Back to Journals » International Journal of General Medicine » Volume 14
Circulating miR-155 and JAK2/STAT3 Axis in Acute Ischemic Stroke Patients and Its Relation to Post-Ischemic Inflammation and Associated Ischemic Stroke Risk Factors
Authors Adly Sadik N , Ahmed Rashed L, Ahmed Abd-El Mawla M
Received 13 December 2020
Accepted for publication 22 March 2021
Published 21 April 2021 Volume 2021:14 Pages 1469—1484
DOI https://doi.org/10.2147/IJGM.S295939
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Noha Adly Sadik,1 Laila Ahmed Rashed,2 Mai Ahmed Abd-El Mawla3
1Department of Internal Medicine, Faculty of Medicine, Cairo University, Cairo, Egypt; 2Department of Biochemistry and Molecular Biology, Faculty of Medicine, Cairo University, Cairo, Egypt; 3Department of Biochemistry, Faculty of Pharmacy, Cairo University, Cairo, Egypt
Correspondence: Noha Adly Sadik
Department of Internal Medicine, Faculty of Medicine, Cairo University, Dyar City, Middle Hill El Mokattam, Cairo, 11571, Egypt
Tel +201278444526
Email [email protected]
Background: “Micro RNAs and their target genes recently have been identified to play a crucial role in the molecular pathogenesis of post-stroke ischemic cellular injury, which elucidates their new role in ischemic stroke diagnosis and therapy”. Thus, we evaluated the relative serum expression of miR-155, an inflammatory micro RNA, and the mRNAs (JAK2/STAT3) in acute ischemic stroke patients and its associations with the inflammatory cytokine TNF-α and different stroke risk factors.
Subjects and Methods: The relative expression of serum miR-155 and mRNAs (JAK2/STAT3) was assessed using RT-PCR, serum TNF-α was measured using ELIZA in 46 acute ischemic stroke patients and 50 control subjects. Receiver operating characteristic (ROC) curve was constructed to assess the specificity and sensitivity of circulating miR-155, JAK2/STAT3 as biomarkers for acute ischemic stroke.
Results: Circulating miR-155, JAK2/STAT3 were significantly up-regulated among stroke patients (8.5, 2.9, 4.2 fold respectively, P< 0.001) with significant increase in TNF-α (263.8 ± 10.7 pg/mL, P < 0.001). MiR-155, JAK2/STAT3 were positively correlated with TNF-α. MiR-155, JAK2/STAT3 were significantly increased in stroke patients and associated with risk factors such as hypertension, carotid atherosclerosis, and atrial fibrillation. Our study revealed that miR-155 has diagnostic accuracy for acute ischemic stroke where AUC=0.9, (P< 0.001).
Conclusion: The elevated expressions of circulating miR-155, JAK2/STAT3, and TNF-α in acute ischemic stroke patients could trigger post-stroke cellular inflammation. MiR-155 could be used as potential inflammatory biomarker for acute ischemic stroke. However, further clinical studies are still needed to determine the exact role of miRNAs and different signal transduction expressions in the stage of acute ischemic stroke.
Keywords: ischemic stroke, miR-155, Janus kinases, signal transduction, post stroke ischemic injury
Introduction
Stroke is ranked the second leading cause of death worldwide.1 It is also associated with high rate of disability affecting about 50% of survivors.2 Thus, it represents one of the most serious and great public health problems, especially in developing countries.3 Ischemic stroke represents nearly 80% of stroke cases which lead to cerebral ischemia with subsequent loss of brain function and a wide range of other pathophysiological processes.4 Hypertension, diabetes mellitus, obesity, smoking, atrial fibrillation, and cardiovascular diseases have been considered major risk factors of ischemic stroke reported by epidemiological studies.5,6 Most of these risk factors are preventable with proper risk stratification via valid diagnostic biomarkers.7 Stroke-related disabilities have been attributed to late-stage diagnostic methods.8,9 Moreover, therapeutic approaches for treatment using recombinant tissue plasminogen activator (rtPA) or mechanical thrombectomy have a narrow therapeutic window, high cost, and require a highly qualified team.10,11 In severe ischemia, complete loss of function occurs in the central core of the infarcted area surrounded by a viable hypoxic area (penumbra)12 that can be salvaged by introducing suitable treatment at the appropriate time.13 Thus, introduction of new valid biomarkers for early diagnosis and prognosis is one of the most important challenges for stroke prevention.14
Different pathological mechanisms have been presumed to cause neuronal cell death including oxidative stress, excitotoxicity, inflammation, dysfunction of blood brain barrier, and cellular apoptosis.15 Inflammation is one of the main mechanisms involved in the development and continuation of reperfusion injury that enhances cellular and molecular damage that occurs after ischemia.15,16
Micro RNAs (miRNAs) are small non-coding RNAs which mediate post-transcriptional gene regulation by controlling the translation of mRNA into protein.17 In ischemic stroke, miRNAs are involved in multiple cellular functions such as neuronal development, injured tissue repair, remodeling and different neuronal activities.15 MiRNAs and their target genes have a crucial regulatory role in the inflammatory process of post-ischemic reperfusion injury, which has demonstrated their potential use as therapeutic target in ischemic stroke.15 Furthermore, several miRNAs are involved in controlling target genes of ischemic stroke risk factors such as miR-155 in hypertension, miR-144 and miR-223 in diabetes mellitus, miR-33 in hyperlipidemia, and miR-21 and miR-126, and miR-320b in atherosclerosis.18,19 In addition, new circulatory miRNAs such as PC-3p-57664, PC-5p-12969, miR-122-5p, miR-211-5p have been identified as novel biomarkers for early diagnosis of ischemic stroke.20 Accordingly, miRNAs have recently gained great attention for being used as potential biomarkers for early diagnosis, prognosis, and as a therapeutic target for ischemic stroke.14
