Back to Journals » Infection and Drug Resistance » Volume 13
Clinical and Molecular Epidemiologic Characteristics of Ceftazidime/Avibactam-Resistant Carbapenem-Resistant Klebsiella pneumoniae in a Neonatal Intensive Care Unit in China
Authors Zhou J, Yang J, Hu F, Gao K, Sun J, Yang J
Received 4 April 2020
Accepted for publication 24 June 2020
Published 27 July 2020 Volume 2020:13 Pages 2571—2578
DOI https://doi.org/10.2147/IDR.S256922
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Juanjuan Zhou,1,2,* Junwen Yang,1,2,* Fupin Hu,3,4 Kaijie Gao,1,2 Jiufeng Sun,5 Junmei Yang1,2
1Zhengzhou Key Laboratory of Children’s Infection and Immunity, Children’s Hospital Affiliated to Zhengzhou University, Zhengzhou, People’s Republic of China; 2Department of Laboratory Medicine, Children’s Hospital Affiliated to Zhengzhou University, Zhengzhou, People’s Republic of China; 3Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, People’s Republic of China; 4Key Laboratory of Clinical Pharmacology of Antibiotics, Ministry of Health, Shanghai, People’s Republic of China; 5Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention, Guangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Junmei Yang
Zhengzhou Key Laboratory of Children’s Infection and Immunity, Children’s Hospital Affiliated to Zhengzhou University, 33M Waihuan East Road, Longhu, Zhengzhou 450018, People’s Republic of China
Tel +86(371)85515770
Email [email protected]
Jiufeng Sun
Guangdong Provincial Institute of Public Health, Guangdong Provincial Center for Disease Control and Prevention, 160M. Qunxian Road, Guangzhou 510300, People’s Republic of China
Tel +86(020)31051091
Email [email protected]
Background: Ceftazidime/avibactam (CZA)-resistant carbapenem-resistant Klebsiella pneumoniae (CRKP) infections occur in adults worldwide but are rarely observed in neonates. We evaluated the activities of CZA against CRKP and described the clinical and molecular epidemiology of CZA-resistant CRKP in a NICU prior to CZA approval in China.
Methods: A laboratory-based surveillance of CRKP was conducted from July 2017 to June 2018. Clinical data were initially reviewed. Antimicrobial susceptibility was determined by the broth microdilution method. CZA-resistant CRKP isolates were submitted to carbapenemase types screening and multilocus sequence typing.
Results: Over 23.3% (10/43) of CRKP strains were resistant to CZA, MIC50 and MIC90 values being 0.5 μg/mL and > 32μg/mL, respectively. Most neonates shared similar clinical features with cesarean (n=8), preterm birth (n=6), low birth weight (n=5), and exposure to carbapenems/β-lactam (n=8). All CZA-resistant CRKP isolates were highly resistant to most tested drugs except for polymyxin B (POL) and tigecycline (TGC). CZA-resistant CRKP isolates showed greater sensitivity to amikacin (AMK), nitrofurantoin (NIT), levofloxacin (LVX) and ciprofloxacin (CIP), compared with CZA-sensitive CRKP. All CZA-resistant CRKP isolates harbored carbapenemase genes, blakpc-2 (n=5) being predominant, followed by blaNDM-1 (n=4) and blaNDM-5 (n=2). Among these CZA-resistant CRKP isolates, a total of eight different STs were identified. CRKP harboring KPC belonged to ST1419, ST37 and ST11, while NDM types were assigned to ST784, ST1710, ST37 and ST324. Furthermore, other β-lactamase genes including blaSHV and blaCTX-M were also found.
Conclusion: Over 23.3% of CRKP strains isolated from neonates were resistant to CZA. Cesarean, preterm birth, low birth weight, and exposure to carbapenems/β-lactam were similar clinical features of most neonates with CZA-resistant CRKP. The predominant carbapenemases of CZA-resistant CRKP were KPC-2 and NDM-1, and KPC-2 producing K. pneumoniae assigned into 3 STs, which indicate the genetic diversity of clinical CZA-resistant CRKP isolates.
