Back to Journals » ClinicoEconomics and Outcomes Research » Volume 15
Clinical and Economic Burden Associated with Disruptive Surgical Bleeding: A Retrospective Database Analysis
Authors Johnston SS, Afolabi M, Tewari P, Danker W
Received 10 March 2023
Accepted for publication 14 June 2023
Published 3 July 2023 Volume 2023:15 Pages 535—547
DOI https://doi.org/10.2147/CEOR.S411778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Samer Hamidi
Stephen S Johnston,1 Mosadoluwa Afolabi,1 Pranjal Tewari,2 Walter Danker3
1MedTech Epidemiology and Real-World Data Sciences, Johnson and Johnson, New Brunswick, NJ, USA; 2Mu Sigma, Bangalore, India; 3Franchise Health Economics and Market Access, Ethicon, Johnson & Johnson, Raritan, NJ, USA
Correspondence: Stephen S Johnston, MedTech Epidemiology and Real-World Data Sciences, Johnson & Johnson, 410 George Street, New Brunswick, NJ, 08901, USA, Tel +1-443-254-2222, Email [email protected]
Background: Hemostatic agents are used to control surgical bleeding; however, some patients experience disruptive bleeding despite the use of hemostats. In patients receiving hemostats, we compared clinical and economic outcomes between patients with vs without disruptive bleeding during a variety of surgical procedures.
Methods: This was a retrospective analysis of the Premier Healthcare Database. Study patients were age ≥ 18 with a hospital encounter for one of 9 procedures with evidence of hemostatic agent use between 1-Jan-2019 and 31-Dec-2019: cholecystectomy, coronary artery bypass grafting (CABG), cystectomy, hepatectomy, hysterectomy, pancreatectomy, peripheral vascular, thoracic, and valve procedures (first procedure = index). Patients were grouped by presence vs absence of disruptive bleeding. Outcomes evaluated during index included intensive care unit (ICU) admission/duration, ventilator use, operating room time, length of stay (LOS), in-hospital mortality, and total hospital costs; 90-day all-cause inpatient readmission was also evaluated. Multivariable analyses were used to examine the association of disruptive bleeding with outcomes, adjusting for patient, procedure, and hospital/provider characteristics.
Results: The study included 51,448 patients; 16% had disruptive bleeding (range 1.5% for cholecystectomy to 44.4% for valve). In procedures for which ICU and ventilator use is not routine, disruptive bleeding was associated with significant increases in the risks of admission to ICU and requirement for ventilator (all p≤ 0.05). Across all procedures, disruptive bleeding was also associated with significant incremental increases in days spent in ICU (all p≤ 0.05, except CABG), LOS (all p≤ 0.05, except thoracic), and total hospital costs (all p≤ 0.05); 90-day all-cause inpatient readmission, in-hospital mortality, and operating room time were higher in the presence of disruptive bleeding and varied in statistical significance across procedures.
Conclusion: Disruptive bleeding was associated with substantial clinical and economic burden across a wide variety of surgical procedures. Findings emphasize the need for more effective and timely intervention for surgical bleeding events.