MiR-155 is one of the proinflammatory miRNAs that are involved in various physiological and pathological functions such as differentiation of hematopoietic cells, inflammation, immunity, cardiovascular disorders, and malignancy.21 MiR-155 is an essential factor for primary macrophage reaction to various inflammatory mediators.21 In addition, miR-155 regulates the microglia-mediated immune response which is related to various pathological conditions through cytokine production.22 The expression profile of miR-155 is significantly influenced by brain ischemia.23,24 MiR-155 can stimulate atherosclerosis which is one of the major risk factors of ischemic stroke by modulating the expression of various inflammatory mediators in the endothelial cells targeting anti-inflammatory endothelial transcription factor (Ets-1) and angiotensin II type 1 receptor (AT1R).25 Also, miR-155 can induce atherosclerosis by targeting several anti-inflammatory cytokines such as HMG box-transcription protein (HMG1),26 B cell lymphoma (BCL-6) protein,27 and suppressor of cytokine signaling protein 1 (SOCS1).28 In addition, miR-155 expression has a significant role in hypertension which is a major ischemic stroke risk factor, by suppressing endothelial nitric oxide synthase which induces disruption in the endothelium-dependent vasorelaxation of the cardiovascular system.29 Ischemic stroke is an important risk factor for Alzheimer’s disease.14 Increased expression of miR-155 has been identified as a potent modulator of the neuro-inflammatory response in Alzheimer’s disease by controlling T cell function.30 Furthermore, miR-155 has a negative regulatory effect on the blood brain barrier function in ischemic stroke inducing deterioration in the clinical disease outcome.31 Inhibition of miR-155 in experimental models of ischemic stroke has led to significant improvement in the vascular structure, infarct size, and neuronal damage associated with increase in the anti-inflammatory neuronal protective cytokines and decrease in the pro-inflammatory cytokines.32,33
Janus kinases (JAKs) and signal transducers and activators of transcription (STATs) pathway regulate dozens of cellular responses such as proliferation, differentiation, migration, apoptosis and cell survival depending on the signal, tissue, and cellular context.34 Particularly, JAK2/STAT3 pathway has been activated in in vitro and in vivo experimental models of stroke and subsequently promoted numerous genes responsible for many cellular functions that may play a critical role in both neural injury and repair.35 Increased expression of miR-216a targets JAK2 resulting in neuroprotection in ischemic stroke.36 Pharmaceutical inhibition of the JAK2/STAT3 signaling pathway could decrease the inflammatory reaction that occurs after brain ischemia.37 Additionally, activation of STAT3 leads to decreased cerebral recovery, while blocking this pathway leads to better neurological outcomes.34
Based upon these data, epigenetics has gained a lot of attention due to increasing evidence implicating its role in development of many diseases which in turn can be used to harness its potential as biomarker or therapeutic targets.8,9 Therefore, we aimed to evaluate circulating miR-155 and JAK2/STAT3 axis in acute ischemic stroke patients and detect its relation to the pro-inflammatory cytokine TNF-α level as an important biomarker of inflammation in acute ischemic stroke and associated stroke risk factors.
Subjects and Methods
Subjects
Forty-six patients with acute ischemic stroke were admitted to the internal medicine department Kasr Al Ainy medical school hospital, Faculty of Medicine, Cairo University during the interval from November 2017 to June 2018. Patients comprised 25 males and 21 females aged from 48 to 65 years with a mean age of 58.3 years. In addition, 50 sex- and age-matched healthy subjects were recruited as control group with a mean age of 56.8 years, subjects with doubtful history of chronic autoimmune or inflammatory diseases, hypertension, diabetes or smoking were excluded. Acute ischemic stroke patients were diagnosed based on history taking, clinical examination confirmed by brain CT. Patients with hemorrhagic stroke or known to have chronic inflammation, chronic kidney or hepatic diseases or malignant disorders were excluded.
Ethics
The study was carried out consistent with the ethical guidelines of the Declaration of Helsinki 1975 and it was approved by the Research Ethical Committee of the internal medicine department, Faculty of medicine, Cairo University, Egypt. Patients and control subjects were asked to give written informed voluntary consent.
Methods
Clinical Assessments
Full history taking and clinical examination were done for every patient. Systolic and diastolic blood pressures were recorded for each patient and control. The medical records were reviewed to confirm the medical history. Additionally, all patients were subjected to carotid duplex using HDI 5000 machine using 7.5 MHz linear transducer, brain CT, and echocardiography.