Keywords: ceftazidime/avibactam, neonate, drug resistance, clinical features, blaKPC
Introduction
The emergence and rapid global expansion of bacterial multidrug-resistance (MDR) and extensive drug-resistance (XDR) means that only a few antibiotics are effective for clinical anti-infection therapy.1 Increased spread of Carbapenem-resistant Klebsiella pneumoniae (CRKP) has become a serious threat to global public health; in particular, there are limited anti-infective therapeutic options for young patients. Due to safety concerns, carbapenems are the antibiotics of choice for treating MDR, and their widespread use has led to the global dissemination of CRKP and serious clinical outcomes in pediatric patients.2–4 The prevalence of CRKP infections is also increasing among neonates and children.5,6
Ceftazidime/avibactam (CZA), a new combination of cephalosporin and avibactam, a β-lactamase inhibitor, approved by US Food and Drug Administration (FDA) in 2015 and the European Medicines Agency (EMA) in 2016, has shown high activity against Escherichia coli, Pseudomonas aeruginosa and Klebsiella pneumoniae (K. pneumoniae).7,8 CRKP is mainly mediated by the production of carbapenemases, such as Klebsiella pneumoniae carbapenemases (KPC) and New Dehli metallo-β-lactamase (NDM), extended spectrum β-lactamase (ESBL) and AmpC β-lactamases (AmpC).9 Avibactam is a novel β-lactamase inhibitor that can inhibit the activity of Ambler class A (KPC), class C, and some class D β-lactamases (OXA-48) when combined with ceftazidime.10 CZA has significantly higher rates of clinical success than other regimens in the treatment of CRKP.11 CZA is thus regarded as an effective drug for the treatment of CRKP infections. Although CZA has not been recommended for use in pediatric patients, studies have shown that it is well tolerated and efficacious in pediatric patients.12,13 Unfortunately, with the introduction of CZA therapy in clinic, CZA-resistant CRKP infections have emerged in adult patients.14–16 Resistance to CZA in CRKP has been linked to mutations within the Ω-loop in blaKPC17,18 or blaCTX-M.16 However, there are limited data on the clinical presentations and molecular characteristics of CZA-resistant CRKP infections in neonates.
To the best of our knowledge, CZA-resistant CRKP has not previously been reported in neonate patients. In this study, we evaluated the in vitro activity of CZA against CRKP clinical isolates from neonates, and reported for the first time the clinical features and molecular characteristics of CZA-resistant CRKP isolates obtained from neonates in China.
Materials and Methods
Study Population and Bacterial Isolates
A laboratory-based surveillance of CRKP was conducted between July 2017 and June 2018 at Children’s Hospital affiliated to Zhengzhou University, a 2200-bed tertiary-care teaching hospital located in Henan Province, China, with over 95,000 inpatients per year. The neonatal intensive care unit (NICU I), general neonatal wards (NICU II) and premature wards together comprise 200 beds. The strains were isolated from specimens of sputum, urine, blood, alveolar lavage and cerebrospinal fluid. All suspected isolates were confirmed as K. pneumoniae using a Bruker Biotyper MALDI-TOF MS (Bruker Daltonik GmbH, Bremen, Germany). Among these isolates, CRKP isolates were identified as resistant to Imipenem (IMP) or Meropenem (MEM) by broth microdilution method. Escherichia coli ATCC 25,922 was used as a quality control.19
The study protocol complied with the Declaration of Helsinki and was approved by the Children’s Hospital affiliated to Zhengzhou University Ethics Committee. Informed patient consent was not required as this study had no impact on the patients, and patient information was anonymized.
Clinical Data Collection
Data on clinical and epidemiological characteristics of the neonatal patients from whom CRKP isolates were obtained from the electronic medical records. The information included demographics, birth information, invasive procedures, details about their hospital stay, infection or colonization by CRKP, and treatments procedures.
Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing to CZA, ceftolozane/tazobactam (CZT), piperacillin-tazobactam (TZP), Cefoperazone/sulbactam (CSL), piperacillin (PIP), cefazolin (CZO), cefmetazole (CMZ), cefuroxime (CXM), ceftazidime (CAZ), ceftriaxone (CRO), cefepime (FEP), IMP, MEM, ertapenem (ETP), AMK, gentamicin (GEN), NIT, trimethoprim-sulfamethoxazole (SXT), aztreonam (ATM), LVX, CIP, doxycycline (DOX), POL and TGC were performed according to the Clinical and Laboratory Standards Institute protocol (CLSI protocol).19 Bacterial suspensions with a turbidity of 0.5 Mc Turbidities were prepared from colonies cultured for 20 hours on agar and were then diluted with MH broth (1:1000). Each well of a 96-well plate was then inoculated with 100μL inoculum, and then the plate was sealed and incubated for 24 hours at 35°C. The minimum inhibitory concentration (MIC) is the minimum concentration in the well at which there was no visible growth. TGC and POL MICs were interpreted according to EUCAST ECOFFs20 (ECAST) and all the other drug MICs were interpreted according to CLSI M100-S30 criteria.19 WHONET software (5.6) was used for the analysis of susceptibility data.
