Back to Journals » Infection and Drug Resistance » Volume 15
Clinical Analysis of 44 Children with Subacute Necrotizing Lymphadenitis
Authors Zheng Y, Du Y, Zhu WH, Zhao CG
Received 7 December 2021
Accepted for publication 11 March 2022
Published 31 March 2022 Volume 2022:15 Pages 1449—1457
DOI https://doi.org/10.2147/IDR.S351191
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yue Zheng, Yue Du, Wan-Hong Zhu, Cheng-Guang Zhao
Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, 111004, People’s Republic of China
Correspondence: Cheng-Guang Zhao, Department of Pediatrics, Shengjing Hospital of China Medical University, Shenyang, 110004, People’s Republic of China, Tel +8618940255157, Email [email protected]
Objective: To explore the causes, clinical features, and laboratory examination characteristics of subacute necrotizing lymphadenitis to improve the understanding of this disease, reduce misdiagnosis, and make appropriate diagnoses and treatments.
Methods: The clinical data of 44 children with subacute necrotizing lymphadenitis admitted to our hospital from January 2013 to August 2021 were collected. The clinical and laboratory examinations of the disease were systematically analyzed.
Results: All 44 cases aged 6– 13 years and averaged 10.3 ± 2.1 years, including 32 boys and 12 girls, were diagnosed with histiocytic necrotizing lymphadenitis (HNL) by lymph node biopsy. Forty-two cases had palpable superficial lymph nodes, and two showed abdominal lymph node enlargement in the imaging examination. The symptoms might be accompanied by mild hepatomegaly or splenomegaly, emaciation, night sweats, rashes, and other manifestations. Leukopenia and neutropenia occurred. The pathological features were extensive coagulative necrosis of lymph nodes with reactive hyperplasia of histiocytes as well as positive IHCl CD68. Four cases had a relapse, and the prognosis of the other cases was good.
Conclusion: The case with fever and lymph node enlargement of unknown origin and no response to antibiotic treatment should undergo a pathological examination as early as possible for diagnosis and treatment. The disease has a good prognosis in most cases but may recur in a few cases.
Keywords: children, fever, subacute necrotizing lymphadenitis, prognosis
Introduction
Subacute necrotizing lymphadenitis (SNL), also called histiocytic necrotizing lymphadenitis (HNL) or Kikuchi-Fujimoto disease (KFD), is a benign self-limiting lymph node enlargement disease that was first reported by Yoshihide Fujimoto and Masahiro Kikuchi in 1972. Its main clinical manifestations include fever and lymph node enlargement as well as rashes, emaciation, fatigue, night sweats, joint pain, and so on in some cases. This disease has no specific clinical manifestations or auxiliary examinations, so it is often misdiagnosed or diagnosed late. This article retrospectively analyzes the clinical features, laboratory examinations, prognoses, and other aspects of 44 children with SNL admitted to our center to improve the understanding of the disease for early diagnosis and treatment.
Materials and Methods
The clinical data of 44 children with SNL admitted to ShengJing Hospital of China Medical University from January 2013 to August 2021 were collected. All 44 children underwent lymph node biopsies, and their pathogenic manifestations were consistent with HNL. Medical records were consulted to retrospectively analyze their ages, genders, clinical manifestations (including fever, rashes, night sweats, weight loss, fatigue, joint pain, headache, hepatomegaly, and splenomegaly), and local lymph node signs (size, location, texture activity, local tenderness, and other aspects), laboratory examination results [including white blood cell (WBC), C reactive protein (CRP), erythrocyte sedimentation rate (ESR), lactate dehydrogenase (LDH), Alanine aminotransferase (ALT), immunological indicators, autoantibodies, and pathogens], ultrasound results, and pathological characteristics. This study was conducted in accordance with the declaration of Helsinki and approved by the Ethics Committee of Shengjing Hospital of China Medical University. Written informed consent was obtained from the study participants’ guardians prior to study commencement.
SPSS 21.0 software was used for statistical analysis. A P-value of less than 0.05 was considered statistically significant. The measured data were expressed as the average value ± SD, and the calculated data were expressed as a percentage (%).
Results
General Information
The 44 children with SNL including 32 boys and 12 girls aged 6–13 years and with an average age of 10.3 ± 2.1 years.