Blood Sampling
Six mL samples of venous blood were drawn from every patient (within average 12 hours from patient admission) and control subjects in sterile tubes under aseptic conditions. The drawn blood was divided into three portions. First portion of whole blood was kept in EDTA sterile tubes until used for complete blood picture. Second portion of blood was allowed to clot and then centrifuged using benchtop centrifuge (Jenway, UK) at 8000 x g for 5 min to separate the serum which was kept frozen at −80°C until used for RNA extraction and other biochemical analysis. Third portion of blood was kept in sodium fluoride tubes and then centrifuged to separate the plasma until used for measuring blood glucose level.
Biochemical Analysis
Total cholesterol (TC) was measured using established enzymatic methods using Stanbio kit (USA).38 HDL-C was estimated by HDL-C precipitant method.39 Triglycerides (TG) were assessed enzymatically.40 LDL-C concentrations were determined by Friedewald’s formula.41 Serum TNF-α was measured using Sandwich ELISA kit which was supplied by NOVA (China).
RNA Extraction and Reverse Transcription
Total RNA was extracted using MiRNeasy extraction kit (Qiagen, Valencia, CA) using QIAzol lysis reagent according to the manufacturer’s instructions. After extraction, RNA quantity was assessed using the NanoDrop 2000 spectrophotometer (Thermo Scientific, USA) at 260/280 nm. RNA was reverse transcribed to cDNA using miScript II RT Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The total RNA (100 ng) in a final volume of 25 μL RT was used for reverse transcription. The programmed thermal cycler condition was done initially at 25°C for 10 min, then at 37°C for 110 min, and finally at 95°C for 5 s. After that, prepared cDNA was stored at −80°C for further analysis.
Measurement of miR-155 and mRNA Expressions
The quantitative detection of miR-155, mRNAs JAK2/STAT3 was performed using premixed SYBR green PCR master mix (Applied Biosystems, USA). The thermal cycler conditions were programmed as follows: primary hold at 90°C for 10 min, followed by 45 cycles consisting of 3 min at 90°C, 30 s at 95°C, and 1 min annealing extension (40-fold). Annealing temperature was 54–60°C. The housekeeping genes were U6 for miR-155 and β-actin for mRNA. The PCR primer sequences were as follows: miR-155, F: 5ʹ-AACTTGTAAACTCCCTCGACTG-3ʹ, R: 5ʹ-CCTTACGTGACCTGGAGTCG-3ʹ, JAK2 F: 5ʹ-CAATGATAAACAAGGGCAAA TGAT-3ʹ, R: 5ʹ-CTTGGCAATCTTCCGTTGCT-3ʹ. STAT3,F:5׳-GCCAGAGAGC CAGGAGCA-3׳ R:5ʹ-ACACAGATAAACTTGGTCTTCAGGTATG-3ʹ, U6, F: 5ʹ-GTGCTCGCTTCGGCAGCA-3ʹ, R: 5ʹ-CAAAATATGGAACGCTTC-3ʹ, β-actin, F:5׳-GGCTGTATTCCCCTCCATCG-3׳,R: 5ʹ-CCAGTTGGTAACAATGCC ATGT-3ʹ. Expression levels were calculated by the relative quantification method (ΔCt).
Statistical Analysis
Data were analyzed using GraphPad Prism version 7.0 software (USA). The normally distributed data were expressed as mean ± SE, whereas variables with a skewed distribution were presented as median (inter-quartile range). The statistical comparisons between different groups were carried out using unpaired Student’s t-test for parametric data and Mann–Whitney U-test for nonparametric data as expression of miRNA and mRNA. The correlation between variables was evaluated using Spearman’s rank correlation coefficient and Pearson correlation tests (2-tailed). The level of significance was identified at P<0.05. Receiver operating characteristic (ROC) curve was constructed and the area under curve (AUC) was used to assess specificity and sensitivity of the circulating miR-155, mRNAs (JAK2/STAT3) as diagnostic biomarkers for acute ischemic stroke.
Results
The present study included 46 stroke patients; 25 men (54.3%) and 21 women (45.7%) with mean age 58.3 ± 2.1 years, our control group included 26 men (52%) and 24 women (48%) with mean age 56.8 ± 3.1 years, with no statistically significant difference regarding age between the two groups (P=0.08). Table 1 shows the demographic and laboratory data of the studied groups. There were significant differences between stroke patients and healthy subjects in terms of systolic and diastolic blood pressure, fasting blood sugar, cholesterol levels, LDL-C, and ESR (P= 0.01, 0.04, 0.0001, 0.02, 0.02, 0.0001 respectively). The basal cardiovascular assessment of stroke patients revealed that traditional cardiovascular events were frequently reported in 9 patients (19.6%) who were diabetic, 19 (41.3%) who were hypertensive, 14 (30%) who were smokers, and 22 (47.8%) patients who were dyslipidemic. Additionally, 2 (4%) were drug abusers, history of recurrence was present in 6 (13%) patients, positive family history in 4 (9%) patients, and atrial fibrillation was detected in 19 (41.3%) patients. Based on carotid arteries duplex ultrasound, carotid atherosclerosis was detected in 27 (59%) patients and significant carotid stenosis was detected in 7 (15%) patients (Table 1).