Identification of Resistance Genes and Multilocus Sequence Typing (MLST)
Carbapenemase coding genes (blaNDM, blaKPC, blaVIM, blaIMP,blaOXA), β-lactamases coding genes (blaCTX, blaTEM, blaSHV) and plasmid-borne AmpC β-lactamases (blaDHA) were detected by polymerase chain reaction (PCR) as previously described.14 PCR amplicons were sequenced and DNA sequences were compared with those available in the NCBI GenBank database (https://www.ncbi.nlm.nih.gov/genbank/) using BLAST. Multilocus sequence typing (MLST) of CZA-resistant CRKP isolates was performed as described in the MLST Pasteur database (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Data Analysis
All statistical analyses were performed with SPSS 24.0 software. Categorical data were evaluated using chi-square tests or Fisher’s exact test and quantitative data was compared using the Student’s t-test or the Manne-Whitney U-test. P values (two-tailed) of <0.05 were regarded as statistically significant.
Results
Clinical Characteristics of Neonates with CZA-Resistant CRKP Isolates
A total of 300 K. pneumoniae isolates were isolated from 12,040 specimens obtained from 5348 neonate inpatients in the NICU of this hospital, 43 of which were identified as CRKP strains. Resistance to CZA was found in 23.3% (10/43) of these CRKP isolates. Clinical characteristics of neonates with CZA-resistant CRKP were summarized in Table 1. Patients who were colonized or infected with CZA-resistant CRKP had a median age of 30 days (interquartile range, 12.75–30 days), and the male to female ratio was 1.5. The median gestational age of neonate patients with CZA-resistant CRKP was 32.30 weeks (interquartile range, 28.98–39.78 weeks). Most neonate patients were born by Caesarean delivery (n=9), classified as premature (n=6) and very low birth weight (n=5). The most common diseases in neonate patients with CZA-resistant CRKP were pneumonia (n=7) and septicemia (n=4). Eight neonate patients had hematological disease, six neonate patients had neurological diseases and four neonate patients had respiratory dysplasia. Most of the neonates accepted invasive treatment, including tracheal intubation (n=6) and intubation of the stomach (n=3). MEM (n=4) and CSL (n=5) combinations were the most frequently used antibiotics before the presence of CZA-resistant CRKP was laboratory confirmed.
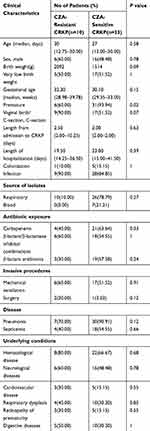 |
Table 1 Clinical Characteristics of Neonatal Patients Infected or Colonized with CZA-Resistant and CZA-Sensitive CRKP Isolates |
In vitro Susceptibilities of CRKP Isolates
23.3% (10/43) of CRKP strain were resistant to CZA, with MIC50 of 0.5 μg/mL and MIC90 of >32 μg/mL, respectively (Table 2). All CRKP isolates were resistant to more than three classes of antibiotic and showed high levels of resistance to β-lactams/β-lactamase inhibitor combinations (100%). Fortunately, rates of resistance to the other tested antimicrobial agents were not so high; AMK resistance: 74.4%, LVX and CIP resistance: 14%, and SXT resistance: 25.5%. All isolates were sensitive to TGC and POL.