Clinical Manifestations (See Table 1)
① Fever: all children had a fever, with a peak body temperature of around 39°C. The fever type was irregular, the duration ranged from 7 to 90 days, and antibiotic treatment was ineffective. ② Lymph nodes (See Table 2): 42 out of 44 cases had palpable superficial lymph nodes, and the lymph node enlargement duration ranged from 10 days to three months. In these 42 cases, 7 had a fever accompanied by lymph node enlargement, 17 had a fever before lymph node enlargement, 14 had lymph node enlargement before a fever, and four saw the doctor only because of fever, and the lymph node enlargement was found by the doctor (the specific time when lymph node enlargement occurred was unclear). Forty cases (90.9%) primarily had cervical lymph node enlargement. Except for one case with unilateral enlargement, the other 39 had bilateral cervical lymph node enlargement. Two cases (4.5%) had axillary lymph node enlargement. The enlarged lymph nodes ranged from 1.7×1.3×1.1-3.8×1.8×1.5cm. They were soft, with good activity, free of adhesion as well as local redness and swelling, and accompanied by tenderness in some cases (33 cases, 75%). Two cases simply showed a fever and were found with celiac lymph node enlargement in the imaging examination, including one with abdominal pain and one without. ③ Others: one case had mild hepatosplenomegaly, and six had mild splenomegaly. Four cases (9.1%) had pleomorphic transient rashes, and there was no pigmentation or desquamation left after the rashes subsided. In these four cases, three showed rashes only on their faces, and one had rashes on both face and wrists. Five cases (11.4%) had emaciation with weight loss ranging from 2–5 kg in the course of the disease, two (4.5%) had night sweats, two (4.5%) experienced fatigue, one (2.3%) had transient hip pain, and one (2.3%) had headaches.
 |
Table 1 General Conditions and Clinical Manifestations |
 |
Table 2 Lymph Node Conditions |
Auxiliary Examinations (See Table 3)
① Forty-four cases had a WBC ranging from 1.47–9.1 × 109/L with an average of 3.5 ± 1.3 × 109/L, including 7 (15.9%) less than 2.5 × 109/L, 11 (25%) of 2.5–3.0 × 109/L, 14 (31.8%) of 3.0–4.0 × 109/L, and 12 (27.3%) over 4.0 × 109/L. All cases had no abnormal white blood cells. Six had mild anemia, while the remaining cases had normal hemoglobin. The BPCs of 44 cases were normal. ② Twenty cases had increased CRP, and 24 had normal CRP. ③ Forty-one out of 44 cases underwent the ESR examination. Thirty-six (81.8%) out of 41 cases had a higher ESR of 23–93 mm/h. ④ Fourteen cases had different degrees of increased ALT levels, with the highest ALT level 190 U/L and the highest Aspartate aminotransferase (AST) level 93 U/L. ⑤ Forty-two cases underwent the LDH test. Their LDH levels were within 233–663 U/L, with different degrees of increase and an average of 389.7 ± 114.8 U/L. ⑥ All cases underwent a series of immunoglobulin tests. It was found that one case (2.3%) had a higher IgG level, nine (20.45%) had higher IgM levels, and one (2.3%) had a lower IgM level. Absolute T lymphocyte count: the CD4/CD8 ratio decreased in nine cases, and the natural killer cell count decreased in six. ⑦ Rheumatism immune indicators: 40 cases underwent the ANA titer and ANA tests. It was found that four cases had positive ANA titers (1:100 for two, 1:160 for one, and 1:320 for one case) and negative ANA test results. Thirty-nine cases underwent the rheumatoid factor examination, with one showing a slight increase. ⑧ Etiological examinations: all cases underwent the tuberculin test or tuberculosis spot test, and all results were negative. Forty-one cases underwent the blood culture test, with no flora growth observed. Two had a streptococcal infection, and three showed positive IgM antibodies against mycoplasma, three had positive IgM antibodies against the herpes virus, two were positive for IgM antibodies against the EB virus, three were positive for the IgM antibodies against the parainfluenza virus, one was positive for the IgA antibodies against the respiratory syncytial virus, and one was positive for the IgA antibodies against adenoviridae. ⑨ Ultrasound findings: all cases underwent an ultrasound examination. The findings showed well-circumscribed lesions in all cases, with a low echo in 21, a low echo in the surrounding area and a high echo in the center of the lesion in 23, and uncalcified foci in all cases (See Figures 1 and 2).