 |
Table 1 Demographic and Laboratory Data of Our Ischemic Stroke Patients and Control Subjects |
Concerning the relative expression of miR-155 in serum obtained from stroke patients and control subjects; it was highly expressed in stroke patients as compared to controls, reaching 8.5 fold change at P value <0.001 (Figure 1A). The relative expression levels of mRNAs (JAK2/STAT3) were highly up-regulated among stroke patients reaching 2.9 and 4.2 fold changes at P values <0.001 and <0.001, respectively as compared to controls, as shown in Figure 1B and C. Furthermore, the mean serum levels of TNF-α in our patients were higher as compared to control subjects (P <0.0001) (Figure 1D).
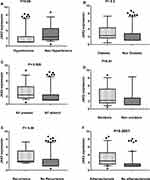
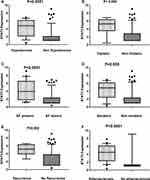
By comparing the relative expression of miR-155 level with the presence or absence of different important risk factors for ischemic stroke such as diabetes, hypertension, smoking, carotid atherosclerosis, stroke recurrence, atrial fibrillation, we found significant association between the relative expression level of miR-155 and hypertension (P=0.01), carotid atherosclerosis (P=0.001) and atrial fibrillation (P= 0.04) (Figure 2). Also, we found significant elevation in the relative expression of JAK2 in acute ischemic patients with history of atrial fibrillation, recurrence, smoking, and carotid atherosclerosis (Figure 3). STAT3 was significantly elevated in patients with hypertension, diabetes, smoking, stroke recurrence, atrial fibrillation, carotid atherosclerosis (Figure 4). While level of TNF-α concentration was significantly higher in stroke patients with hypertension, diabetes, smoking, stroke recurrence, atrial fibrillation, carotid atherosclerosis (Figure 5).
On performing Spearman correlation we found that miR-155 was positively correlated with STAT3 (r=0.71, p < 0.0001), JAK2 (r=0.73, p < 0.0001) and TNF-α (r =0.71, p < 0.0001), also, STAT3 was positively correlated to JAK2 (r=0.58, p < 0.001) and TNF-α (r=0.67, p < 0.001), in addition, JAK2 was positively correlated to TNF-α (r=0.52, p < 0.0001) among our studied subjects, as shown in Figure 6. Additionally, miR-155 was positively correlated with age of patients, duration of stroke in hours, systolic blood pressure, total cholesterol levels, and ESR among ischemic stroke patients. JAK2 was positively correlated with age of patients, duration of stroke in hours, blood pressure and HDL-C levels; STAT3 was positively correlated with systolic blood pressure and HDL-C, and TNF-α was correlated positively with age of patients, duration of stroke in hours, blood pressure, triglycerides, HDL-C, and ESR levels among ischemic stroke patients (Table 2).
 |
Table 2 The Correlation of Circulating miR-155, JAK2, STAT3 and TNF-α with Other Parameters Among Ischemic Stroke Patients |
The potential utility of circulating miR-155, JAK2/STAT3 as biomarkers for stroke patients was determined. Our analysis indicated that, at cut off point 1.75, miR-155 had sensitivity= 85.7% and specificity= 100% and at cut off point 1.25, JAK2 had sensitivity= 61.9% and specificity= 84.2 and at cut off point 2.05, STAT3 had sensitivity= 83.3% and specificity= 67.8, as shown in Figure 7.
Discussion
Recently, many cellular changes have been implicated in stroke pathogenesis and several miRNAs have been found to play a crucial role in the atherosclerosis process and have different expression levels according to the stroke etiology,42,43 which elucidates their new role in ischemic stroke diagnosis and therapy. Various studies documented the essential role of miR-155 as a pro-inflammatory trigger inducing the inflammatory signal transduction cascade in atherosclerosis.42,44,45 Activation of JAK2/STAT3 signaling pathway stimulates the release of the inflammatory mediators such as TNF-α in the peripheral macrophages which in turn increase the inflammatory reaction.46 Thus, we evaluated the relative serum expression of miR-155 and the mRNAs (JAK2/STAT3) in acute ischemic stroke patients and its associations with the inflammatory biomarker TNF-α levels and different stroke risk factors.
