Back to Journals » Clinical Ophthalmology » Volume 17
Classification of Subtypes of Vernal Keratoconjunctivitis by Cluster Analysis Based on Clinical Features
Authors Fujita H , Ueno T , Suzuki S , Harada K , Tsukahara-Kawamura T , Ozaki H, Uchio E
Received 21 July 2023
Accepted for publication 13 October 2023
Published 31 October 2023 Volume 2023:17 Pages 3271—3279
DOI https://doi.org/10.2147/OPTH.S431798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hideaki Fujita, Tomohiro Ueno, Shuji Suzuki, Kazuhiro Harada, Tomoko Tsukahara-Kawamura, Hiroaki Ozaki, Eiichi Uchio
Department of Ophthalmology, Fukuoka University School of Medicine, Fukuoka, Japan
Correspondence: Eiichi Uchio, Department of Ophthalmology, Fukuoka University School of Medicine, 7-45-1 Nanakuma, Jonan-ku, Fukuoka, 814-0180, Japan, Tel +81 92 801 1011, Fax +81 92 865 4445, Email [email protected]
Background: Vernal keratoconjunctivitis (VKC) is a refractory ocular allergic disorder that mainly affects boys. A few studies have attempted to develop a classification of subtypes of VKC. In this study, we investigated a computational approach called cluster analysis to separate VKC cases into groups based on clinically relevant characteristics.
Methods: In total, 41 consecutive patients clinically diagnosed with VKC at the Department of Ophthalmology of Fukuoka University Hospital were included. Patients were treated with immunosuppressive eye drops without simultaneous corticosteroid eye drops, except for the occurrence of exacerbations. Collated variables were age at onset, clinical score of ocular lesions at baseline, clinical score of ocular lesions at final visit, clinical score of atopic dermatitis (AD) at baseline, frequency of exacerbations of VKC, serum total IgE level and peripheral blood eosinophil count.
Results: VKC patients were grouped into three clusters by cluster analysis, and cluster 1, 2, and 3 comprised 25, 9 and 7 cases, respectively. There were differences in the incidence of complications of AD and age at onset among the clusters; therefore, we named the three clusters for better understanding as traditional VKC (cluster 1), early-onset atopic keratoconjunctivitis (AKC)/VKC (cluster 2) and puberty-onset AKC (cluster 3).
Conclusion: We found in this study that VKC in childhood has three phenotypes which were previously unknown. Our findings may help to establish precision medicine by focusing on the phenotype of each case to develop individualized medicine to prevent exacerbations.
Keywords: vernal keratoconjunctivitis, subtype, atopic dermatitis, atopic keratoconjunctivitis, cluster analysis
Introduction
Vernal keratoconjunctivitis (VKC) is a chronic ocular inflammatory disorder which is most prevalent among males, with onset typically occurring before age 10 and resolving at puberty. VKC is characterized by severe inflammation affecting both the cornea and conjunctiva, such as hyperemia, chemosis, photophobia, mucous discharge, giant papillae of the upper tarsal conjunctiva, limbal Horner-Trantas dots, superficial keratopathy and corneal shield ulcers.1,2 Although the symptoms of VKC usually persist despite treatment, it generally subsides with the onset of puberty,3 but some therapeutic measures may be required beyond this age to control the course of the disease, especially in those cases associated with atopic dermatitis (AD), in whom permanent changes to the ocular surface may occur, accompanied by permanent visual impairment.4 Although cases have been treated with medical management and environmental control, varying responses to similar medication have been observed in VKC,5 and the reasons underlying the variation in clinical outcomes among VKC cases have been poorly studied and little understood. Therefore, there is a need to develop a novel classification of subtypes of VKC. The prognosis may differ according to the disease phenotype of VKC;1 however, to our knowledge, no attempt has been made to classify patients into subgroups based on VKC features and demographic characteristics.