 |
Table 3 Laboratory Examination |
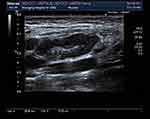 |
Figure 1 Ultrasonogram of lymph node. |
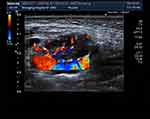 |
Figure 2 Rheography of lymph node. |
Pathological Examinations
① Forty-four cases underwent bone marrow aspiration examinations and had infected or normal bone marrow images. Five cases had visible hemophagocytic histiocytes. ② All cases underwent the lymph node biopsies, with results consistent with the manifestations of SNL: most structures in the lymph nodes were destroyed; extensive coagulative necrosis in the cortical and paracortical areas of the lymph nodes was accompanied by a reactive proliferation of histiocytes; there were a large number of nuclear fragments as well as karyopyknosis and karyolysis cells in the necrotic foci; histiocyte infiltration was observed, but neutrophil infiltration was not, and the IHC CD68 was positive (see Figures 3–5).
 |
Figure 3 Pathological image of lymph node (4X). |
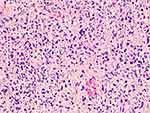 |
Figure 4 Pathological image of lymph node (40X). |
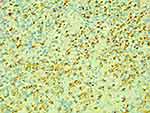 |
Figure 5 Pathological CD68 IHC image of lymph node (40X). |
Treatment and Outcomes
The time from onset to diagnosis of the 44 children was between 14 and 100 days, with an average of 37.1 ± 13.8 days (restricted by conditions, the pathological diagnosis cycle was 7–14 days). None of these cases recovered after regular antibiotic treatment for one to four weeks. Then they underwent lymph node biopsies and were diagnosed with SNL. The body temperatures of four cases gradually stabilized 14–42 days after the onset of the disease, and their lymph nodes gradually shrank and remitted spontaneously. No hormones were administered in the initial treatment. The remaining 40 cases were treated with intravenous methylprednisolone or oral prednisolone 1.5–2 mg/kg·d. Their body temperatures gradually stabilized within one to three days, and the dosage of hormone was gradually reduced and finally stopped one to three months later. One out of 44 cases was lost to follow-up, while the remaining 43 cases were followed up for two months to eight years, with four cases having a relapse. In the four cases, one was not treated with hormones in the initial treatment. This case had fever accompanied by enlarged and painful lymph nodes again eight months after the disease and was then confirmed as HNL by a lymph node biopsy. The body temperature gradually stabilized, and the lymph nodes steadily disappeared after hormones were administered. The case has been followed up for 6.5 years, with no relapse observed. Another three cases were treated with hormones in the initial treatment. One case had a relapse during the application of hormones one month after the disease and improved after hormones were increased. Then the amount of hormone was gradually reduced until it was stopped three months later. The case has been followed up for one year, with no relapse observed. One case had a relapse four months after the disease and got better after repeated hormone treatment. The case has been followed up for 14 months, with no relapse observed. Another case had an Antinuclear antibody (ANA) titer of 1:320 in the initial treatment. The cervical lymph nodes disappeared, and the routine blood examination, CRP, and ESR results returned to normal after hormone treatment for 17 days, and hormones were stopped three months later. The case was subsequently followed up, with an ANA titer staying positive, no CTD-related symptoms observed, and normal anti-dsDNA antibody and complement. Four years later, the case had a relapse, with an ANA titer of 1:640 and was complicated by Hashimoto’s thyroiditis. The condition got better after the case was treated with hormones again. The case has been followed up for four years, with an ANA titer of 1:80, normal anti-dsDNA antibody and complement, and no relapse observed. In the study, the remaining three cases with positive ANA titers have shown no Systemic lupus erythematosus (SLE) manifestations but negative anti-dsDNA antibody and normal complement since follow-up.
Discussion
Mainly manifested as fever and lymph node enlargement, SNL is a benign and self-limiting disease. Cases have been reported worldwide, but Asians are more likely to develop the disease. Compared with the general population, HNL patients often have specific HLA-II, especially HLA-DPA1 and HLA-DPB1 alleles. These alleles are more common in Asians but rare or missing in Caucasians, explaining why this gene is more common in Asians.1 The disease occurs more frequently in women aged 20–35.2 Previous studies have shown that the disease has a gender ratio of approximately 1.25–2:1 (female:male).3,4 However, children with the disease have increasingly been reported in recent years. This study included 44 children aged 6–13 years and an average age of 10.3 ± 2.1 years. There were more boys than girls, which was different from previous studies. This may be a characteristic of SNL in children.
