Back to Journals » OncoTargets and Therapy » Volume 13
CK19 Promotes Ovarian Cancer Development by Impacting on Wnt/β-Catenin Pathway
Authors Lu Q, Qu H, Lou T, Liu C, Zhang Z
Received 18 December 2019
Accepted for publication 12 March 2020
Published 24 March 2020 Volume 2020:13 Pages 2421—2431
DOI https://doi.org/10.2147/OTT.S242778
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Carlos E Vigil
Qi Lu, Hong Qu, Tong Lou, Chongdong Liu, Zhenyu Zhang
Department of Obstetrics and Gynecology, Chao-yang Hospital, Capital Medical University, Beijing, People’s Republic of China
Correspondence: Zhenyu Zhang; Chongdong Liu
Department of Obstetrics and Gynecology, Beijing Chao-yang Hospital, Capital Medical University, 8 Gongtinanlu, Chaoyang District, Beijing 100020, People’s Republic of China
Tel +86 13801237287
Fax +86 10 85231507
Email [email protected]; [email protected]
Background: Epithelial ovarian cancer (EOC) is the most common type of ovarian cancer and is the most lethal gynecologic malignancy. Cytokeratin 19 (CK19) is a small type I cytokeratin. The aim of this study is to explore the functional role of CK19 and its underlying mechanism in EOC.
Methods: The expression levels of CK19 in EOC tissues were identified by Western blotting and RT-PCR assay. Transwell assay and CCK-8 proliferation assay were used to assess the invasion, migration and proliferation abilities of overexpressed or knockdown CK19 of ovarian cancer cells. We also detected the related genes of Wnt/β-catenin signal pathway, including β-catenin, TCF7, LEF1, c-MYC and cyclin D1 in the transfected ovarian cancer cells by Western blotting and RT-PCR assay.
Results: The results demonstrated that CK19 was upregulated in EOC tissue. CK19 was verified to promote the invasion, proliferation and migration of ovarian cancer cells. Additionally, CK19 activates the Wnt/β-catenin signaling pathway by upregulated β-catenin, TCF7, LEF1, c-MYC and cyclin D1.
Conclusions: In summary, this is the first study to investigate the role of CK19 in EOC. These findings provide a potential new therapeutic target for the clinical diagnosis and treatment of ovarian cancer.
Keywords: CK19, Wnt/β-catenin, ovarian cancer, SKOV3
Background
In 2018, the worldwide incidence of ovarian cancer was 6.6/100,000.1 Epithelial ovarian cancer (EOC) is the most common type of ovarian cancer. Because the ovaries are hidden in the abdominal cavity and there are no effective early screening methods, 80% of EOC are diagnosed after the cancer has widely metastasized. With an overall five-year survival rate of only 38–40%, epithelial ovarian cancer is the most lethal gynecologic malignancy, and the eighth leading cause of cancer-related deaths among women worldwide.1–3
Cytokeratins (CKs) make up the largest subgroup of intermediate filament proteins and represent the most abundant proteins in epithelial cells.4 They participate in the formation of the cell skeleton and play an important role in the response to stress reaction, cell signaling and apoptosis.5 Cytokeratin 19 (CK19) is a small type I cytokeratin, which lacks the tail domain common among cytokeratins. It is comprised of 399 amino acids with a molecular weight of 44 kilodaltons, and contains a 13 amino acid extension of the alpha-helical rod.6 CK19 is mainly expressed in ductal epithelial and gastrointestinal epithelia.6 It is also expressed in a subset of hepatocellular carcinomas with poor prognosis as a biliary/hepatic progenitor cell marker and is thought to reflect the cell of origin.7 Qu et al have reported that CK19 is upregulated in EOC by quantitative proteomics.8 However, Ju et al have reported that silencing of CK19 increased cell proliferation, migration, invasion, and survival in human breast cancer cells. They also confirmed that silencing of CK19 increased tumor formation in a xenograft model.9 These studies have shown that modulation of CK19 expression led to different effects on cell function depending on the cancer cell type; however, there were few studies about CK19 expression in EOC. Therefore, extensive molecular studies on CK19 are required to elucidate its role in EOC.