While various studies have been reported on the immunological or allergological features of VKC,6–10 it would seem to be of considerable value for clinical ophthalmologists if the clinical subtypes could be classified based mainly on the clinical features, with at least addition of laboratory findings that can be carried out in daily clinical practice. Therefore, we investigated a computational approach called cluster analysis, performed by grouping all cases of VKC together, then separating them into groups based on their statistical similarity of clinically relevant characteristics. The aim of the present study was also to propose a model of discrimination between VKC phenotypes different from the traditional one,2,3 which enabled us to investigate the possibility of establishing a classification of clinical subtypes of VKC, especially before puberty, using clinical findings derived from long-term observation of cases of VKC while minimizing clinical diagnostic bias.
Materials and Methods
Patients
This cross-sectional case series study was approved by the Institutional Review Board of Fukuoka University School of Medicine (approval number: 2017Ml40) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all subjects enrolled in this study. This study conforms to the reporting requirements of the STROBE guidelines.
In total, 41 consecutive juvenile patients aged 4 to 16 years at the onset of symptoms, diagnosed with VKC and treated for at least one year in the Department of Ophthalmology of Fukuoka University Hospital (Fukuoka, Japan), located in the west region of Fukuoka City which has an approximate population of 300,000, were included in this study. The diagnosis of VKC was based on the Japanese guidelines for allergic conjunctival diseases.11 In summary, VKC was diagnosed by the presence of proliferative change, such as typical cobblestone excrescences (giant papillae of the upper palpebral conjunctiva more than 1 mm in size), and corneal lesions, such as stromal lipid deposition or pannus formation, and cases of limbal type were excluded in this study, as while limbal lesions are associated with proliferative change, they comprise only 5% of VKC cases in our hospital.12 Exclusion criteria were bacterial conjunctivitis, viral keratoconjunctivitis or other unclassified conjunctivitis such as phlyctenular or toxic conjunctivitis and dry eye disease, and subjects wearing contact lenses were also excluded.
Ocular Clinical Grading and Clinical Observations
Clinical evaluation of ocular findings was carried out according to the ocular clinical grading system reported recently.13 Among ten objective ocular clinical findings of conjunctival, limbal, and corneal lesions, three findings, conjunctival papillae, conjunctival giant papillae and corneal epithelial lesions, were each graded on a 4-point scale (0 = none, 1 = mild, 2 = moderate, and 3 = severe; left and right eyes separately in each case), and reference clinical pictures are shown in the guidelines.13 Details of the scoring criteria of the three findings are shown in Table 1. The total score of the three findings, with a maximum of 9, taking the score of the more severe side in bilateral cases, was used as the clinical score. The clinical scores were recorded at each visit to our clinic; however, those at the first visit and final visit were adopted for this study.
 |
Table 1 Criteria for Clinical Evaluation of Allergic Ocular Findings |
Patients with VKC were treated according to the guidelines11 with modifications; in brief, immunosuppressive eye drops, mainly tacrolimus (ciclosporin in the other patients), were used as basic treatment without simultaneous corticosteroid eye drops. Most cases could be controlled by increasing or decreasing the instillation of immunosuppressive eye drops (four times a day, once a week), combined with anti-allergic eye drops in some cases. This prolonged use of tacrolimus ophthalmic suspension 0.1%, namely proactive therapy reported previously,14 entails instillation of low doses of drugs to prevent recurrences; this is similar to the medical treatment of AD using tacrolimus ointment.15 Exacerbation of VKC was determined as worsening of the grade of giant conjunctival papillae or corneal epithelial lesions to at least moderate, meaning elevated giant papillae extending over less than half of the upper palpebral conjunctiva or superficial punctate keratitis with filamentary debris,11 because local or systemic corticosteroid treatment is necessary in these cases. The frequency of exacerbations of VKC was calculated as the total number of exacerbations during the observation period.
Atopic Dermatitis
Atopic dermatitis was diagnosed according to the criteria proposed by Hanifin and Rajka,16 by the presence of four items: (1) itching, (2) chronic course of more than 1 year, (3) atopic history, and (4) typical lesions of AD. The clinical severity of AD lesions was graded by dermatologists as (I) mild, (II) moderate, or (III) severe, according to the distribution of lesions, response to therapy, frequency of relapse, and the clinical course, as reported elsewhere.17 Grade I, II and III were assigned a score of 1, 2 and 3, respectively, and non-atopic cases were assigned a score of 0 for statistical evaluation.