Keywords: health resource utilization, hospital costs, burden of bleeding, real-world evidence, RWE, hemostatic agent
Introduction
Bleeding, a common intra- and post-operative surgical complication, may range from mild to severe in both its intensity and impact.1 At the extreme, surgery-related bleeding increases mortality risk and has also been associated with increased use of high-intensity and expensive health-care services (eg, intensive care unit [ICU] admissions), longer operating times and hospital stays, elevated risk of a reoperation, and higher all-cause direct health-care costs.2–4 A variety of hemostatic agents are routinely used to control bleeding during surgical procedures in hospitals in the United States; however, some patients experience disruptive bleeding despite the use of hemostats.2 A previous study by Corral et al documented an association of disruptive bleeding with higher mortality risk, as well as greater healthcare resource use and total hospital costs among patients who had received a hemostatic agent during one of eight surgical procedures deemed by surgeons to carry substantial risk of bleeding. However, the surgeries evaluated in that study were all performed via an open surgical approach and all in calendar year 2012; since that time, additional hemostatic agents have become available in the US and a systematic shift toward minimally invasive surgeries (MIS) has occurred.5–7
These fundamental changes in the surgical landscape suggest a need to revisit the question of how surgery-related disruptive bleeding may impact outcomes. Therefore, the current study was undertaken to examine the contemporary association of disruptive bleeding with healthcare resource utilization, in-hospital mortality, and total hospital costs among patients who received hemostats during selected surgical procedures. Consistent with the aforementioned analysis conducted by Corral et al, the procedures selected for study represent a wide variety of surgical specialties, including cardiovascular (coronary artery bypass graph, valve, and peripheral valve procedures), hepatopancreatobiliary/general surgery (hepatectomy, pancreatectomy, and cholecystectomy), gynecology (hysterectomy), thoracic (lung resection), and urology (cystectomy).2
Materials and Methods
Data Source
We conducted this retrospective observational study using electronic health record and hospital billing data from the Premier® Healthcare Database ((PHD), Premier, Inc., NC, USA).8 PHD is an all-payer, United States (US) population-based research database that contains inpatient and outpatient hospital billing records from over 900 hospitals and health systems that participated in the Premier Healthcare Performance Improvement Alliance at the time the data were obtained. The hospitals in the PHD are nationally representative with respect to bed size, geographic region, location (urban/rural) and teaching status. Source data include hospital discharge-level information on patient demographics, primary and secondary diagnoses, treatments (eg, procedures, medical devices/supplies, and medications), length of hospital stay, and discharge disposition, as well as information on facility and provider characteristics. For each encounter, the PHD also contains hospital cost data corresponding to a date-stamped log of all billed items by cost-accounting department. This analysis of the PHD was conducted under an exemption from Institutional Review Board oversight for US-based studies using de-identified health-care records, as dictated by Title 45 Code of Federal Regulations (45 CFR 46.101(b)(4)).9
Patient Selection
We selected patients aged 18 years or older at the time of their first (index) inpatient or outpatient discharge for one of nine procedures of interest between January 1, 2019, and December 31, 2019: coronary artery bypass grafting (CABG), cholecystectomy, cystectomy, hepatectomy, hysterectomy, lung resection, pancreatectomy, peripheral vascular procedures (PVP), valve repair/replacement.
For the primary analysis, we included patients with evidence of hemostatic agent use during the index procedure; patients who met the other study selection criteria regardless of whether or not they had received a hemostatic agent were also examined in a sensitivity analysis. For both the primary and sensitivity analysis, we included only patients whose index procedure was performed at a hospital that contributed data to the PHD for at least 90 days post-discharge. We excluded patients whose index admission occurred through transfer from a different hospital, those with missing discharge status or sex, or those with zero or negative billed amounts for total, room and board, and/or supply costs.
Measurement of Disruptive Bleeding and Outcome Variables
We classified each patient into one of two mutually exclusive cohorts based on the presence or absence of at least one event indicative of disruptive bleeding during the index procedure.2 We identified disruptive bleeding events using the International Classification of Diseases, 10th Revision, Clinical Modification/Procedure Classification System (ICD-10-CM/PCS) diagnosis and procedure codes for hemorrhage or hematoma complicating a procedure and interventions to control bleeding; charges billed for use of hemovac drainage devices; charges billed for use of erythropoietin; blood product transfusions; and charges billed for cryoprecipitates, fresh frozen plasma, red blood cells, plasma, platelets, and whole blood. We required bleeding-related diagnoses to not be designated as “present on admission”, an indicator that delineates conditions that were pre-existing upon admission vs those that were diagnosed acutely during the admission.
Outcomes evaluated during index included operating room time, intensive care unit (ICU) admission, ICU duration, ventilator use, length of stay, in-hospital mortality, and total hospital costs (ie, costs of the index admission from the hospital perspective); 90-day all-cause inpatient readmission was also evaluated.