The Wnt/β-catenin signaling pathway is one of the most relevant pathways involved with cancer stem cells and the role of Wnt/β-catenin signaling pathway in cancer stem cells is to regulate cell proliferation, migration and apoptosis. It also has been reported to be abnormally activated in various types of cancers, including ovarian cancer.10–13
In the present study, we explored the functional role of CK19 and its underlying mechanism in EOC. We demonstrated that CK19 was upregulated in EOC tissues. CK19 was verified to promote the invasion, proliferation and migration of OC cells. Additionally, Wnt/β-catenin signaling pathway abnormally activation was implicated in the tumor promotion role of CK19 in OC cells.
Materials and Methods
Human Tissue Samples and Cell Culture
A total of 15 normal ovarian tissue and 15 epithelial ovarian cancer tissue were used for quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis. All tissue specimens were obtained from the Beijing Chaoyang hospital from May 2017 to May 2018. The clinicopathology information of the patients are listed in Table 1. The samples were frozen at −80°C until analysis. The study was approved by the ethics committee of Beijing Chaoyang hospital, and the inform consent was signed by all the participants.
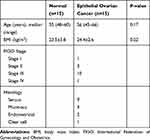 |
Table 1 Clinicopathology Information of the Patients |
The cell lines used in this study included the human ovarian cancer cells SKOV3, OVCAR3 and CAOV3 were obtained from the Medical Research Center of Beijing Chaoyang Hospital. The use of all the cell lines had ethical or institutional review board approval. All cells were cultured in RPMI-1640 (Gibco, Carlsbad, CA, USA) medium supplemented with 10% or 20% fetal bovine serum (FBS, Gibco) and 1% penicillin (Sigma-Aldrich, Inc., St-Louis, MO, USA) in a humidified 5% CO2 incubator at 37°C.
Western Blotting
Proteins were extracted following the manufacturer’s instructions using the protein extraction kit (Beyotime Institute of Biotechnology, Haimen, China). Protein concentrations were determined by the BCA Protein assay. Extracted protein were subjected to 10% SDS-PAGE. Proteins were transferred to nitrocellulose membranes (EMD Millipore, Billerica, MA, USA) and incubated overnight with the following antibodies:CK19 (Abcam, USA), GAPDH (Abcam, USA), β-catenin (Abcam, USA), c-MYC (Abcam, USA), and cyclin D1 (Abcam, USA). Following this, they were washed three times with TBST at room temperature on a decolorization shaker for 5 min each time. The membranes were incubated with specific Horseradish peroxidase (HRP)-conjugated secondary antibodies (DingTaiChangSheng, Beijing). Films were scanned using a calibrated Bio-Rad GS 800 densitometer, and signals were analyzed using ImageJ.
RNA Isolation and Quantitative Reverse Transcription PCR (RT-PCR)
The total RNA was isolated from the tissue or cultured cells by using Trizol agent (Thermo Fisher Scientific) following the instructions of manufacture. The concentration and purity of the RNA samples were measured using NanoDrop2000 (Thermo Scientific). The RNA was reverse transcribed into cDNA using the miScript II Reverse Transcription (RT) kit from Qiagen according to the manufacturer’s protocol with 1000ng total RNA. Subsequently, RT-PCR was conducted using the SYBR Green PCR kit for mRNA containing ROX as the reference dye in the ABI 7500 RT-PCR system. Accordingly, GAPDH was used as internal control for the normalization and quantification of the mRNA. The primers used for RT-PCR are listed in Table 2.
 |
Table 2 List of Primers Used for Quantification of Specific Gene Expression |
CK19 Stable Overexpressed in SKOV3 Cells
SKOV3 were infected with a lentiviral vector carrying CK19 plasmids or the scrambled sequence at an MOI of 30. After 72 hrs of infection, cells infected with lentiviral vectors encoding enhanced green fluorescent protein (eGFP) were observed via florescence microscopy to essess transduction efficiency and eGFP expression. Cells with 80% infection efficiency and better proliferative ability were selected and expanded through puromycin treatment. The efficiency of overexpressed of CK19 was confirmed by Western blotting and RT-PCR.
