL Puresense Redensity® 1 [Instructions for use]. Geneva: Teoxane SA; 2022.
9. Profhilo®[Instructions for use]. Lugano: IBSA Pharmaceuticals; 2021.
10. Di Bernardo G, Di Bernardo B. Prediction of Treatment Outcomes for Neck Rejuvenation Utilizing a Unique Classification System of Treatment Approach Using a 1440-nm Side-Firing Laser. Aesthet Surg J. 2018;38(suppl_2):S43–S51.
11. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012;4(3):253–258.
12. Stern R, Maibach HI. Hyaluronan in skin: aspects of aging and its pharmacologic modulation. Clin Dermatol. 2008;26(2):106–122.
13. Stuhlmeier KM, Pollaschek C. Differential effect of transforming growth factor beta (TGF-beta) on the genes encoding hyaluronan synthases and utilization of the p38 MAPK pathway in TGF-beta-induced hyaluronan synthase 1 activation. J Biol Chem. 2004;279(10):8753–8760.
14. Bernstein EF, Underhill CB, Hahn PJ, Brown DB, Uitto J. Chronic sun exposure alters both the content and distribution of dermal glycosaminoglycans. Br J Dermatol. 1996;135(2):255–262.
15. Weiss JM, Renkl AC, Sleeman J, et al. CD44 variant isoforms are essential for the function of epidermal Langerhans cells and dendritic cells. Cell Adhes Commun. 1998;6(2–3):157–160.
16. Wortsman X, Jemec G, eds. Dermatologic Ultrasound with Clinical and Histologic Correlations. New York: Springer: Springer Science & Business Media; 2013.
17. Stern R, Jedrzejas MJ. Hyaluronidases: their genomics, structures, and mechanisms of action. Chem Rev. 2006;106(3):818–839.
18. Ronan SJ, Eaton L, Lehman A, Pilcher B, Erickson CP. Histologic Characterization of Polymethylmethacrylate Dermal Filler Biostimulatory Properties in Human Skin. Dermatol Surg. 2019;45(12):1580–1584.
19. Wang F, Garza LA, Kang S, et al. In vivo stimulation of de novo collagen production caused by cross-linked hyaluronic acid dermal filler injections in photodamaged human skin. Arch Dermatol. 2007;143(2):155–163.
20. Litwiniuk M, Krejner A, Speyrer MS, Gauto AR, Grzela T. Hyaluronic Acid in Inflammation and Tissue Regeneration. Wounds. 2016;28(3):78–88.
21. Gao Y, Sun Y, Yang H, et al. A Low Molecular Weight Hyaluronic Acid Derivative Accelerates Excisional Wound Healing by Modulating Pro-Inflammation, Promoting Epithelialization and Neovascularization, and Remodeling Collagen. Int J Mol Sci. 2019;20(15):3722.
22. Ailhaud G. Tissue secretion systems in the adipose tissue. Rev Prat. 1994;44(13):
23. Nadra K, André M, Marchaud E, et al. A hyaluronic acid-based filler reduces lipolysis in human mature adipocytes and maintains adherence and lipid accumulation of long-term differentiated human preadipocytes. J Cosmet Dermatol. 2021;20(5):1474–1482.
24. Wollina U. Midfacial rejuvenation by hyaluronic acid fillers and subcutaneous adipose tissue–a new concept. Med Hypotheses. 2015;84(4):
25. Majewska L. Synergy of stabilized and nonstabilized hyaluronic acid soft tissue fillers in skin density and skin thickness enhancement. Dermatol Ther. 2022;35(11):e15885. doi:10.1111/dth.15885
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