Measurement of Patient, Procedure, and Hospital/Provider Characteristics
In order to characterize the study cohort and adjust for underlying differences between patients with vs without disruptive bleeding in the analysis of outcomes, we measured the following characteristics during index: patients’ age, sex, race/ethnicity, marital status, payer type (eg, Medicare, Medicaid, Commercial, and other), comorbidity burden as quantified by the Charlson Comorbidity Index (CCI) Score10 and Elixhauser comorbidities;11,12 surgical indication; hospital bed size, US Census region, urban vs rural location, teaching vs non-teaching status, procedure volume for the index procedure type under evaluation, and physician specialty.
Statistical Analysis
We used generalized linear models (GLM) to quantify the association between disruptive bleeding and the study outcomes, adjusting for all patient, procedure, and hospital/provider characteristics described above. A log link and binomial error distribution were used for binary outcomes; a log link and negative binomial error distribution were used for count outcomes; a log link and gamma error distribution were used for total hospital costs. We conducted separate models for each of the nine types of index procedures. A p-value of ≤0.05 was used as the threshold for statistical significance. We performed all analyses with StataSE 16 (StataCorp, College Station, Texas, US).
Results
Figure 1 shows the sample size attrition associated with each step of patient selection. Among 349,763 patients with at least one hospital discharge for a procedure of interest in the 2019 calendar year, 304,074 patients met the final patient selection criteria for the broad sensitivity analysis population. Of these 304,074 patients, 51,448 patients had received a hemostatic agent during their index procedure and therefore contributed data for the primary analysis.
 |
Figure 1 Patient selection. |
Primary Analysis
Figure 2 shows patient counts and the incidence proportion of disruptive bleeding during the index admission for each of the nine procedures of interest. The number of patients included for the primary analysis ranged from 635 for cystectomy to 22,121 for hysterectomy. Overall, 8444 (16%) patients had disruptive bleeding during their index admission, while the remaining 43,004 patients had no evidence of disruptive bleeding. The incidence proportion of disruptive bleeding during index admission ranged from 1.5% of patients who underwent cholecystectomy to 44.4% of patients with valve procedures.
 |
Figure 2 Incidence proportion of disruptive bleeding during index admission. |
Table 1 shows selected patient characteristics, stratified by patients with vs without disruptive bleeding; these groups differed in terms of demographics, type of insurance coverage, and comorbidity burden. Compared with patients without disruptive bleeding, patients with disruptive bleeding were on average nearly 10 years older, more likely to be male, have Medicare coverage, and carried a greater comorbidity burden.
 |
Table 1 Selected Patient Characteristics Among Patients in Whom a Hemostat Was Used |
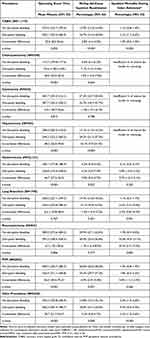
Table 2 shows the adjusted association of disruptive bleeding with mean operating room time, 90-day all-cause inpatient readmission rates, and inpatient mortality rates, stratified by index surgical procedure. Disruptive bleeding was associated with significantly longer operating room times for patients undergoing CABG, cholecystectomy, hepatectomy, hysterectomy, PVP, and valve procedures (incremental increases ranging from 22.6 minutes for CABG to 48.2 minutes for hepatectomy, all p≤0.05). Disruptive bleeding was also associated with statistically significant increased risks of 90-day all-cause readmission for patients undergoing CABG, hepatectomy, hysterectomy, PVP, and valve procedures (incremental absolute risk increases ranging from 2.0% for hysterectomy to 16.1% for hepatectomy, all p≤0.05). Finally, disruptive bleeding conferred a substantial and significantly significant increase in the risk of inpatient mortality during the index admission for patients undergoing CABG, cystectomy, lung resection, pancreatectomy, and valve procedures (incremental absolute risk increases ranging from 1.0% for CABG to 28.4% for cystectomy, all p≤0.05).