Our study revealed that increased expression of miR-155 in acute ischemic stroke patients was associated with significant elevation in the relative expression of JAK2/STAT3 with significant increase in the inflammatory cytokine TNF-α. Also, miR-155 and mRNAs (JAK2/STAT3) were positively correlated with TNF-α levels, one of the most important inflammatory biomarkers related to ischemic stroke.36,47 We also found that miR-155 was positively correlated with ESR that was significantly increased in our patients. ESR is an independent inflammatory marker for early prediction of poor stroke outcomes.48,49 Also, there was a significant association between miR-155 and mRNAs’ (JAK2/STAT3) expression. This direct relation between miR-155 and STAT3 was also reported by Escobar et al in another inflammatory model (uveitis) which revealed that STAT3 binds directly to the miR-155 locus and that STAT3 is required for miR-155 expression and that mature miR-155 expression is defective in the absence of STAT3 via Chromatin Immunoprecipitation (ChIP) Analyses technique.50 As JAK2 is an upstream to STAT3, we believe that miR-155 and STAT3/JAK2 form an axis that promotes post-stroke cellular inflammation. Accordingly, we hypothesize that triggering JAK2/STAT3 axis and TNF-α may be one of the underlying mechanisms of the inflammatory role of miR-155. Thus, targeting this axis may be beneficial for treatment. The inflammatory response that occurs after brain ischemia is a complementary process of both brain injury and recovery, where elevated levels of inflammatory mediators accompanied by reduced levels of anti-inflammatory neuro-protective mediators have been linked to poor prognosis.10,51
We found a significant association between the relative expression of circulating miR-155 and atherosclerosis. This is in agreement with Li et al who reported increased expression of miRNA-155 in the plasma and atherosclerotic veins of atherosclerosis patients.52 MiR-155 is involved in the atherosclerotic process inducing macrophage cell differentiation and stimulation.53 Nazari-Jahantigh et al reported a direct association between inhibition of miR-155 expression and decrease in plaque size and macrophage number.27 Additionally, we found a significant increase in the expression of JAK2/STAT3 in patients with atherosclerosis. JAK2/STAT3 signaling pathway is involved in the inflammatory process associated with atherosclerosis controlling the monocyte macrophage differentiation which is a crucial step in augmenting atherosclerosis.54,55 These results elucidate the important role of miR-155 and JAK2/STAT3 signaling pathway in the process of atherosclerosis, a major risk factor of ischemic stroke.
Regarding hypertension, one of the most important modifiable risk factors of stroke, accumulating evidence suggests that miR-155 is related to AT1R that could control blood pressure by modulating TGF-β1 signaling pathway.56 Also, Johnson et al documented the crucial role of STAT3 for angiotensin II induced hypertension via its effect on vascular endothelium and oxidative stress.57 In our study, we found a significant increase in circulating miR-155 and STAT3 among stroke patients with hypertension in comparison to patients without hypertension, along with a significant positive correlation with systolic blood pressure, in agreement with earlier studies.58,59 Our findings determined the significant role of miR-155 and STAT3 in ischemic stroke pathogenesis through controlling the arterial blood pressure and its validity to be used as biomarkers for risk stratification.
In our study we found a significant association between miR-155 and total cholesterol levels. Thus, increased expression of miRNA-155 which is associated with hypercholesterolemia may at least partly emphasize the link between atherosclerosis and stroke which was previously reported in our study. Similarly, Faccini et al found a significant association between miR-155 and cholesterol in patients with coronary artery disease.60 Our study revealed a significant negative correlation between JAK2/STAT3, TNF-α and HDL-C. The presence of dyslipidemia stimulates the release of inflammatory cytokines such as TNF-α which results in activation of JAK2/STAT3 pathway which aggravates atherosclerosis, and by inhibiting the JAK2/STAT3 pathway, the formation of lipid particles inside the vessels as well as the formation of atheromatous plaque can be prevented.61
Atrial fibrillation has a well-described role in the pathogenesis of stroke. In our study, we explored a novel significant relation between circulating miR-155 and atrial fibrillation in stroke patients. These results are supported by Tran et al who reported that miR-155 increases the secretion of nerve growth factor (NGF) signaling and the inflammatory cytokine IL-8 from the epicardial adipose tissue which potentiates the development and maintenance of atrial fibrillation.62 Currently, there is no available biomarker for early diagnosis of atrial fibrillation. Studies have found that miRNAs can serve as a good biomarker for diagnosis.63 The miRythm study evaluated 86 circulating miRNAs in atrial fibrillation patients and they found that circulating miR-21 and miR-150 were down-regulated in atrial fibrillation patients compared to controls, and suggested that studying miRNAs can be an important method to understand more about cardiac gene regulation.64 Importantly, our study pointed out that miR-155 may provide additional information for reassessing atrial fibrillation, which may trigger stroke. Regarding JAK2/STAT3, we also found a significant increase in their expression in stroke patients with atrial fibrillation. Interestingly, Xue et al reported up-regulation in STAT3 expression in the atrial tissue in rheumatic heart disease patients with atrial fibrillation compared to sinus patients.65 In vivo inhibition of STAT3 decreased the atrial fibrosis present in an animal model, which elucidates the important role of STAT3 as an essential mediator in the fibrotic process and atrial remodeling.66
MiR-144 and miR-223 were linked to diabetes in ischemic stroke patients.67 In our study we did not find any significant association between miR-155 and diabetes, consistent with Fichtlscherer et al’s study which was done on patients with coronary artery disease.68 On the contrary. we found significant increase in STAT3 expression and TNF-α level in diabetic stroke patients. Yang et al also documented significant increase in STAT3 expression and TNF-α level in diabetic patients with macrovascular complications which are related to the inflammatory role of JAK2/STAT3 pathway in inflammation.69
Smoking is considered one of the major cardiovascular risk factors that induce endothelial dysfunction and atherogenesis.70 Smoking can increase the expression of miR-155 in the endothelial cells which induce the release of inflammatory mediators and cell death.71 Also, smoking can cause impairment in miR-155 expression that induces acute lung injury by targeting SOCS1 and emphysema.72,73 In our, study we did not find a significant association between smoking and miR-155 expression. On the contrary, we found a significant increase in STAT3/JAK2 expression and smoking. The relation between STAT3 and nicotine in atherosclerosis model was studied by Xu et al, who reported a direct relation between STAT3 and nicotinic acetylcholine receptors α1 (nAChRα1) and inhibition of these interactions decreases nicotine-induced atherosclerosis by targeting Akt/mTOR pathway.74
We assessed the relation between circulating miR-155 and mRNAs (JAK2/STAT3) and stroke recurrence. Only mRNAs (JAK2/STAT3) were associated with stroke recurrence. A previous study documented the association of miR-17 and stroke recurrence among five miRNAs significantly associated with acute stroke.75 Other studies have linked genetic polymorphisms of miRNAs to stroke recurrence.76,77 This means that there is no direct relation between significant expression of specific miRNA and disease outcome, and there are still unknown mechanisms which play a significant role in miRNAs’ profiling along the course of the disease.
