Back to Journals » International Journal of Women's Health » Volume 15
Clinical and Management Dilemmas Concerning Early-Stage Cervical Cancer in Pregnancy – A Case Report
Authors Kurniadi A , Setiawan D, Kireina J, Suardi D, Salima S , Erfiandi F, Andarini MY
Received 10 May 2023
Accepted for publication 1 July 2023
Published 27 July 2023 Volume 2023:15 Pages 1213—1218
DOI https://doi.org/10.2147/IJWH.S420801
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Everett Magann
Andi Kurniadi,1 Dani Setiawan,1 Jessica Kireina,1 Dodi Suardi,1 Siti Salima,1 Febia Erfiandi,1 Mia Yasmina Andarini2
1Department of Obstetrics and Gynecology, Universitas Padjadjaran, Bandung, Indonesia; 2Faculty of Medicine, Universitas Islam Bandung, Bandung, Indonesia
Correspondence: Andi Kurniadi, Department of Obstetrics and Gynecology, Universitas Padjadjaran, Bandung, Indonesia, Tel +62 812 2028 6197, Email [email protected]
Background: Cervical cancer in pregnancy is rare and its management remains a formidable challenge. Clinical upstaging is a serious concern. Presentation may mimic pregnancy-related conditions, thus delaying diagnosis and leading to an advanced stage at presentation. In addition, concerns regarding chemotherapy safety in pregnancy may hinder its administration. Definitive therapy may also be delayed due to pregnancy.
Case Report: A 37-year-old G3P2A0 10– 11 weeks pregnant woman was diagnosed with stage IB2 cervical cancer. We originally planned to perform neoadjuvant chemotherapy with paclitaxel 175mg/m2 and carboplatin 6 AUC every 21 days followed by caesarean section and radical hysterectomy. However, preoperatively, the tumor had grown further and progressed to stage IIB. Postpartum radiotherapy was thus indicated. Lower segmental caesarean section along with bilateral salpingectomy and ovarian transposition were performed. Radiotherapy was administered through external beam radiation therapy and brachytherapy. The patient delivered a small for gestational age male baby with no abnormalities. At 2-month follow-up, the infant appeared generally healthy.
Conclusion: Cancer diagnosis during pregnancy adversely impacts women’s physical and psychological states. Symptoms may mimic pregnancy-related conditions, thus delaying diagnosis. Its management involves a multidisciplinary team to protect both maternal and fetal health.
Keywords: cervical cancer, pregnancy, cancer in pregnancy, neoadjuvant chemotherapy
Introduction
Cancer during pregnancy is defined as any tumor found during pregnancy or in the immediate postpartum period.1 It is considered a very rare incident, occurring in 1/1000 pregnancies per year, accounting for around 0.07–0.1% of all malignant tumors. Melanoma and breast cancer are the most prevalent malignancies associated with pregnancy, followed by gynecological cancers, lymphomas, and leukemias.2 Cervical cancer is the most common gynecological cancer encountered during pregnancy.3 Around 1–3% of cervical cancer cases are diagnosed during pregnancy or postpartum.4 However, cervical cancer in pregnancy is still considered a rare occurrence, with an estimated incidence of 0.1–12.0 per 10.000 pregnancies.5 Most patients are diagnosed in early stage due to the wide availability of prenatal screening. Furthermore, advanced diseases might hinder conception.4,6 Common cervical cancer symptoms, ie painless vaginal bleeding, pelvic and lower back pain, or urinary frequency, might resemble pregnancy-related conditions causing a delay in diagnosis. In addition, antenatal care is often neglected by pregnant women, especially in developing countries, thus hindering tumor detection and leading to advanced stage upon presentation.5,6
The rarity of cervical cancer in pregnancy made large-scale studies hard to be conducted. Effective management guidelines for cervical cancer in pregnancy have been developed in recent years.7,8 However, these guidelines are mostly based on case studies and expert commentary. Therefore, treatment of cervical cancer in pregnancy remains a formidable challenge. The choice between deferring treatment until the patient’s pregnancy reaches full term or opting for immediate treatment is difficult for both the patient and the physician. Additionally, the safety of chemotherapy during pregnancy continues to raise numerous concerns, impeding its administration. Management of cervical cancer in pregnancy should involve a multidisciplinary team and should be individualized based on each patient’s gestational age and staging.5
Herein, we report a case of stage IB2 cervical cancer first diagnosed at 10–11 weeks of gestation. We originally planned to treat her with neoadjuvant chemotherapy followed by caesarean section and radical hysterectomy, but she ended up receiving radiation due to clinical-stage progression to stage IIB. With this case report, we wish to shed light on the rarity of this case, gaps in our knowledge, dilemmas we faced, and approaches taken to address them.