Table 3 shows the adjusted association of disruptive bleeding with admission to ICU, duration of ICU stay, and ventilator use, stratified by index surgical procedure. With the exception of CABG and valve procedures, for which admission to ICU is routine, disruptive bleeding was associated with statistically significant increased risks of admission to ICU in all other procedures (incremental absolute risk increases ranging from 2.1% for hysterectomy to 20.4% for cystectomy, all p≤0.05) and longer durations of ICU stay (incremental increases ranging from 0.3 days for hysterectomy to 1.6 days for cystectomy, all p≤0.05). Disruptive bleeding was also associated with statistically significant higher risks of ventilator use among patients undergoing hepatectomy, hysterectomy, lung resection, pancreatectomy, and PVP procedures (incremental absolute risk increases ranging from 4.7% for hysterectomy to 21.3% for hepatectomy, all p≤0.05).
 |
Table 3 Adjusted Association of Disruptive Bleeding with Admission to ICU, Duration of ICU Stay, and Ventilator Use, Stratified by Index Surgical Procedure Among Patients in Whom a Hemostat Was Used |
Table 4 shows the adjusted association of disruptive bleeding with total hospital cost and length of stay for index admission, stratified by index surgical procedure. Disruptive bleeding was uniformly associated with statistically significant increases in total hospital costs for all procedures (incremental increases ranging from $3329 for cholecystectomy to $14,762 for valve procedures, all p≤0.05), and increases in length of stay for all procedures except lung resection (incremental increases ranging from 1.4 days for CABG to 6.3 days for cholecystectomy, all p≤0.05).
 |
Table 4 Adjusted Association of Disruptive Bleeding with Total Hospital Cost and Length of Stay for the Index Admission, Stratified by Index Surgical Procedure |
Sensitivity Analysis of Broad Population
Results of sensitivity analysis in the broad population (ie, not restricted to patients with hemostatic agent use) are presented in the Supplement. As in the primary analysis, with few exceptions disruptive bleeding was associated with statistically significant increases in the risks or magnitude of all study outcomes.
Discussion
This analysis of over 50,000 patients undergoing a wide variety of surgical procedures demonstrates the substantial clinical and economic burden associated with disruptive bleeding for US patients and hospitals.
The occurrence and impact of disruptive bleeding during routine surgeries has been studied previously, although the relevant literature is relatively sparse. Previous studies have provided somewhat more limited insights than the current study as they were often focused on a single type of procedure or specific geography/patient population (eg, critically ill patient), and all are based on older data and therefore they do not reflect the current surgical landscape.2–4,13–17 The current study, for instance, shares a study design and data source common with the study published by Corral et al in 2015, but whereas that study included only open procedures, this study also included minimally invasive surgeries, a change made to better reflect the contemporary surgical landscape. Comparing results from these two studies suggests that there may have been some improvements in the procedure-specific incidence of disruptive bleeding in the intervening time. Specifically, the earlier study reported disruptive bleeding in 56% (versus 34%) of CABG patients, 68% (versus 44%) of cardiac valve surgery patients, 39% (versus 2%) of cholecystectomy patients, 60% (versus 24%) of cystectomy patients and 50% (versus 24%) in patients with pancreatic surgery. Not surprisingly, the incremental cost of bleeding is higher now than that previously reported for procedures that are still primarily performed using open approaches (ie, incremental costs reported in Corral et al vs current study for CABG: $8910 vs $9504; for cardiac valve procedures: $13,286 vs $14,762). For the procedures evaluated in both studies and which have recently largely shifted to minimally invasive approaches (eg, cholecystectomy, cystectomy, pancreatic surgery, and hysterectomy) the incremental cost of bleeding is from $1888 to $8289 lower than previously reported. Future studies may be designed to quantify temporal trends in the incidence and incremental costs of disruptive bleeding and to identify factors that shaped these trends, but it seems reasonable to suggest that improvements in surgical techniques, including an increasing array of hemostatic agents and greater use of minimally invasive approaches, have played a role.