CK19 Knockdown in SKOV3 Cells by Small Interfering RNA
Transfection of siRNA was conducted using Genmute reagent (SignaGen, ML) according to the manufacturer’s protocol. In this study, siRNAs targeting human CK19 were transiently transfected into SKOV3 cells, with nontargeting control siRNA used as negative controls. Cells were harvested 24 or 48hrs later.
Cell Migration and Invasion Assays
Cell migration assays were conducted using 8-μm pore size Transwell chambers (Costar, Cambridge, MA, USA). The lower chamber was filled with medium containing 10% FBS. 4*103 cells were suspended in 1640 with 0.1% FBS and plated into the upper chamber. After 20 hrs, the number of cells on the bottom surface of the polycarbonate membranes was counted visually using crystal violet dye and a light microscope. Cell invasion assays were conducted using the same procedure except that the upper chamber was coated with Matrigel (50g/mL, BD Bioscience, San Jose, CA, USA), and 4*103 cells were suspended into the upper chamber. Finally, the number of stained cells at the lower surface of the membrane was counted on a light microscope.
Cell Proliferation Assays
Cells were seeded at 2*103 cells/well in 96-well plates. Cell viability was assessed at 0, 24, 48 and 72hrs. Then, 10ul of CCK-8 was added to each well and incubated with cells for 2hrs. The OD was measured on a microplate reader at 450nm.
Xenografts
Animal care was provided in accordance with the Guide for the Care and Use of Laboratory Animals. 2×106 cells of SKOV3 explanted cells transfected with negative control and CK19 overexpressed were injected in 6-week-old female BALB/c nude mice (n=4/group) in 200μL of PBS. Tumor volume was calculated according to the following formula: V =0.5×D×d×d, where D represents length and d represents the width of tumors. Tumor volume was measured every 3–4 days using an electronic caliper, and at day mice were sacrificed and tumors were removed and weighed. Each tumor was used for RNA extraction and protein extraction.
Statistical Analyses
SPSS version 22.0 (IBM Corporation, Armonk, NY, USA) was used for the statistical analyses. All quantitative data were expressed as Mean±SD. Independent-samples t-tests were used for comparisons of variables between groups. Correlations between the detected markers were assessed using Spearman’s rank correlation. P-values of <0.05 were considered statistically significant.
Results
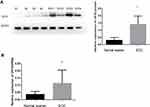
CK19 Expression Level Is Upregulated in EOC Tissues
In order to evaluate the expression levels of CK19 in EOC tissues, we performed Western blotting and RT-PCR assay. Significantly, we discovered that higher expression levels of CK19 protein and CK19mRNA were expressed in EOC tissues than in normal ovarian tissue (Figure 1A and B, P<0.05)
CK19 Protein and mRNA Expression Level in Different Human Ovarian Cancer Cell Lines
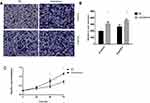
To explore the biological function of CK19 in ovarian cancer development, three established human ovarian cancer cell lines, SKOV3, OVCAR-3 and CAOV-3 were used in our experiments. Western blotting showed that all cell lines expressed CK19, and, SKOV3 cells expressing the highest levels of CK 19 than other cell lines (Figure 2). As SKOV3 cell had the highest expression, we selected SKOV3 cell for further experiments.
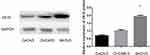 |
Figure 2 CK19 expression was the highest in SKOV3 cell line. *P<0.05. |
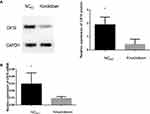
The Expression of CK19 Decreased After Transfection of siRNA
We silence CK19 expression in SKOV3 cells by transfecting siRNA. To exam the expression level of CK19 protein and CK19 mRNA after transfection, we performed Western blotting and RT-PCR assay. Results showed that CK19 protein and CK19 mRNA were markedly downregulated in SKOV3 cell compared with negative control (NCKD) group (Figure 3).