Serological Analysis
Serum total IgE level and peripheral blood eosinophil count were measured in each case at the first visit to our hospital. The normal range of serum total IgE was < 110 IU/mL at 4–6 years old, and < 170 IU/mL at 7 years old or older. The normal range of blood eosinophil count was 1–9%. Cut-off levels of these parameters are not established for allergic conjunctival diseases (ACD).
Classification Methods
We used divisive hierarchical cluster analysis18 to identify subgroups of patients with similar characteristics. Hierarchical clustering methods are categorized into agglomerative (bottom-up) and divisive (top-down) procedures. Divisive procedures begin by considering a group that includes all samples, which is divided into several groups in subsequent stages until all groups comprise only a single sample.19 Collated variables for cluster analysis were as follows: age at onset, clinical score of ocular lesions at baseline, clinical score of ocular lesions at final visit, clinical score of AD at baseline, frequency of exacerbations of VKC, serum total IgE level and peripheral blood eosinophil count. We performed Ward’s hierarchical cluster analysis with dissimilarity measures of the squared Euclidean distance for the data. Cluster analysis is an “automatic” statistical analysis based on primarily the data of samples, and cluster formation was performed with a kind of black box process without any direction such as number of clusters, etc. The validity of the number of clusters was assessed by several methods based on inter- and intra-cluster “scatter” indicators.20–22
Comparison of Clinical Characteristics and Clinical Outcome Among Clusters
Differences in clinical characteristics and laboratory data among clusters were tested using analysis of variance. All tests were two-sided, and p < 0.05 was considered to indicate statistical significance. All statistical evaluations including cluster analysis were performed using Excel Toukei-Kaiseki software (BellCurve, Tokyo, Japan).
Results
Demographic Features of Patients
Baseline demographic and clinical characteristics of the 41 patients are shown in Table 2. The observation period ranged from 12 to 119 months, with mean ± standard deviation (SD) of 28.6 ± 14.6 months. Twenty-two cases (54%) were observed for 18 to 36 months, and only two cases of early onset, aged 5 years old, were followed for more than 100 months. The overall prevalence of AD was 36.6% (15/41).
 |
Table 2 Baseline Characteristics of Included Patients |
Cluster Analysis of Patients with VKC
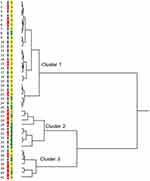
All patients with VKC were evaluated by cluster analysis and grouped into three clusters. The dendrogram is shown in Figure 1, and clinical and laboratory findings of the three cluster groups of VKC patients are shown in Table 3. Cluster 1, 2 and 3 contained 25, 9 and 7 cases, respectively. In the analysis of variance among the three clusters, significant differences were observed in all items (p < 0.05) except for clinical score of ocular lesions at baseline. Cluster 2 and cluster 3 included 5 (56%) and 5 patients (71%) with AD, respectively; in contrast, AD cases comprised 20% of cluster 1. Severity of AD was lowest in cluster 1. Most parameters showed the highest value in cluster 3, except for age at onset and peripheral blood eosinophil count. Peripheral blood eosinophil count was similar in cluster 2 and cluster 3. Age at onset was higher in cluster 1 and cluster 3 than in cluster 2. Cluster 1 showed an obviously lower percent eosinophil count than the other clusters. Serum total IgE level was higher in cluster 2 and cluster 3 compared with cluster 1. From these results, we named the three clusters for better understanding as traditional VKC (cluster 1), early-onset atopic keratoconjunctivitis (AKC)/VKC (cluster 2) and puberty-onset AKC (cluster 3). As shown above, a treatment regimen using tacrolimus eye drops in a proactive way was universal among the clusters.
 |
Table 3 Clinical and Laboratory Findings of Three Cluster Groups of VKC Patients |
Characteristics of Three Clusters
The characteristics of these three clusters are summarized in Table 4.