The primary clinical manifestations of HNL include fever and lymph node enlargement as well as rashes, emaciation, fatigue, night sweats, joint pain, and so on in some cases. Enlarged lymph nodes are most often found in the neck but can also be seen in the armpits, groin, abdomen, and other areas. Laboratory examinations may show fewer leucocytes and higher ESR and LDH levels in some children without specific serum markers. Due to the lack of specificity of its clinical manifestations and auxiliary examinations, the general understanding of the disease in the early stage is insufficient. Lymph node enlargement is common in pediatrics. About 38–45% of healthy children may have cervical lymph node enlargement,5 which is more common during infection. In addition, it is often confused with lymphoma, infectious mononucleosis, lymph node tuberculosis, systemic lupus erythematosus, and other diseases clinically, with high missed diagnosis and misdiagnosis rates. The time from onset to diagnosis of the cases in this study was between 14 and 100 days, with an average of 37.1 ± 13.8 days. Most of the cases were transferred to multiple hospitals before the final diagnosis. Lymph node biopsy is the most reliable way to diagnose the disease. Therefore, patients with fever accompanied by painful lymph node enlargement and low WBC should undergo a lymph node biopsy as soon as possible. In addition, 75% of the cases at our center had lymph node tenderness, but 25% still had painless enlargement. In this case, it should be differentiated from lymphoma and other diseases, and lymph node biopsy is the most crucial differentiation approach. In addition, SNL manifests with a low WBC, but about 27.3% of the cases in this study had a normal WBC of >4.0 × 109/L. Therefore, the possibility of SNL still cannot be excluded even if the WBC is not low. Besides, in the cases at our center, two had no superficial lymph node enlargement. They went to the doctor only because of long-term fever, and abdominal lymph node enlargement was finally found with imaging examinations. Therefore, for a patient with low WBC and fever, attention shall be paid to abdominal or mediastinal lymph node enlargement even if superficial lymph node enlargement is not palpable in the physical examination.
In this study, some children tested positive for pathogens, including streptococcus, adenovirus, and the EB, parainfluenza, mycoplasma, herpes simplex, and respiratory syncytial viruses which are consistent with previous studies.6,7 At present, the cause of the disease has not been fully understood. It is generally believed that lymph node necrosis is an excessive immune response mediated by T cells under the stimulation of pathogens instead of being directly caused by viruses and pathogens.8 In addition, the onset of HNL is related to autoimmune factors, including SLE, Still’s disease, Sjogren’s syndrome, polymyositis, rheumatoid arthritis, granulomatous polyangiitis, and Grave’s disease.6,9 SLE is widely recognized as being related to the onset of HNL. Studies have shown that patients with SLE can develop HNL during the course of the disease, and it is believed that HNL may be related to or part of SLE.8 Necrotizing lymphadenitis and SLE-related lymphadenitis have similar immunophenotypes and histological characteristics. There are also studies reporting the development of SLE during the follow-up of HNL.9,10 Four cases in this group had positive ANA titers. However, only one case has kept a positive ANA titer currently in the follow-up, but no evidence of development into SLE was found. This may be relevant with a small number of observed cases or a short follow-up duration.
HNL usually has an acute or subacute onset, a prolonged course duration, no response to antibiotic and antiviral treatments, and poor response to treatments with non-steroidal anti-inflammatory drugs. HNL is considered a benign and self-limiting disease. Patients with this disease generally recover well, but some may have multiple organ damage complications. Some scholars have reported that some patients with HNL died of pulmonary hemorrhage and focal myocardial necrosis.11,12 It has also been reported that various diseases caused by abnormal immune function may be secondary to this disease.13 Therefore early diagnosis and treatment are crucial. At present, there are no standard HNL treatment guidelines. Past data shows that lymph node excision is not only a means of diagnosis but also a treatment method. The clinical symptoms of some patients will be quickly relieved after lymph node excision.14 In this study, the body temperatures of four cases gradually stabilized after lymph node excision, which might be relevant with treatment but could not exclude the disease reaching the self-healing course. According to previous experience, treatment with glucocorticoids has a certain effect in shortening the duration of symptoms and/or mitigating the severity of the disease.15 After 40 children in this study were administered glucocorticoids, their body temperatures quickly stabilized, and their lymph nodes gradually shrank, significantly shortening the course of the disease.