The Expression of CK19 Increased After Transfection of Lentiviral Vector
We designed lentiviral vector to overexpress CK19 expression in SKOV3 cells. To exam the expression level of CK19 protein and CK19 mRNA after transfection, we performed Western blotting and RT-PCR assay. Results showed that CK19 protein and CK19 mRNA were significantly upregulated in SKOV3 cell compared with negative control (NC) group (Figure 4).
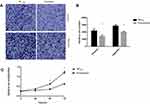
Knockdown of CK19-Inhibited Ovarian Cancer Cells Invasion, Migration and Proliferation Capacity
Transwell assay demonstrated that the invasive capacity of SKOV3 cells with knockdown of CK19 was significantly reduced. Then, we explored the effects on migration of SKOV3 knockdown CK19. Results showed that the cell migration rate of SKOV3 was decreased after knockdown of CK19 (Figure 5A and B). CCK-8 assay results showed a significant reduction in the rate of SKOV3 cell proliferation after CK19 knockdown (Figure 5C).
Overexpression of CK19 Promote Ovarian Cancer Cells Invasion, Migration and Proliferation Capacity
Transwell assay demonstrated that the invasive capacity of SKOV3 cells with overexpressed CK19 was significantly enhanced. Then, we explored the effects on migration of SKOV3 cells with overexpression of CK19. Results showed that the cell migration rate of SKOV3 was increased after CK19 overexpressed (Figure 6A and B). CCK-8 assay results showed a significant heightened rate of SKOV3 cell proliferation after CK19 overexpressed (Figure 6C).
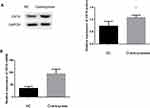
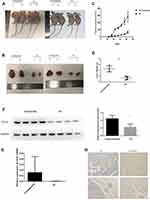
CK19 Overexpression Promote Tumor Growth in vivo
To recapitulate in vivo the results obtained in vitro, 2×106 negative control and CK19 overexpressed transfected SKOV3 cells were injected in the flank of nude mice and monitored. CK19 overexpression was confirmed before cell implantation and at the end of the experiment. 7 days post-injection, all mice developed tumors. Significant differences in tumor volume were visible at 15 days post-injection and maintained through the experiment. Mice were sacrificed at day 42 and tumors were weighted and imaged, finding larger tumors in the CK19 overexpressed group. Furthermore, CK19 expression levels were upregulated in the tumor tissues of the mice injected with the CK19 overexpressed cells at the end of the experiment (Figure 7).
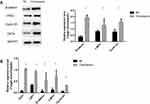
Wnt/β-Catenin Signaling Pathway Is Abnormally Activated in Overexpressed CK19 Ovarian Cancer Cells
To find out the potential mechanism of CK19 in ovarian cancer cells, we detected related genes on the Wnt/β-catenin signaling pathway. Results verified that CK19 overexpression increased the protein expression levels of β-catenin, c-MYC and cyclin D1. We also demonstrated that CK19 overexpression enhanced the Wnt/β-catenin signal pathway-related mRNA expression levels of TCF7, LEF1, β-catenin, cyclin D1 and c-MYC in SKOV3 cells (Figure 8)
Discussion
CK19 is a carcinoma marker that releases from viable epithelial tumor cells and it is also used as a marker for the detection of tumor cells disseminated in lymph nodes, peripheral blood, and bone marrow. Its positivity could be considered as prognostic indicator.5 CK19 plays a key role in hepatocellular carcinoma with the expression of invasion-related/metastasis-related markers.7 In lung cancer, CK19 intracellularly binds to HER2 to promote HER2 activation.14 CK19 has been discovered to be involved in the development of a variety of tumors, including colorectal neoplasm, nonmedullary thyroid carcinoma and breast cancer, as well as cervical carcinoma.15 However, the underlying role of CK19 and regulatory mechanism in EOC remains unclear. In the present study, a significant increase of CK19 expression level in EOC tissues was demonstrated. Furthermore, it was confirmed in cell experiments that overexpression of CK19 promoted the proliferation, invasion and migration of SKOV3 cells, and the knockdown of CK19 expression reduced the proliferation, invasion, and migration of SKOV3 cells. These data suggested that CK19 is indeed an oncogene that plays a vital role in OC progression.