Case Report
A 37-year-old G3P2A0 10–11 weeks pregnant woman was referred to our gynecological oncology department with cervical cancer. She complained of contact bleeding since 1 month ago. She reported no abdominal or pelvic pain, no vaginal discharge, and no mass. She denied any medical and surgical histories. On physical examination, we found an exophytic 3×2 cm carcinomatous lesion without vaginal or parametrial involvement. The lesion displayed contact bleeding. Cervical biopsy revealed the diagnosis of squamous cell carcinoma. Ultrasonography result showed a single live fetus. She was eventually diagnosed with stage IB2 cervical cancer based on International Federation of Gynecology and Obstetrics (FIGO) staging of cervical cancer 2019.9
The patient was adamant about maintaining her pregnancy and refused surgery. The potential risks and complications of her choice were outlined, and she signed an informed consent form. Following much deliberation with a multidisciplinary team (MDT), we planned to give her neoadjuvant chemotherapy with paclitaxel 175mg/m2 and carboplatin 6 AUC every 21 days followed by caesarean section and radical hysterectomy whilst closely monitoring the growth of her tumour and the progress of her pregnancy. The patient received 5 cycles of chemotherapy from 14 to 34 weeks gestational age. The only side effect that she experienced throughout her chemotherapy cycles was anemia.
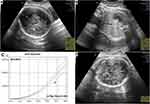
At 35–36 weeks gestational age, the patient came for her routine antenatal care. Fetomaternal ultrasonography was performed and the result indicated small for gestational age with estimated fetal weight <10th percentile on fetal growth curve (Figure 1A–D), adequate amniotic fluid (single deepest pocket of 4.58), normal Doppler cerebroplacental ratio, MCA PI 1.08 S/D 2.75, PSV:60.12 cm/s, A. UMB PI 1.03 S/D 2.65.
Given that foetal lung maturity is achieved at 34 weeks gestation and that a minimum of 3-week interval between the last cycle of chemotherapy (34 weeks) and delivery is recommended for cervical cancer in pregnancy, her caesarean section was scheduled at 38 weeks gestation. The interval between termination and last chemotherapy cycle will allow both maternal and fetal bone marrow to recuperate.5,7
At 38 weeks gestational age, during preoperative physical examination, we found that the exophytic carcinomatous lesion had grown to 3×4 cm and infiltrated both sides of the parametrium. The cancer-free space had been reduced by 50% in the left parametrium. Her diagnosis progressed to stage IIB cervical cancer. Therefore, after careful MDT discussion, we opted for postpartum radiotherapy as her definitive treatment. Under general anesthesia, we performed lower segmental caesarean section followed by bilateral salpingectomy and ovarian transposition. Ovarian transposition was performed prior to pelvic radiation to help preserve ovarian function.
A live 2330 gr, 45 cm male baby (small for gestational age, below the 10th percentile on the Lubchenco curve) was born with APGAR score at 1 and 5 minute of 7 and 9. No abnormalities were detected in the neonate’s physical examination, hematological panel, or biochemical analysis. The patient was discharged after seven days, and the abdominal incision healed well. She then underwent 25 sessions of external beam radiotherapy (EBRT) followed by 3 sessions of brachytherapy. Two months post-surgery, the infant was followed up and appeared to be in good general condition.