Over and above, ROC analysis was used to explore the fact that ischemic stroke patients with over-expression of miR-155 and mRNAs (STAT3/JAK2) are at a higher risk for ischemic stroke development as the calculated cut-off value, sensitivity and specificity are appropriate for the discrimination between patients and controls. Moreover, miR-155 and mRNAs (STAT3/JAK2) were strongly correlated with several risk factors associated with stroke. Therefore, we suggest that miR-155 and mRNAs (STAT3/JAK2) could be accessible complementary biomarkers in order to improve the diagnostic performance of stroke. There were some limitations in our study, the relatively small number of patients studied, also, it was important to assess these biomarkers again after stabilization of patients and detect its association with patient outcomes. The significant association between some stroke risk factors and miR-155 and target genes still raises the question about the main mechanism that leads to this significant expression, there are still unknown molecular mechanisms in miRNAs’ profiling that have to be elucidated. Therefore, we still need further studies to answer these questions.
Conclusion
In light of the presented findings, we can conclude that circulating miR-155 expression and mRNAs (JAK2/STAT3) are significantly elevated in acute ischemic stroke patients and significantly correlated to the inflammatory cytokine TNF-α which could collectively trigger post-stroke inflammation. Also, there were significant associations between miR-155 and mRNAs’ (JAK2/STAT3) expressions and important risk factors of stroke such as hypertension, AF, dyslipidemia, smoking, and atherosclerosis. These significant associations could help us to know more about the role of different stroke risk factors in the pathogenesis of ischemic stroke. Furthermore, miR-155 could be used as complementary inflammatory biomarker for differentiating ischemic stroke patients from healthy subjects, and targeting miRNAs could control a variety of coding genes which constitute the future of gene therapy for post-stroke recovery. However, further clinical studies are still needed to determine the exact role of miRNAs and different signal transduction expressions in the stage of post-ischemic stroke injury, which may shed more light on debated mechanisms of stroke and new approaches for management.
Funding
This research was self-funded and did not receive specific grant from any funding agency.
Disclosure
There are no conflicts of interest.
References
1. Heron M. Deaths: leading causes for 2004. Natl Vital Stat Rep. 2007;56:1–96.
2. Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ. Global and regional burden of disease and risk factors,2001: systematic analysis of population health data. Lancet. 2006;367(9524):1747–1757. doi:10.1016/S0140-6736(06)68770-9
3. Adogu POU, Ubajaka CF, Emelumadu OF, Alutu COC. Epidemiologic transition of diseases and health-related events in developing countries: a review. Am J Med Sci Med Sci. 2015;5(4):150–157.
4. Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinical identifiable subtypes of cerebral infarction. Lancet. 1991;337(8756):1521–1526. doi:10.1016/0140-6736(91)93206-O
5. Béjot Y, Bailly H, Durier J, Giroud M. Epidemiology of stroke in Europe and trends for the 21st century. La Presse Médicale. 2016;45:e391–e398. doi:10.1016/j.lpm.2016.10.003
6. Ekker MS, Boot EM, Singhal AB, et al. Epidemiology, aetiology, and management of ischaemic stroke in young adults. Lancet Neurol. 2018;17:790–801. doi:10.1016/S1474-4422(18)30233-3
7. Powers WJ. Acute ischemic stroke. N Engl J Med. 2020;383:252–260. doi:10.1056/NEJMcp1917030
8. Pearce WJ. Epigenetics: an expanding new piece of the stroke puzzle. Transl Stroke Res. 2011;2:243–247. doi:10.1007/s12975-011-0094-0
9. Kassis H, Shehadah A, Chopp M, Zhang ZG. Epigenetics in stroke recovery. Genes. 2017;8:89. doi:10.3390/genes8030089
10. Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi:10.1186/1479-5876-7-97
11. Kadir RRA, Bayraktutan U. Urokinase plasminogen activator: a potential thrombolytic agent for ischaemic stroke. Cell Mol Neurobiol. 2020;40:347–355. doi:10.1007/s10571-019-00737-w
12. Roitbak T. Silencing a multifunctional microRNA is beneficial for stroke recovery. Front Mol Neurosci. 2018;11:58. doi:10.3389/fnmol.2018.00058
13. Bulygin KV, Beeraka NM, Saitgareeva AR, et al. Can miRNAs be considered as diagnostic and therapeutic molecules in ischemic stroke pathogenesis?—Current status. Int J Mol Sci. 2020;21(18):6728. doi:10.3390/ijms21186728
14. Vijayan M, Reddy PH. Peripheral biomarkers of stroke: focus on circulatory microRNAs. Biochim Biophys Acta (BBA)-Mol Basis Dis. 2016;1862:1984–1993. doi:10.1016/j.bbadis.2016.08.003
15. Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M. Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. J Stroke. 2017;19(2):166–187. doi:10.5853/jos.2016.01368
16. Nakka VP, Prakash-babu P, Vemuganti R. Crosstalk between endoplasmic reticulum stress, oxidative stress, and autophagy: potential therapeutic targets for acute CNS injuries. Mol Neurobiol. 2016;53:532–544. doi:10.1007/s12035-014-9029-6
17. O’Brien J, Hayder H, Zayed Y, Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Front Endocrinol (Lausanne). 2018;9:402. doi:10.3389/fendo.2018.00402
18. Eyileten C, Wicik Z, De Rosa S, et al. MicroRNAs as diagnostic and prognostic biomarkers in ischemic stroke—A comprehensive review and bioinformatic analysis. Cells. 2018;7:249. doi:10.3390/cells7120249
19. Vijayan M, Reddy PH. Non-coding RNAs based molecular links in type 2 diabetes, ischemic stroke, and vascular dementia. J Alzheimers Dis. 2020;75:353–383. doi:10.3233/JAD-200070
20. Vijayan M, Kumar S, Yin X, et al. Identification of novel circulatory microRNA signatures linked to patients with ischemic stroke. Hum Mol Genet. 2018;27(13):2318–2329. doi:10.1093/hmg/ddy136
21. Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792:497–505. doi:10.1016/j.bbadis.2009.02.013
22. Cardoso AL, Guedes JR, Pedroso de Lima MC. miR-155 modulates microglia-mediated immune response by down-regulating SOCS-1 and promoting cytokine and nitric oxide production. Immunology. 2012;135:73–88. doi:10.1111/j.1365-2567.2011.03514.x
23. Lim KY, Chua JH, Tan JR, et al. MicroRNAs in cerebral ischemia. Transl Stroke Res. 2010;1:287–303. doi:10.1007/s12975-010-0035-3
24. Liu Y, Zhang J, Han R, Liu H, Sun D, Liu X. Downregulation of serum brain specific microRNA is associated with inflammation and infarct volume in acute ischemic stroke. J Clin Neurosci. 2015;22:291. doi:10.1016/j.jocn.2014.05.042
25. Zhu N, Zhang D, Chen S, et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis. 2011;215:286–293. doi:10.1016/j.atherosclerosis.2010.12.024
26. Tian FJ, An LN, Wang GK, et al. Elevated microRNA-155 promotes foam cell formation by targeting HBP1 in atherogenesis. Cardiovasc Res. 2014;103:100–110. doi:10.1093/cvr/cvu070
27. Nazari-Jahantigh M, Wei Y, Noels H, et al. MicroRNA-155 promotes atherosclerosis by repressing Bcl6 in macrophages. J Clin Invest. 2012;122:4190–4202. doi:10.1172/JCI61716
28. Yao R, Ma YL, Liang W, et al. MicroRNA-155 modulates Treg and Th17 cells differentiation and Th17 cell function by targeting SOCS1. PLoS One. 2012;7:e46082. doi:10.1371/journal.pone.0046082
29. Sun H-X, Zeng D-Y, Li R-T, et al. Essential role of microRNA-155 in regulating endothelium-dependent vasorelaxation by targeting endothelial nitric oxide synthase. Hypertension. 2012;60:1407–1414. doi:10.1161/HYPERTENSIONAHA.112.197301
30. Guedes JR, Custodia CM, Silva RJ, de Almeida LP, de Lima MCP, Cardoso AL. Early miR-155 upregulation contributes to neuroinflammation in Alzheimer’s disease triple transgenic mouse model. Hum Mol Genet. 2014;23:6286–6301. doi:10.1093/hmg/ddu348
31. Lopez‐Ramirez MA, Wu D, Pryce G, et al. MicroRNA‐155 negatively affects blood–brain barrier function during neuroinflammation. FASEB J. 2014;28(6):2551–2565. doi:10.1096/fj.13-248880
32. Pena-Philippides JC, Caballero-Garrido E, Lordkipanidze T. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation. 2016;13:1–16. doi:10.1186/s12974-016-0753-x
33. Zhang L, Liu C, Huang C, Xiaohui X, Teng J. miR-155 knockdown protects against cerebral ischemia and reperfusion injury by targeting MafB. Biomed Res Int. 2020;2020:11.