A wide variety of hemostatic agents are available to clinicians, including those with mechanical, biological, and clot-forming properties, delivered through patches, sealants, and powders. A recent retrospective cost analysis by Ramirez et al estimated that the use of active hemostatic agents to reduce bleeds and the need for transfusions resulted in per-patient savings of $19,472 by reducing blood product use in cardiac, thoracic, and ortho–ortho spine surgeries.17 However, the present study suggests that even with the use of hemostatic agents, disruptive bleeding can occur with significant consequences. Although future investigation is needed to reveal the specific underlying factors that influence the risk of disruptive bleeding even with the use of hemostatic agents, the present study’s findings suggest there may be room for improvement in terms of hemostatic agent choice optimization and more effective and timely intervention for surgical bleeding events.
Limitations
As with any research study, an understanding of key data and/or design limitations provides important context for interpreting results. First, the selection of patients and measurement of many covariates and outcomes relied on diagnosis and procedure codes recorded within the PHD. Although these data are primarily recorded to support clinical and billing activity, they may be subject to measurement error. Second, in the primary analysis, all patients received hemostatic agents, suggesting that at least some amount of surgical bleeding took place to necessitate intervention. The classification of patients into the disruptive bleeding group relied on the presence of diagnoses, procedures, and use of products related bleeding; although the documentation of such information may simply be reflective of the surgical bleeding that originally necessitated the use of a hemostatic agent, the significant association of disruptive bleeding with the study outcomes suggests that our classification was indeed a meaningful differentiator. Third, mortality is typically incompletely captured in real-world data sources, such as administrative claims and health systems data. This issue was mitigated by limiting our analysis to deaths that occurred during the facility visit for the index procedure. Although our results provide an important perspective on the role of disruptive bleeding in shaping mortality risk, we acknowledge that the overall procedure-related mortality risk is likely greater than that reported here since some patients may die post-discharge. Fourth, drawing on a prior published definition,2 we defined bleeding events based on a combination of diagnoses for hemorrhage or hematoma complicating a procedure, procedures codes for interventions to control bleeding, and use of other interventions such as hemovac drainage devices, erythropoietin, and blood products. As Premier Healthcare Database does not have information on the specific clinical indication for which the erythropoietin was used, it is possible that some uses may have been for indications aside from intra- or post-operative bleeding per-se; however, this accounted for a relatively small number of patients (N=937 across all surgical procedures) and therefore over-identification of bleeding events would likely be minimal and potentially offset by false-negative instances of bleeding (ie, bleeding occurred but was not clinically documented). On balance, it is unlikely that the estimated incremental burden associated with disruptive bleeding would be heavily influenced by inclusion or exclusion of these cases. Finally, we note that PHD is an all-payer database that is generally considered nationally representative of US hospitals; however, the present results may not necessarily generalize to all hospitals or international settings.
Conclusions
The incidence of disruptive bleeding among patients in whom a hemostat was used varied by procedure type and was associated with substantial clinical and economic burden across a wide variety of surgical procedures. These findings emphasize the need for more effective and timely intervention for surgical bleeding events.
Acknowledgments
The authors would like to acknowledge Sally Wade of Wade Outcomes Research and Consulting for proving writing support for the manuscript, which was funded by Johnson & Johnson. The abstract of this paper was presented at the 2022 38th Annual International Conference for Pharmacoepidemiology, Bella Center Copenhagen, August 24–28, 2022, Copenhagen, Denmark, as a poster presentation with interim findings. The poster’s abstract was published in “ABSTRACTS of ICPE 2022” in Pharmacoepidemiology and Drug Safety: Abstract 1011 https://onlinelibrary.wiley.com/doi/full/10.1002/pds.5518
Funding
This study was funded by Johnson & Johnson (Ethicon, Somerville, NJ).
Disclosure
SSJ and WD are employees and stockholders of Johnson & Johnson. PT is an employee of Mu Sigma. MA and PT provided data and analysis support under contract to Johnson & Johnson. The authors report no other conflicts of interest in this work.