What is the molecular mechanism of the CK19 in OC? In the late 1990s, the importance of the Wnt pathways in the embryonic development of the ovary was established. Wnt-4 demonstrated a critical role in embryonic ovarian development.16 Wnt-7a was shown to affect the sex-specific differentiation of the reproductive tract.17 These results verified the abnormal Wnt pathway expression may be involved in ovarian tumorigenesis in humans. The Wnt/β-catenin signal pathway in normal cells, β-catenin acts as a cytoskeletal protein at the cell membrane to form a complex with E-cadherin to maintain adhesion among cells of the same type and to prevent cell migration.18 β-catenin can accumulate in the cytoplasm, and when the cytoplasmic concentration of β-catenin reaches a certain level, it can be transferred to the nucleus. In the nucleus, β-catanin binds to the transcription factor family TCF/LEFs, which activates protooncogenes such as cyclin D1 and c-Myc, leading to cell proliferation, differentiation and maturation.19 Alterations affecting Wnt pathway proteins on the cell membrane, in the cytoplasm, and in the nucleus have been shown to play important roles in the tumorigenesis of ovarian cancer.20 Saha et al demonstrated that CK19 expression was increased in colon cancer and breast cancer, and the knockdown of CK19 led to the suppression of cancer via downregulation of Wnt signaling pathway.21 It has been well documented that c-MYC transcription factor regulates numerous malignant cell processes, including cell cycle progression, metastasis and angiogenesis in many types of cancer. The overexpression of c-MYC protein is likely to promote the process of malignancy, and is associated with high recurrence, poor overall survival in the patients with ovarian cancer.22 In the present study, we detected Wnt related genes, including β-catenin, LEF1, TCF7, c-MYC and cyclin D1 in overexpressed CK19 of SKOV3 cells, the results showed that the expression levels of the related genes were all increased. All these data demonstrated that overexpression of CK19 may activate the Wnt/β-catenin signaling pathway in SKOV3 cells.
Conclusion
In summary, this is the first study to investigate the role of CK19 in OC. Results showed that CK19 activates the Wnt/β-catenin signaling pathway and promotes the development of epithelial ovarian cancer. These findings provide a potential new therapeutic target for the clinical diagnosis and treatment of ovarian cancer.
Data Sharing Statement
The data sets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics and Consent Statement
Ethical approval for the study was granted by the Medical Ethics Committee of Beijing Chao-Yang Hospital in accordance with the Declaration of Helsinki, and the written informed consent was obtained from each participant involved. Cell lines in the study were obtained from the Medical Research Center of Beijing Chaoyang Hospital. The use of all the cell lines had ethical or institutional review board approval. Xenograft experiments were approved by Animal Research Ethics Committee of Capital Medical University, in accordance with the Guide for the Care and Use of Laboratory Animals.
Author Contributions
Qi Lu, Hong Qu, and Tong Lou performed the experiments, analysed the data and drafted the experiments; Qi Lu wrote the paper; Chongdong Liu and Zhenyu Zhang conceived and designed the experiments and revised the paper. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This study was supported by Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding-YangFan Project (Project No. ZYLX201713).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953. doi:10.1002/ijc.v144.8
2. Alemani C, Weir HK, Varreria H, et al. Global surveillance of cancer survival 1995–2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010. doi:10.1016/S0140-6736(14)62038-9