 |
Table 4 Characteristics of Three Clusters |
Cluster 1 (n = 25, 61.0%) - traditional VKC. This was the largest group; it consisted of subjects with a median age at onset of up to around puberty, and included female patients with a male to female sex ratio of 21: 4. Clinical resolution was obtained in most cases by the last follow-up, and fewer AD patients were included. Typically showing a low peripheral blood eosinophil count and serum total IgE level, this group corresponded to VKC that undergoes spontaneous resolution at puberty, from a previous study of this disease.2,3
Cluster 2 (n = 9, 21.9%) - early-onset AKC/VKC. The subjects had a younger median age at onset of 6 years, and a high peripheral blood eosinophil count was the specific characteristic of this group. Male to female sex ratio was 7: 2. The median clinical scores of ocular lesions at baseline and the final visit were low compared with those in cluster 3, indicating that they showed a favorable clinical outcome, with clinical resolution during follow-up. On close observation, this group comprised two subgroups; all of the subgroup (case 26 – case 29) patients had AD, while only one patient in the subgroup (case 30 – case 34) was complicated with AD (Figure 1). Therefore, this group might comprise combined subgroups of juvenile onset severe spectrum of ACD, and we named this AKC/VKC.
Cluster 3 (n = 7, 17.1%) - puberty-onset AKC. Cases in this group were relatively late-onset, similar to cluster 1, and all cases were male. The majority had AD (71%) and this group had a high median clinical score of ocular lesions at onset (6), while the median clinical score of ocular lesions at the final visit was highest (4) among the three groups, suggesting this to be the most clinically severe subtype. The median frequency of exacerbations of VKC (1.5) was highest, indicating the refractory nature of VKC in this group. As shown by the high clinical score of ocular lesions at the final visit (Table 3), some patients did not show clinical resolution, and treatment with immunosuppressive eye drops was continued in these cases.
Discussion
Hierarchical clustering analysis in this study identified three clusters, which consisted of “traditional VKC” (cluster 1), “early-onset AKC/VKC” (cluster 2), and “puberty-onset AKC” (cluster 3), as presented in the Results section. This finding that VKC, which has been considered an allergic disease that especially occurs in childhood, falls into three phenotypic groups is open to at least two interpretations. The first is that the presence of AD might influence the clinical course of this disorder, because two clusters including the name AKC (clusters 2 and 3) showed higher serum total IgE levels and peripheral blood eosinophil counts than those in late-onset VKC (cluster 1), indicating that cases in these groups had an atopic background despite the absence of apparent dermatological findings such as AD. The second interpretation is that timing of onset is another differentiating element of VKC, because early-onset AKC/VKC (cluster 2) showed lower frequency of recurrence, similar to that in traditional VKC (cluster 1), than late-onset AKC (cluster 3). Considering the small number in each cluster, even though statistically significant differences among the groups were found for certain parameters, such as the onset of VKC or the complication of AD, it is difficult to explain in detail the meaning of each cluster from the results of this study, because cluster analysis is basically an automatic statistical method, as shown in the Materials and methods section.
There is controversy regarding AKC in childhood. AKC was defined by Hogan in 1952 as allergic keratoconjunctivitis occurring in association with atopic eczema.23 Distinguishing between VKC and AKC can be challenging. Historically, AKC is rarely recognized as a diagnostic entity before puberty and is thought to occur predominantly in adults.24 If a young patient were to present with AKC-like symptoms and AD, they might be diagnosed with VKC. We agree with this standpoint; therefore, the title of this paper includes only “vernal keratoconjunctivitis”.25 In contrast, Ebihara et al proposed that VKC cases with any history of AD should be diagnosed as AKC, regardless of patient age.26 VKC generally resolves at puberty; however, in some cases, it is thought that VKC may “evolve” into AKC in adulthood.27 AKC was included in the disease name of cluster 2 and cluster 3, but, as noted above, all juvenile cases with giant papillae in this study were diagnosed as VKC at their onset, but not AKC, even though they were complicated with AD. As noted by Frankland et al in an earlier study in the 70’s that VKC was an atopic disease,28 the prevalence of AD among cases of VKC ranged between 15% and 52% in the past literature,29–31 and the rate in the present study, 36%, was in accordance with these studies. Based on these combined reports, we consider that VKC might not be a uniform clinical entity, but composed of several subtypes other than traditionally considered VKC affecting mainly boys in the first decade of life and generally subsiding with the onset of puberty.1 The name AKC/VKC (cluster 2) typically reflects the ambiguous border between AKC and VKC, especially in puberty, as shown in this study. The puberty onset group of patients (cluster 2) might be classified into two groups in a future study.