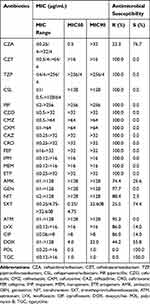 |
Table 2 In vitro Activities of Ceftazidime-Avibactam and Comparator Antimicrobial Agents Tested Against 43 CRKP Isolates (μg/mL) |
Of note, the antibiotic resistance spectrum of CZA-resistant CRKP to non-β-lactams was different from that of CZA-sensitive CRKP. For example, the CZA-resistant CRKP group had lower resistance rates than CZA-sensitive CRKP group to AMK (30% VS 87.8%), LVX (40% VS 100%), CIP (70% vs 100%) and NIT (50% VS 100%) (P<0.05). The MIC50 values against these drugs in the CZA-resistant CRKP group were lower than those in the CZA-sensitive CRKP group (Table 3).
 |
Table 3 Antimicrobial Activities of CZA-Resistant and CZA-Sensitive CRKP Isolates |
Genotyping and MLST
Of the 10 CZA-resistant CRKP isolates with carbapenemase genes, five harbored blakpc-2, four harbored blaNDM-1 and two harbored blaNDM-5. Of note, one isolate harbored blaNDM-5 and blakpc-2. Of interest, the CZA-resistant CRKP group had different carbapenemase genes to the CZA-sensitive CRKP group. For example, the CZA-sensitive CRKP group had a higher positive rate to blakpc-2 than CZA-resistant CRKP group (32/33 vs 5/10), but a lower positive rate to blaNDM (0/33 vs 6/10) (P<0.05). Overall, all CZA-resistant CRKP isolates carried ESBL genes with nine isolates carrying SHV-11 and 10 isolates carrying CTX-M-14. Among the 10 CZA-resistant CRKP isolates, a total of 8 different STs were identified in this study (Table 4). The KPC-2 positive isolates were assigned to types ST241, ST37 and ST11, while isolates carrying NDM-5 and KPC-2 were assigned to ST782. The NDM-1 positive isolates were assigned to types ST784, ST1710, ST37 and ST324, and NDM-5 positive isolates were assigned to ST1419.
 |
Table 4 Genotypes and MLST of CZA-Resistant and CZA-Sensitive CRKP Isolates |
Discussion
Data from the Chinese Bacterial Resistance Surveillance Network (CHINET) showed that the prevalence of CRKP isolates in neonates increased from 2.2% in 2005 to 14.0% in 2017.22 This increasing tendency was associated with a higher mortality ranging from 20% to 64% among pediatric cases in China.17,23 CZA has been viewed as a valuable antibiotic against CRKP in American and European countries,18,24 and the administration of CZA appears to be well tolerated and efficacious in pediatric patients.12,13 In this study, the vitro activity of CZA against CRKP isolated from neonates was 77.7%. Two recent multicenter studies also showed that the vitro activity of CZA against CRKP in adults is high.25,26 Any decrease in susceptibility to CZA, a novel agent with minimal previous clinical use even in adults, is of concern. To the best of our knowledge, this is the first report of the vitro activity of CZA-resistant CRKP isolated from neonates, including premature neonates. Therefore, the novel findings of rather high resistance isolates to CZA in neonates need to pay extra more attention to summarize clinical characteristics of infected patients, to find any mechanism behind and track any potential transmission in NICU.
Furthermore, our study is first to report the clinical epidemiologic characteristics of infected or colonized CZA-resistant CRKP isolates from neonates including premature neonates. Very few studies have previously described the clinical features of CZA-resistant CRKP. Generally speaking, only the clinical features of individual infections in adults have been described.15,16 Most CZA-resistant CRKP strains in our study were isolated from respiratory tract specimens, and the most common disease was pneumonia. A large proportion of neonates in this study shared similar clinical features with Caesarean section deliveries, preterm births, low birth weights, and exposure to carbapenems/cephalosporin. Exposure to carbapenems/β-lactam antibiotics is considered as a risk factor associated with nosocomial infections with Carbapenem-Resistant Enterobacteriaceae.27
In addition, the distinction of antimicrobial activities of CZA-resistant and CZA-sensitive CRKP isolates was compared. The rate of resistance to AMK, NIT, LVX and CIP in the CZA-resistant CRKP group was lower than that in the CZA-sensitive CRKP group. This may be due to the positive of blakpc being lower in CZA-resistant than CZA-sensitive CRKP group. In a previously reported pediatric study, CRKP carrying blakpc showed higher resistance rates to AMK, LVX and NIT, compared with CRKP carrying blaNDM.28 The above-mentioned antibiotics may thus be an option for treating infections caused by CZA-resistant CRKP carrying blaNDM.