Discussion
Cervical cancer is the most common gynecological cancer in pregnancy.3 Symptoms of cervical cancer in pregnancy are the same as in the non-pregnant population.5 In the present case, our patient only complained of 1-month-long contact bleeding with no other additional symptoms. Early-stage cervical cancer may present as asymptomatic and can only be detected through cervical cytology. Visible signs and symptoms may include abnormal vaginal bleeding and discharge, dyspareunia, and abnormal cervix. Haematuria, urinary incontinence, lower limb edema, and alterations in bladder and bowel habits are additional symptoms that may be associated with local extension to pelvic organs. Bone pain or other systemic symptoms like weight loss, weakness, or loss of appetite may be signs of more distant metastasis. Pain in the flank or loin may indicate the presence of ureteral blockage. The abovementioned symptoms resemble pregnancy-related conditions, thus hindering diagnosis process.5,10 Furthermore, during pregnancy, gynecological examination is limited, causing a higher chance of misdiagnosis. Therefore, obstetricians should always consider cervical cancer in pregnant or postpartum women presenting with alarming symptoms such as vaginal bleeding. In these cases, the patients should be closely monitored, with gynecological examination and cytology screening carried out when necessary.
Staging cervical cancer in pregnancy is crucial in developing a personalized management plan for each patient. There is still very limited knowledge regarding the management of cervical cancer during pregnancy, as it is a very rare occurrence. Additionally, there are no large-scale research or randomized controlled trials available to establish high-grade recommendations. Recently, third international consensus meeting of members of the International Network on Cancer, Infertility and Pregnancy (INCIP) in collaboration with other international experts issued guidelines for gynecologic cancers in pregnancy. Management of cervical cancer in pregnancy varies according to the patient’s gestational age at the time of diagnosis, the extent of the tumor, and the patient’s desires concerning her pregnancy.7 Contrary to popular belief, most studies found no differences in prognosis between pregnant and non-pregnant women when it comes to cervical cancer. Thus, pregnancy-preserving options should always be considered, while non-preserving management reserved only for those with advanced disease (stage IIB or higher or with lymph node metastases).7,11
Our patient was first diagnosed with stage IB2 cervical cancer at 10–11 weeks of gestation. For stage IB2 diagnosed in less than 22nd weeks of gestation, two options can be considered: (1) pelvic lymphadenectomy followed by either chemotherapy or follow-up and (2) neoadjuvant chemotherapy (NACT) to help shrink and manage the disease.7 It is noteworthy to remember that chemotherapy is contraindicated in the first trimester of pregnancy because it interferes with organogenesis and has been associated with 10–20% increased risk of major fetal malformation. Thus, NACT should only be given following the completion of first trimester. Chemotherapy is generally feasible after 14 weeks of gestation because the incidence of fetal malformations is comparable to that of the general population.12 The effectiveness of platinum agents (cisplatin and carboplatin) and taxanes (such as paclitaxel) in treating cervical cancer has been widely reported.13 The carboplatin and paclitaxel regimen is preferred compared to cisplatin and paclitaxel because it is better tolerated with reduced risk of ototoxicity and nephrotoxicity.5,14 In this present case, after thorough discussion with a multidisciplinary team, we opted for neoadjuvant chemotherapy with paclitaxel 175mg/m2 and carboplatin 6 AUC as a 3-weekly regimen started at 14 weeks gestational age.
Fetal toxicity of chemotherapy depends on the amount of drugs passed on the fetus. Chemotherapy can affect growing fetus either directly or indirectly through the placenta. After organogenesis, chemotherapy can still negatively impact the fetal developing central nervous system, hematological system, eyes, and genitalia. Furthermore, chemotherapy can suppress both maternal and fetal bone marrow, causing anemia that can eventually affect fetal growth.10,15,16 The only chemotherapy side effect that our patient experienced was anemia. The results of our patient’s ultrasonography at 35–36 weeks gestational age suggested the presence of small for gestational age (defined as EFW or AC below the 10th percentile of given reference range (Figure 1C)), which was further confirmed by the baby’s birth weight (small for gestational age based on Lubchenco curve).17 This fetal growth disturbance might be a direct effect of chemotherapy-induced anemia. Our patient delivered a male baby without any abnormalities found in his physical examination, hematological panel, or biochemical analysis. This case supports the growing body of evidence that claims paclitaxel/carboplatin three-weekly regimen as safe for offspring. However, a longer follow-up period and larger studies are needed to truly determine this.