The phenotypic differences identified in this study could be further investigated in a future study, in conjunction with pathophysiological data, because differences occur throughout the course of the disease and may affect the clinical outcome in adulthood, especially in cases with AD - cluster 3 (puberty-onset AKC). Clinical exacerbations could not be avoided by proactive therapy using tacrolimus eye drops in cluster 3, all of which were male cases with AD whose onset was later on average than in cluster 2. We expect that most cases belonging to cluster 3 relate to a phenotype of AKC in adults that has early onset around puberty.32 Considering the refractory features observed in cluster 3, our findings could help identify the clinical characteristics and understand the similarity of pathogenic mechanisms within each cluster, which could contribute to better ways of managing VKC in childhood. Some cases of VKC that resulted in refractory AKC in adulthood were observed.27 Chronological analysis of VKC throughout the clinical course from onset to after puberty will clarify differences in the clinical outcome among the clusters identified in this study. This observational study is in progress.
To establish precision medicine, it is important to focus on the phenotype of each case in order to develop individualized medicine to prevent exacerbations. In contrast, the clinical outcome in early-onset AKC/VKC (cluster 2) was generally favorable and cases had fewer exacerbations and the disease tended to be self-limiting, although their allergological background was similar to that of cluster 3 (Table 3). Future observational studies with long-term follow-up from birth may reveal the reason.
There are several limitations of this study. Firstly, the study population was small. VKC is basically a rare ocular disorder. A questionnaire-based survey by practicing ophthalmologists was conducted in several European countries in 2002, and the prevalence of VKC was estimated to be 0.003% - 0.09%.27 They concluded that the best estimate of VKC prevalence in Western Europe is 3.2/10,000 inhabitants, and the prevalence of VKC with corneal complications is 0.8/10,000 inhabitants.22 The prevalence of VKC varies greatly depending on the region of the world. High prevalence rates are reported especially for regions with warm dry climates, eg, Middle Eastern countries, central and South Africa, and Mediterranean countries. In contrast, western EU nations have a low prevalence of VKC.33 We herein report a single-center study that included patients treated in a tertiary consulting eye center that provides care to approximately 300,000 inhabitants surrounding our hospital (in west Fukuoka area), and 12% of the population of Fukuoka City is reported to comprise children under 12 years old.34 It is reported that the prevalence of ACD in children under 12 years in Japan is 11%.35 In Japan, the prevalence of VKC in ACD patients under medical care was reported to be 3.8%.13 It is difficult to calculate the exact number of cases of VKC in this region of Fukuoka City. Assuming that all cases affected with ACD in west Fukuoka under 12 years old were treated medically, the estimated number of ACD juvenile cases in west Fukuoka is 3960 (300,000 x 0.12×0.11), and 41 cases accounted for 1.0% of 3960 cases. Considering these factors, our study population of 41 cases is not far from the estimated number of cases that need continued medical care by a tertiary consulting eye center because of their severe illness. Enlargement of the study population is needed to verify and confirm the results of this study, possibly on a nationwide basis. Secondly, among the variables selected, the clinical score of ocular lesions at the final visit and the frequency of exacerbations of VKC could not be predicted at the first visit from other variables, and we need to wait until the end of the observation period. Therefore, we should understand that these subtypes cannot be distinguished at the start of treatment of childhood cases of VKC. However, our present results would give physicians a useful and meaningful standpoint that VKC might be divided into several subtypes depending on, at least, age at onset, clinical scores of ocular lesions and AD, serum total IgE level and peripheral blood eosinophil count at baseline, without carrying out invasive laboratory analyses. Therefore, a future longitudinal, multi-center study is warranted to establish the robustness of the cluster patterns obtained here.
Acknowledgments
This work was supported by a Grant-in-Aid for Encouragement of Scientists (21K09709) from the Ministry of Education, Science, Sports, and Culture of Japan. We thank Dr W Gray for editing this manuscript.