References
1. Thomas AB, Shammas RL, Orr J, et al. An assessment of bleeding complications necessitating blood transfusion across inpatient plastic surgery procedures: a nationwide analysis using the national surgical quality improvement program database. Plast Reconstr Surg. 2019;143:1109e–17e. doi:10.1097/PRS.0000000000005537
2. Corral M, Ferko N, Hollmann S, Broder MS, Chang E. Health and economic outcomes associated with uncontrolled surgical bleeding: a retrospective analysis of the premier perspectives database. Clinicoecon Outcomes Res. 2015;7:409–421. doi:10.2147/CEOR.S86369
3. Johnston SS, Jamous N, Mistry S, et al. Association of in-hospital surgical bleeding events with prolonged hospital length of stay, days spent in critical care, complications, and mortality: a retrospective cohort study among patients undergoing neoplasm-directed surgeries in English hospitals. Clinicoecon Outcomes Res. 2021;13:19–29. doi:10.2147/CEOR.S287970
4. Al-Attar N, Johnston S, Jamous N, et al. Impact of bleeding complications on length of stay and critical care utilization in cardiac surgery patients in England. J Cardiothorac Surg. 2019;14:64. doi:10.1186/s13019-019-0881-3
5. Akhtar-Danseh -G-G, Akhtar-Danesh N, Finley C. Uptake and survival effects of minimally invasive surgery for lung cancer: a population-based study. Eur J Surgl Oncol. 2021;47:1791–1796. doi:10.1016/j.ejso.2021.01.002
6. Williams AD, Sun T, Kakade S, Wong SL, Shulman LN, Carp NZ. Comparison of open and minimally invasive approaches to colon cancer resection in compliance with 12 regional lymph node harvest quality measure. J Surg Oncol. 2021;123:986–996. doi:10.1002/jso.26298
7. Unruh KR, Bastawrous AL, Bernier GV, et al. Evaluating the regional uptake of minimally invasive colorectal surgery: a report from the surgical care outcomes assessment program. J Gastrointest Surg. 2021;25:2387–2397. doi:10.1007/s11605-020-04875-1
8. Premier Applied Sciences. Premier healthcare database white paper: data that informs and performs; 2020.
9. 45 CFR 46.104 -- Exempt research. Available from: https://www.ecfr.gov/current/title-45/subtitle-A/subchapter-A/part-46/subpart-A/section-46.104.
10. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi:10.1016/0895-4356(92)90133-8
11. Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi:10.1097/00005650-199801000-00004
12. van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47:626–633. doi:10.1097/MLR.0b013e31819432e5
13. Lauzier F, Arnold DM, Rabbat C, et al. Risk factors and impact of major bleeding in critically ill patients receiving heparin thromboprophylaxis. Intensive Care Med. 2013;39:2135–2143. doi:10.1007/s00134-013-3044-3
14. Stone GW, Clayton TC, Mehran R, et al. Impact of major bleeding and blood transfusions after cardiac surgery: analysis from the Acute Catheterization and Urgent Intervention Triage strategY (ACUITY) trial. Am Heart J. 2012;163:522–529. doi:10.1016/j.ahj.2011.11.016
15. Christensen MC, Krapf S, Kempel A, von Heymann C. Costs of excessive postoperative hemorrhage in cardiac surgery. J Thorac Cardiovasc Surg. 2009;138:687–693. doi:10.1016/j.jtcvs.2009.02.021
16. Stokes ME, Ye X, Shah M, et al. Impact of bleeding-related complications and/or blood product transfusions on hospital costs in inpatient surgical patients. BMC Health Serv Res. 2011;11:135. doi:10.1186/1472-6963-11-135
17. Ramirez GM, Castillo G, Ramirez AM. The economic burden of bleeds and transfusions in selected surgeries: a retrospective multi center analysis from the us perspective. AJBSR. 2019;2:211–215. doi:10.34297/AJBSR.2019.02.000610
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.