Caesarean section is the recommended mode of delivery as it reduces the risk of cancer recurrence in the episiotomy site and avoids active pushing that may cause pathological fractures or increased intracranial pressure in cases of metastases. Furthermore, with vaginal delivery, the tumor may obstruct the birth canal and cause massive bleeding during delivery.7,18,19 The time of the delivery must be carefully considered to ensure that the patient is not neutropenic. It is advisable to wait a minimum of three weeks between the final dosage of chemotherapy and delivery to enable time for maternal and fetal bone marrow regeneration. In addition, chemotherapy is not advised after 35 weeks of gestation to reduce the risk of spontaneous labor in patients who may be neutropenic.5,7 Our patient received 5 cycles of chemotherapy from 14 to 34 weeks gestational age and her caesarean section was scheduled 4 weeks after her last cycle of chemotherapy (at 38 weeks of gestation).
In our case, during preoperative physical examination, we found that our patient’s tumor has grown and her diagnosis changed to stage IIB cervical cancer. The efficacy of NACT has long remained a mystery, with only a few trials investigating this matter.14 Previous studies found that the rate of optimum response (reduction of tumor volume by >50%) following NACT was 78.8% in stage IB bulky tumors, however it reduced to only 60.7% in tumors with largest diameter of >2 cm.20,21 Our case questions the effectiveness of NACT as her lesion increased in size after NACT completion.
According to the present guideline, for patients with stage IB3 or above, once fetal maturation is achieved, delivery by caesarean section should be followed by either surgery or radiation.7 For this patient, we eventually decided on radiation as her definitive treatment. Therefore, after performing lower segmental caesarean section, we performed bilateral salpingectomy and ovarian transposition to help preserve ovarian function during radiation therapy. After that, the patient underwent 25 radiotherapy sessions followed by 3 sessions of brachytherapy. Transposing the ovaries above the pelvic brim and outside the planned radiotherapy field will permit subsequent retrieval of oocytes for in vitro fertilization and reduce the risk of premature menopause. Bilateral salpingectomy should be considered because it may reduce future risks of epithelial cancers, improve adnexa mobilization, and avoid tubal damage and hydrosalpinx formation due to radiation therapy or postoperative adhesion.5
Our report has its limitation. As per the time of this writing, follow-up was only done for up to 2-months post-surgery. A longer follow-up period and larger studies are needed to prove NACT safety for both the mother and child.
Conclusion
Cancer diagnosis during pregnancy is difficult and may be delayed as symptoms of cervical cancer often mimic pregnancy-related conditions. Its management is complex and multidisciplinary. Striking the right balance between preserving maternal and fetal health remains a challenge.
Ethics Approval and Informed Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request. The institutional review board has determined that our case report is exempted from an ethical review.