Disclosure
The authors report no conflict of interest in this work.
References
1. Bonini S, Bonini S, Lambiase A, et al. Vernal keratoconjunctivitis revisited: a case series of 195 patients with long-term follow-up. Ophthalmology. 2000;107(6):1157–1163. doi:10.1016/S0161-6420(00)00092-0
2. Buckley RJ. Vernal keratoconjunctivitis. Int Ophthalmol Clin. 1988;28(4):303–308. doi:10.1097/00004397-198802840-00009
3. Foster CS. The pathophysiology of ocular allergy: current thinking. Allergy. 1995;50(Suppl 21):6–9. doi:10.1111/j.1398-9995.1995.tb04250.x
4. Uchio E, Miyakawa K, Ikezawa Z, Ohno S. Systemic and local immunological features of atopic dermatitis patients with ocular complications. Br J Ophthalmol. 1998;82(1):82–87. doi:10.1136/bjo.82.1.82
5. Kosrirukvongs P, Vichyanond P, Wongsawad W. Vernal keratoconjunctivitis in Thailand. Asian Pac J Allergy Immunol. 2003;21(1):25–30.
6. Shoji J, Inada N, Sawa M. Antibody array-generated cytokine profiles of tears of patients with vernal keratoconjunctivitis or giant papillary conjunctivitis. Jpn J Ophthalmol. 2006;50(3):195–204. doi:10.1007/s10384-005-0319-4
7. Micera A, Di Zazzo A, Esposito G, et al. Quiescent and active tear protein profiles to predict vernal keratoconjunctivitis reactivation. Biomed Res Int. 2016;2016:9672082. doi:10.1155/2016/9672082
8. Montan PG, Scheynius A, van der Ploeg I. Similar T helper Th2-like cytokine mRNA expression in vernal keratoconjunctivitis regardless of atopic constitution. Allergy. 2002;57(5):436–441. doi:10.1034/j.1398-9995.2002.13375.x
9. Zicari AM, Nebbioso M, Zicari A, et al. Serum levels of IL-17 in patients with vernal keratoconjunctivitis: a preliminary report. Eur Rev Med Pharmacol Sci. 2013;17(9):1242–1244.
10. Leonardi A, Daull P, Garrigue JS, et al. Conjunctival transcriptome analysis reveals the overexpression of multiple pattern recognition receptors in vernal keratoconjunctivitis. Ocul Surf. 2021;19:241–248.
11. Takamura E, Uchio E, Ebihara N, et al. Japanese guideline for allergic conjunctival diseases. Allergol Int. 2011;60(2):191–203. doi:10.2332/allergolint.11-RAI-0335
12. Neumann E, Gutmann MJ, Blumenkrantz N, Michaelson IC. A review of four hundred cases of vernal keratoconjunctivitis. Am J Ophthalmol. 1959;47(2):166–172. doi:10.1016/S0002-9394(14)76417-7
13. Miyazaki D, Takamura E, Uchio E, et al. Japanese guidelines for allergic conjunctival diseases 2020. Allergol Int. 2020;69(3):346–355. doi:10.1016/j.alit.2020.03.005
14. Hirota A, Shoji J, Inada N, Shiraki Y, Yamagami S. Evaluation of clinical efficacy and safety of prolonged treatment of vernal and atopic keratoconjunctivitis using topical tacrolimus. Cornea. 2022;41(1):23–30. doi:10.1097/ICO.0000000000002692
15. Wollenberg A, Reitamo S, Girolomoni G, et al. Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy. 2008;63(7):742–750. doi:10.1111/j.1398-9995.2007.01406.x-i1
16. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;60(Suppl 92):44–47. doi:10.2340/00015555924447
17. Ikezawa Z, Miyakawa K, Komatsu H, et al. A probable involvement of rice allergy in severe type of atopic dermatitis in Japan. Acta Derm Venereol. 1992;72(Suppl 176):103–107.