3. Mor G, Alvero A. The duplicitous origin of ovarian cancer. Ramban Maimonides Med. J. 2013;4:e0006.
4. Omary MB, Ku NO, Strnad P, Hanada S. Toward unraveling the complexity of simple epithelial keratins in human disease. J Clin Invest. 2009;119(7):1794–1805. doi:10.1172/JCI37762
5. Alix-Panabieres C, Vendrell J-P, Slijper M, et al. Full-length cytokeratin-19 is released by human tumor cells: a potential role in metastatic progression of breast cancer. Breast Cancer Res. 2009;11(3):R39. doi:10.1186/bcr2326
6. Bader BL, Magin TM, Hatzfeld M, Franke WW. Amino acid sequence and gene organization of cytokeratin no.19, an exceptional tail-less intermediate filament protein. EMBO J. 1986;5(8):1865–1875. doi:10.1002/embj.1986.5.issue-8
7. Govaere O, Komuta M, Berkers J, et al. Keratin 19: a key role player in the invasion of human hepatocellular carcinomas. Gut. 2014;63(4):674–685. doi:10.1136/gutjnl-2012-304351
8. Qu H, Chen YL, Cao GM, et al. Identification and validation of differentially expressed proteins in epithelial ovarian cancers using quantitative proteomics. Oncotarget. 2016;7(50):83187–83199. doi:10.18632/oncotarget.v7i50
9. Ju JH, Yang W, Lee KM, et al. Regulation of cell proliferation and migration by keratin 19 induced nuclear import of early growth response-1 in breast cancer cells. Clin Cancer Res. 2013;19(16):4335–4346. doi:10.1158/1078-0432.CCR-12-3295
10. Nusse R, Fuerer C, Ching W, Harnish K, Logan C, Zeng A, ten Berge D and Kalani Y. Wnt signaling and stem cell control. Cold Spring Harb Symp Quant Biol. 2008;73:59–66. doi:10.1101/sqb.2008.73.035
11. Valenta T, Hausmann G, Basler K. The many faces and functions of β-catenin. EMBO J. 2012;31:2714–2736.
12. Liu J, Pan S, Hsieh MH, et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci USA. 2013;110(50):20224–20229. doi:10.1073/pnas.1314239110
13. Arend RC, Londono-Joshi AI, Straughn JM
14. Ohtsuka T, Sakaguchi M, Yamamoto H, et al. Interaction of cytokeratin 19 head domain and HER2 in the cytoplasm leads to activation of HER2-Erk pathway. Sci Rep. 2016;6(1):39557. doi:10.1038/srep39557
15. Bremmer F, Strobel P, Jarry H, et al. CK19 is a sensitive marker for yolk sac tumours of the testis. Diagn Pathol. 2015;10(1):7. doi:10.1186/s13000-015-0243-y
16. Vainio S, Heikkila M, Kispert A, Chin N, McMahon AP. Female development in mammals is regulated by Wnt-4 signalling. Nature. 1999;397(6718):405–409. doi:10.1038/17068
17. Parr BA, McMahon AP. Sexually dimorphic development of the mammalian reproductive tract requires Wnt-7a. Nature. 1998;395(6703):707–710. doi:10.1038/27221
18. Kimura M, Nakajima-Koyama M, Lee J, Nishida E. Transient expression of WNT2 promotes somatic cell reprogramming by inducing β-catenin nuclear accumulation. Stem Cell Rep. 2016;6(6):834–843. doi:10.1016/j.stemcr.2016.04.012
19. Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene. 2017;36:1461–1473.
20. Arend RC, Londono-Joshi AI, Michael Straughn J
21. Saha SK, Yin YF, Chae HS, Cho SG. Opposing regulation of cancer properties via KRT19-mediated differential modulation of Wnt//β-catenin/Notch signaling in breast and colon cancers. Cancers. 2019;11(1):1–20. doi:10.3390/cancers11010099
22. Liu X, Yu Y, Zhang J, et al. HDAC1 silencing in ovarian cancer enhances the chemotherapy response. Cell Physiol Biochem. 2018;48(4):1505–1518. doi:10.1159/000492260
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.