The further genetic analysis of those CZA-resistant CRKP strains based on the definition of CZA-resistant CRKP provided valuable additional information. CZA has been shown in vitro to inactive Ambler class A and C (including KPC) and some class D β-lactamases. However, CZA does not inhibit metallo-β-lactamases (including NDM).29 The major mechanism of CRKP resistance to carbapenems is the production of carbapenemase. Although KPC is the predominant carbapenemase in adults,30 the prevalent carbapenemase is different in pediatric patients. IMP-4 was the prevalent carbapenemase in 2010–2012, while NDM-1 was the most common carbapenemase in 2013–2014.31 KPC-2 was found to be the predominant carbapenemase in another study.28 In this study, the most frequently detected carbapenemase was KPC-2 (n=5) followed by NDM-1 (n=4). Production of different carbapenemases in Carbapenem-resistant Enterobacteriaceae (CRE) in different regions has also been reported.32 Our data agree with the previous conclusion in archived studies, but extend it with new information on neonates. Our findings indicate that identification of the carbapenemase genotype is essential, prior to the clinical application of CZA therapy.
CZA resistance has previously been associated with mutations in blaKPC (D179Y)26 and blaCTX-M (P179S).16 In some studies, resistance emerged after CZA therapy.14,33 Here, however, CZA was only approved for clinical anti-infection therapy in China in May 2019, so the resistance that has emerged cannot be attributed to CZA drug use, as none of the neonates received CZA treatment. CZA does not inhibit NDM,29 which explains all six NDM-producing CZA-resistant CRKP isolates. While resistance to CZA did not emerge, CZA sensitivity was somewhat lower in a case series of neonates and children <5 years of age patients with CRKP treated with CZA.13 Resistance emerged in 14% of adult patients with CRKP treated with CZA,11 and the predominant carbapenemase was KPC. As intestinal carriage of carbapenemase is an important source of transmission,34 patient-to-patient transmission may be a significant factor in other CZA-resistant CRKP isolates in this study. However, none of the reported mutations in blaKPC or blaCTX-M was detected in other KPC-producing K. pneumoniae in our study. Zhang, Humphries et al suggest this may be due to overexpression of the blaKPC gene.26,35 Nevertheless, this will require further study in the future to address any potential mechanisms of CZA-resistant CRKP-producing KPC.36
The transmission route of neonatal infection was tracked by using genetic markers, MLST. ST11, ST37 and ST241 of CZA-resistant CRKP predominated in NICU of our hospital. Our MLST data indicated that CZA-resistance is not limited to K. pneumoniae ST258,18 ST30714 or ST10115 found in European and North America, but can also emerge rapidly in other clonal backgrounds, such as ST11,26 ST37 and ST241 found in this China-based study but already successfully disseminates worldwide. Although the dissemination mechanism is still unclear, our findings highlight the importance of long-term active resistance surveillance of CZA-resistant CRKP in neonate patients.
Conclusion
We have reported the emergence of CZA-resistant CRKP strains among neonates prior to the approval of CZA for clinical use in China, and have described the clinical features and molecular characteristics of CZA-resistant CRKP strains were analyzed. Our findings emphasize the importance of clinical awareness, effective infection control measures and epidemiologic surveillance to prevent the onward transmission of CZA-resistant CRKP isolated from neonates.