In conclusion, patients with fever and lymph node enlargement of unknown origin and no response to antibiotic treatment should undergo lymph node biopsy as soon as possible for early diagnosis and treatment. The disease has a good prognosis in most cases but may recur in a few cases. Therefore, long-term follow-up should be made.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Tanaka T, Ohmori M, Yasunaga S, Ohshima K, Kikuchi M, Sasazuki T. DNA typing of HLA class II genes (HLA-DR, -DQ and -DP) in Japanese patients with histiocytic necrotizing lymphadenitis (Kikuchi’s disease). Tissue Antigens. 1999;54:246–253. doi:10.1034/j.1399-0039.1999.540305.x
2. Deaver D, Naghashpour M, Sokol L. Kikuchi-Fujimoto disease in the United States: three case reports and review of the literature [corrected]. Mediterr J Hematol Infect Dis. 2014;6(1):e2014001. doi:10.4084/mjhid.2014.001
3. Lin HC, Su CY, Huang CC, Hwang CF, Chien CY. Kikuchi’s disease: a review and analysis of 61 cases. Otolaryngol Head Neck Surg. 2003;128(5):650–653. doi:10.1016/S0194-5998(02)23291-X
4. Kucukardali Y, Solmazgul E, Kunter E, Oncul O, Yildirim S, Kaplan M. Kikuchi-Fujimoto Disease: analysis of 244 cases. Clin Rheumatol. 2007;26(1):50–54. doi:10.1007/s10067-006-0230-5
5. Lelii M, Senatore L, Amodeo I, et al. Kikuchi-Fujimoto disease in children: two case reports and a review of the literature. Ital J Pediatr. 2018;44(1):83. doi:10.1186/s13052-018-0522-9
6. Baziboroun M, Bayani M, Kamrani G, Saeedi S, Sharbatdaran M. Kikuchi-Fujimoto disease in an Iranian woman; a rare but important cause of lymphadenopathy. Arch Acad Emerg Med. 2019;7(1):e3.
7. Chong Y, Lee JY, Thakur N, Kang CS, Lee EJ. Strong association of torque teno virus/torque teno-like minivirus to Kikuchi-Fujimoto lymphadenitis (histiocytic necrotizing lymphadenitis) on quantitative analysis. Clin Rheumatol. 2020;39(3):925–931. doi:10.1007/s10067-019-04851-4
8. Horino T, Ichii O, Terada Y. Is recurrent Kikuchi-Fujimoto disease a precursor to systemic lupus erythematosus? Rom J Intern Med. 2019;57(1):72–77. doi:10.2478/rjim-2018-0028
9. Sopeña B, Rivera A, Chamorro A, et al. Clinical association between Kikuchi’s disease and systemic lupus erythematosus: a systematic literature review. Semin Arthritis Rheum. 2017;47(1):46–52. doi:10.1016/j.semarthrit.2017.01.011
10. Salamat S, Chan J, Jolly K, et al. Kikuchi-Fujimoto Disease and Prognostic Implications. Head Neck Pathol. 2020;14(1):272–275. doi:10.1007/s12105-019-01026-0
11. Koybasi S, Saydam L, Gungen Y. Gungen, histiocytic necrotizing lymphadenitis of the neck. Am J Otolaryngol. 2003;24(5):344–347. doi:10.1016/S0196-0709(03)00061-9
12. Scagni P, Morello M, Pagliero R, Pecco P. Benign paroxysmal torticollis of infancy. A case report Minerva Pediatr. 2006;58(5):499–501.
13. Taguri AH, McIlwaine GG. Bilateral panuveitis: a possible association with Kikuchi-Fujimoto disease. Am J Ophthalmol. 2001;132(3):419–421. doi:10.1016/S0002-9394(01)00934-5
14. Lee KY, Yeon YH, Lee BC. Kikuchi-Fujimoto disease with prolonged fever in children. Pediatrics. 2004;114(6):752–756. doi:10.1542/peds.2004-0485
15. Jang YJ, Park KH, Seok HJ. Management of Kikuchi’s disease using glucocorticoid. J Laryngol Otol. 2000;114(9):709–711. doi:10.1258/0022215001906561
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