18. Edelbrock C, McLaughlin B. Hierarchical cluster analysis using intraclass correlations: a mixture model study. Multivariate Behav Res. 1980;15(3):299–318. doi:10.1207/s15327906mbr1503_5
19. Lorr M, Suziedelis A. A cluster analytic approach to MMPI profile types. Multivariate Behav Res. 1982;17(3):287–299. doi:10.1207/s15327906mbr1703_1
20. Westgate PM. Intra-cluster correlation selection for cluster randomized trials. Stat Med. 2016;35(19):3272–3284. doi:10.1002/sim.6922
21. Fraboni M, Cooper D. Six clustering algorithms applied to the WAIS-R: the problem of dissimilar cluster results. J Clin Psychol. 1989;45(6):932–935. doi:10.1002/1097-4679(198911)45:6<932::AID-JCLP2270450617>3.0.CO;2-T
22. Tonidandel S, Overall JE. Determining the number of clusters by sampling with replacement. Psychol Methods. 2004;9(2):238–249. doi:10.1037/1082-989X.9.2.238
23. Hogan MJ. Atopic keratoconjunctivitis. Trans Am Ophthalmol Soc. 1952;50:265–281.
24. Brémond-Gignac D, Nischal KK, Mortemousque B, Gajdosova E, Granet DB, Chiambaretta F. Atopic keratoconjunctivitis in children: clinical features and diagnosis. Ophthalmology. 2016;123(2):435–437. doi:10.1016/j.ophtha.2015.07.012
25. Calonge M, Herreras JM. Clinical grading of atopic keratoconjunctivitis. Curr Opin Allergy Clin Immunol. 2007;7(5):442–445. doi:10.1097/ACI.0b013e3282efd0bf
26. Ebihara N, Ohashi Y, Uchio E, et al. A large prospective observational study of novel cyclosporine 0.1% aqueous ophthalmic solution in the treatment of severe allergic conjunctivitis. J Ocul Pharmacol Ther. 2009;25(4):365–372. doi:10.1089/jop.2008.0103
27. Bremond-Gignac D, Donadieu J, Leonardi A, et al. Prevalence of vernal keratoconjunctivitis: a rare disease? Br J Ophthalmol. 2008;92(8):1097–1102. doi:10.1136/bjo.2007.117812
28. Frankland AW, Easty D. Vernal kerato-conjunctivitis: an atopic disease. Trans Ophthalmol Soc U K. 1971;91:479–482.
29. Kausar A, Akhtar N, Akbar N. Epidemiological aspects of allergic conjunctivitis. J Ayub Med Coll Abbottabad. 2022;34(1):135–140. doi:10.55519/JAMC-01-9432
30. Jongvanitpak R, Vichyanond P, Jirapongsananuruk O, Visitsunthorn N, Pacharn P. Clinical characteristics and outcomes of ocular allergy in Thai children. Asian Pac J Allergy Immunol. 2022;40(4):407–413. doi:10.12932/AP-160519-0564
31. Ajaiyeoba AI. Prevalence of atopic diseases in Nigerian children with vernal kerato-conjunctivitis. West Afr J Med. 2003;22(1):15–17. doi:10.4314/wajm.v22i1.27971
32. Jay JL. Clinical features and diagnosis of adult atopic keratoconjunctivitis and the effect of treatment with sodium cromoglycate. Br J Ophthalmol. 1981;65(5):335–340. doi:10.1136/bjo.65.5.335
33. Miyazaki D, Fukagawa K, Okamoto S, et al. Epidemiological aspects of allergic conjunctivitis. Allergol Int. 2020;69(4):487–495. doi:10.1016/j.alit.2020.06.004
34. Population by age of Fukuoka City. Fukuoka City. Available from: https://www.city.fukuoka.lg.jp/soki/tokeichosa/shisei/toukei/jinkou/tourokujinkou/TourokuJinko_kubetsu.html.
35. Nishima S, Odajima H, Ohta K, et al. Nishi-nihon shogaku jidou ni okeru arerugi shikkan yushoritsu chosa 1992, 2002, 2012 nen no hikaku. [A study on the prevalence of allergic diseases in school children in Western districts of Japan - comparison between the studies in 1992, 2002 and 2012 with the same methods and same districts]. Nihon Shouni Arerugi Gakkaishi. 2013;27(2):149–169. Japanese.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.