Acknowledgments
We thank Dr Joy Fleming (Institute of Biophysics, Chinese Academy of Sciences) for linguistic assistance and helpful discussions during the preparation of this manuscript. This work was funded by grants from the Medical Science and Technology Project of Henan Province (2018020607, 2018020698).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perez F, El Chakhtoura NG, Papp-Wallace KM, et al. Treatment options for infections caused by carbapenem-resistant Enterobacteriaceae: can we apply “precision medicine” to antimicrobial chemotherapy? Expert Opin Pharmacother. 2016;17(6):761–781. doi:10.1517/14656566.2016.1145658
2. Ding Y, Wang Y, Hsia Y, et al. Systematic review of carbapenem-resistant Enterobacteriaceae causing neonatal sepsis in China. Ann Clin Microbiol Antimicrob. 2019;18(1):36. doi:10.1186/s12941-019-0334-9
3. Diaz A, Ortiz DC, Trujillo M, et al. Clinical characteristics of Carbapenem-resistant Klebsiella pneumoniae infections in ill and colonized children in Colombia. Pediatr Infect Dis J. 2016;35(3):237–241. doi:10.1097/INF.0000000000000987
4. Folgori L, Bielicki J. Future challenges in pediatric and neonatal sepsis: emerging pathogens and antimicrobial resistance. J Pediatr Intensive Care. 2019;8(1):17–24. doi:10.1055/s-0038-1677535
5. Esposito EP, Gaiarsa S, Del Franco M, et al. A novel IncA/C1 group conjugative plasmid, encoding VIM-1 metallo-beta-lactamase, mediates the acquisition of Carbapenem resistance in ST104 Klebsiella pneumoniae isolates from neonates in the intensive care unit of V.Monaldi hospital in Naples. Front Microbiol. 2017;8:2135. doi:10.3389/fmicb.2017.02135
6. Logan LK, Renschler JP, Gandra S, et al. Carbapenem-Resistant Enterobacteriaceae in children, United States, 1999–2012. Emerg Infect Dis. 2015;21(11):2014–2021. doi:10.3201/eid2111.150548
7. Zhou M, Chen J, Liu Y, et al. In vitro activities of Ceftaroline/avibactam, Ceftazidime/avibactam, and other comparators against pathogens from various complicated infections in China. Clin Infect Dis. 2018;67(suppl_2):S206–S16. doi:10.1093/cid/ciy659
8. Kazmierczak KM, de Jonge BLM, Stone GG, et al. In vitro activity of ceftazidime/avibactam against isolates of Pseudomonas aeruginosa collected in European countries: INFORM global surveillance 2012–15. J Antimicrob Chemother. 2018;73(10):2777–2781. doi:10.1093/jac/dky267
9. Potter RF, D’Souza AW, Dantas G. The rapid spread of carbapenem-resistant Enterobacteriaceae. Drug Resist Updat. 2016;29:30–46. doi:10.1016/j.drup.2016.09.002
10. Sharma R, Park TE, Moy S. Ceftazidime-avibactam: a novel Cephalosporin/ beta-lactamase inhibitor combination for the treatment of resistant gram-negative organisms. Clin Ther. 2016;38(3):431–444. doi:10.1016/j.clinthera.2016.01.018
11. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against Carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883–17. doi:10.1128/AAC.00883-17
12. Bradley JS, Armstrong J, Arrieta A, et al. Phase I study assessing the pharmacokinetic profile, safety, and tolerability of a single dose of Ceftazidime-avibactam in hospitalized pediatric patients. Antimicrob Agents Chemother. 2016;60(10):6252–6259. doi:10.1128/AAC.00862-16
13. Iosifidis E, Chorafa E, Agakidou E, et al. Use of Ceftazidime-avibactam for the treatment of extensively drug-resistant or pan drug-resistant Klebsiella pneumoniae in neonates and children <5 years of age. Pediatr Infect Dis J. 2019;38(8):812–815. doi:10.1097/INF.0000000000002344
14. Gaibani P, Campoli C, Lewis RE, et al. In vivo evolution of resistant subpopulations of KPC-producing Klebsiella pneumoniae during ceftazidime/avibactam treatment. J Antimicrob Chemother. 2018;73(6):1525–1529. doi:10.1093/jac/dky082
15. Gottig S, Frank D, Mungo E, et al. Emergence of ceftazidime/avibactam resistance in KPC-3-producing Klebsiella pneumoniae in vivo. J Antimicrob Chemother. 2019;74(1):3211–3216. doi:10.1093/jac/dkz330
16. Both A, Buttner H, Huang J, et al. Emergence of ceftazidime/avibactam non-susceptibility in an MDR Klebsiella pneumoniae isolate. J Antimicrob Chemother. 2017;72(9):2483–2488. doi:10.1093/jac/dkx179
17. Chiotos K, Han JH, Tamma PD. Carbapenem-resistant Enterobacteriaceae infections in children. Curr Infect Dis Rep. 2016;18(1):2. doi:10.1007/s11908-015-0510-9
18. Seifert H, Korber-Irrgang B, Kresken M, et al. In-vitro activity of ceftolozane/tazobactam against Pseudomonas aeruginosa and Enterobacteriaceae isolates recovered from hospitalized patients in Germany. Int J Antimicrob Agents. 2018;51(2):227–234. doi:10.1016/j.ijantimicag.2017.06.024
19. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing.