Consent for Publication
Written consent was obtained from patients regarding the subsequent publication of their anonymized data for research purposes.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Perrone AM, Bovicelli A, D’Andrilli G, Borghese G, Giordano A, De Iaco P. Cervical cancer in pregnancy: analysis of the literature and innovative approaches. J Cell Physiol. 2019;234(9):14975–14990. doi:10.1002/jcp.28340
2. Hepner A, Negrini D, Hase EA, et al. Cancer during pregnancy: the oncologist overview. World J Oncol. 2019;10(1):28–34. doi:10.14740/wjon1177
3. Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet. 2012;379(9815):558–569. doi:10.1016/S0140-6736(11)60829-5
4. Nocarová L, Ondruš D. Cervical cancer in pregnancy. Klin Onkol. 2020;33(4):268–273. doi:10.14735/amko2020268
5. Howe T, Lankester K, Kelly T, Watkins R, Kaushik S. Cervical cancer in pregnancy: diagnosis, staging and treatment. Obstet Gynaecol. 2022;24(1):31–39. doi:10.1111/TOG.12783
6. Van Calsteren K, Vergote I, Amant F. Cervical neoplasia during pregnancy: diagnosis, management and prognosis. Best Pract Res Clin Obstet Gynaecol. 2005;19(4):611–630. doi:10.1016/j.bpobgyn.2005.03.002
7. Amant F, Berveiller P, Boere IA, et al. Gynecologic cancers in pregnancy: guidelines based on a third international consensus meeting. Ann Oncol. 2019;30(10):1601–1612. doi:10.1093/annonc/mdz228
8. Morrison J, Balega J, Buckley L, et al. British Gynaecological Cancer Society (BGCS) uterine cancer guidelines: recommendations for practice. Eur J Obstet Gynecol Reprod Biol. 2022;270:50–89. doi:10.1016/j.ejogrb.2021.11.423
9. Bhatla N, Berek JS, Cuello Fredes M, et al. Revised FIGO staging for carcinoma of the cervix uteri. Int J Gynecol Obstet. 2019;145(1):129–135. doi:10.1002/IJGO.12749
10. Rhaidouni MA, Outifa Y, Abderrahmane MC, et al. Cervical cancer in pregnant women: a case report. Sch Int J Obstet Gynecol. 2021;4(4):95–98. doi:10.36348/sijog.2021.v04i04.003
11. Vandervange N, Weverling G, Ketting B, Ankum W, Samlal R, Lammes F. The prognosis of cervical cancer associated with pregnancy: a matched cohort study. Obstet Gynecol. 1995;85(6):1022–1026. doi:10.1016/0029-7844(95)00059-Z
12. Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy. Lancet Oncol. 2004;5(5):283–291. doi:10.1016/S1470-2045(04)01466-4
13. Bradley K, Crispens MA, Frederick P. NCCN clinical practice guidelines in oncology (NCCN Guidelines®) cervical cancer; 2021.
14. Guo Y, Zhang D, Li Y, Wang Y. A case of successful maintained pregnancy after neoadjuvant chemotherapy plus radical surgery for stage IB3 cervical cancer diagnosed at 13 weeks. BMC Pregnancy Childbirth. 2020;20(1):1–4. doi:10.1186/s12884-020-02895-y
15. Köhler C, Oppelt P, Favero G, et al. How much platinum passes the placental barrier? Analysis of platinum applications in 21 patients with cervical cancer during pregnancy. Am J Obstet Gynecol. 2015;213(2):206.e1–206.e5. doi:10.1016/j.ajog.2015.02.022
16. Kozuki N, Lee AC, Katz J. Moderate to severe, but not mild, maternal anemia is associated with increased risk of small-for-gestational-age outcomes 3. J Nutr. 2012;142(2):358–362. doi:10.3945/jn.111.149237
17. Lees CC, Stampalija T, Baschat A, et al. ISUOG Practice Guidelines: diagnosis and management of small-for-gestational-age fetus and fetal growth restriction. Ultrasound Obstet Gynecol. 2020;56(2):298–312. doi:10.1002/uog.22134
18. Leproux C, Awazu E, Boughalem E, Blouet A. Invasive cervical cancer diagnosed in an 8-month pregnant woman: a case report. J Mol Clin Med. 2020;3(4):109. doi:10.31083/j.jmcm.2020.04.902
19. Iavazzo C, Karachalios C, Iavazzo PE, Gkegkes ID. The implantation of cervical neoplasia at postpartum episiotomy scar: the clinical evidence. Irish J Med Sci. 2015;184(1):113–118. doi:10.1007/s11845-014-1208-y
20. Robova H, Rob L, Halaska MJ, et al. High-dose density neoadjuvant chemotherapy in bulky IB cervical cancer. Gynecol Oncol. 2013;128(1):49–53. doi:10.1016/j.ygyno.2012.10.002
21. Robova H, Halaska MJ, Pluta M, et al. Oncological and pregnancy outcomes after high-dose density neoadjuvant chemotherapy and fertility-sparing surgery in cervical cancer. Gynecol Oncol. 2014;135(2):213–216. doi:10.1016/j.ygyno.2014.08.021
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.