20. Sweden: European committee on antimicrobial susceptibility testing; 2018. Available from: http://www.eucast.org/clinical_breakpoints/.
21. Tian D, Pan F, Wang C, et al. Resistance phenotype and clinical molecular epidemiology of carbapenem-resistant Klebsiella pneumoniae among pediatric patients in Shanghai. Infect Drug Resist. 2018;11:1935–1943. doi:10.2147/IDR.S175584
22. Guo Y, Hu F, Zhu D, et al. Antimicrobial resistance changes of carbapenem-resistant Enterobacteriaceae strains isolated from children. Zhonghua Er Ke Za Zhi. 2018;12:907–914.
23. Daikos GL, Tsaousi S, Tzouvelekis LS, et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother. 2014;58(4):2322–2328. doi:10.1128/AAC.02166-13
24. Sutherland CA, Nicolau DP. Susceptibility profile of Ceftolozane/tazobactam and other parenteral antimicrobials against Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa from US hospitals. Clin Ther. 2015;37(7):1564–1571. doi:10.1016/j.clinthera.2015.05.501
25. Yin D, Wu S, Yang Y, et al. Results from the China Antimicrobial Surveillance Network (CHINET) in 2017 of the in vitro activities of Ceftazidime-avibactam and Ceftolozane-tazobactam against clinical isolates of Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2019;63(4):e02431–18. doi:10.1128/AAC.02431-18
26. Zhang P, Shi Q, Hu H, et al. Emergence of ceftazidime/avibactam resistance in carbapenem-resistant Klebsiella pneumoniae in China. Clin Microbiol Infect. 2020;26(1):
27. Wang B, Pan F, Wang C, et al. Molecular epidemiology of Carbapenem-resistant Klebsiella pneumoniae in a paediatric hospital in China. Int J Infect Dis. 2020;93:311–319. doi:10.1016/j.ijid.2020.02.009
28. Nour I, Eldegla HE, Nasef N, et al. Risk factors and clinical outcomes for carbapenem-resistant Gram-negative late-onset sepsis in a neonatal intensive care unit. J Hosp Infect. 2017;97(1):52–58. doi:10.1016/j.jhin.2017.05.025
29. Nichols WW, de Jonge BL, Kazmierczak KM, et al. In vitro susceptibility of global surveillance isolates of Pseudomonas aeruginosa to Ceftazidime-avibactam (INFORM 2012 to 2014). Antimicrob Agents Chemother. 2016;60(8):4743–4749. doi:10.1128/AAC.00220-16
30. Wang Q, Wang X, Wang J, et al. Phenotypic and genotypic characterization of Carbapenem-resistant Enterobacteriaceae: data from a longitudinal large-scale CRE study in China (2012–2016). Clin Infect Dis. 2018;67(suppl_2):S196–S205. doi:10.1093/cid/ciy660
31. Dong F, Zhang Y, Yao K, et al. Epidemiology of Carbapenem-resistant Klebsiella pneumoniae bloodstream infections in a Chinese children’s hospital: predominance of New Delhi metallo-beta-lactamase-1. Microb Drug Resist. 2018;24(2):154–160. doi:10.1089/mdr.2017.0031
32. Logan LK, Weinstein RA. The epidemiology of Carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis. 2017;215(suppl_1):S28–S36. doi:10.1093/infdis/jiw282
33. Giddins MJ, Macesic N, Annavajhala MK, et al. Successive emergence of Ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother. 2018;62(3):e02101–e02117. doi:10.1128/AAC.02101-17
34. Viau R, Frank KM, Jacobs MR, et al. Intestinal carriage of Carbapenemase-producing organisms: current status of surveillance methods. Clin Microbiol Rev. 2016;29(1):1–27. doi:10.1128/CMR.00108-14
35. Humphries RM, Hemarajata P. Resistance to Ceftazidime-avibactam in Klebsiella pneumoniae due to porin mutations and the increased expression of KPC-3. Antimicrob Agents Chemother. 2017;61(6):e00536–17. doi:10.1128/AAC.00537-17
36. Lee Y, Kim YA, Kim D, et al. Risk factors of community-onset extended-spectrum beta-lactamase-producing Klebsiella pneumoniae bacteremia in South Korea using National Health Insurance claims data. Int J Antimicrob Agents. 2019;54(6):723–727. doi:10.1016/j.ijantimicag.2019.09.005
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