34. Chen Q, Lv J, Yang W, et al. Targeted inhibition of STAT3 as a potential treatment strategy for atherosclerosis. Theranostics. 2019;9(22):6424–6442. doi:10.7150/thno.35528
35. Liang Z, Wu G, Fan C, et al. The emerging role of signal transducer and activator of transcription 3 in cerebral ischemic and hemorrhagic stroke. Prog Neurobiol. 2016;137:1–16. doi:10.1016/j.pneurobio.2015.11.001
36. Tian YS, Zhong D, Liu QQ, et al. Upregulation of miR-216a exerts neuroprotective effects against ischemic injury through negatively regulating JAK2/STAT3-involved apoptosis and inflammatory pathways. J Neurosurg. 2018;130(3):977–988. doi:10.3171/2017.5.JNS163165
37. Wu Y, Xu J, Xu J, Zheng W, Chen Q, Jiao D. Study on the mechanism of JAK2/STAT3 signaling pathway-mediated inflammatory reaction after cerebral ischemia. Mol Med Rep. 2018;17(4):5007–5012. doi:10.3892/mmr.2018.8477
38. Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–475. doi:10.1093/clinchem/20.4.470
39. Lopes-virella ME, Stone P, Elliss S. Cholesterol determination in high density lipoprotein separated by three different methods. Clin Chem. 1977;23:882–884. doi:10.1093/clinchem/23.5.882
40. Neri BP, Frings CS. Improved method for determination of triglycerides in serum. Clin Chem. 1973;19(10):1201–1202. doi:10.1093/clinchem/19.10.1201
41. Friedewald WT, Levy RL, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi:10.1093/clinchem/18.6.499
42. Virtue A, Mai J, Yin Y, et al. Structural evidence of anti-atherogenic microRNAs. Front Biosci. 2011;16:3133–3145. doi:10.2741/3901
43. Tsai PC, Liao YC, Wang YS, Lin HF, Lin RT, Juo SH. Serum microRNA-21and microRNA-221 as potential biomarkers for cerebrovascular disease. J Vasc Res. 2013;50:346–354. doi:10.1159/000351767
44. Graff JW, Dickson AM, Clay G, McCaffrey AP, Wilson ME. Identifying functional microRNAs in macrophages with polarized phenotypes. J Biol Chem. 2012;287:21816–21825. doi:10.1074/jbc.M111.327031
45. Yao Y, Li G, Wu J, Zhang X, Wang J. Inflammatory response of macrophages cultured with Helicobacter pylori strains was regulated by miR-155. Int J Clin Exp Pathol. 2015;8:4545–4554.
46. Liu H, Yao YM, Yu Y, Dong N, Yin HN, Sheng ZY. Role of Janus kinase/signal transducer and activator of transcription pathway in regulation of expression and inflammation-promoting activity of high mobility group box protein 1 in rat peritoneal macrophages. Shock. 2007;27:55–60. doi:10.1097/01.shk.0000233197.40989.31
47. Pan J, Meijie Q, Yongfang L, et al. MicroRNA-126-3p/-5p overexpression attenuates blood–brain barrier disruption in a mouse model of middle cerebral artery occlusion. Stroke. 2020;51:619–627. doi:10.1161/STROKEAHA.119.027531
48. Chamorro A, Vila N, Ascaso C, et al. Early prediction of stroke severity role of the erythrocyte sedimentation rate. Stroke. 1995;26(4):573–576.
49. Singh AS, Atam V, Yathish BE, et al. Role of erythrocyte sedimentation rate in ischemic stroke as an inflammatory marker of carotid atherosclerosis. J Neurosci Rural Pract. 2014;5(1):40–45. doi:10.4103/0976-3147.127870
50. Escobar T, Yu CR, Muljo SA, Egwuagu CE. STAT3 activates miR-155 in Th17 cells and acts in concert to promote experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2013;54(6):4017–4025. doi:10.1167/iovs.13-11937
51. Perera MN, Ma HK, Arakawa S, et al. Inflammation following stroke. J Clin Neurosci. 2006;13:1–8. doi:10.1016/j.jocn.2005.07.005
52. Li X, Kong D, Chen H, et al. miR-155 acts as an anti-inflammatory factor in atherosclerosis-associated foam cell formation by repressing calcium-regulated heat stable protein 1. Sci Rep. 2016;6:21789. doi:10.1038/srep21789
53. Nazari-Jahantigh M, Wei Y, Schober A. The role of microRNAs in arterial remodelling. Thromb Haemost. 2012;107:611–618. doi:10.1160/TH11-12-0826
54. Vasamsetti SB, Karnewar S, Kanugula AK, Thatipalli AR, Kumar JM, Kotamraju S. Metformin inhibits monocyte-to-macrophage differentiation via AMPK-mediated inhibition of STAT3 activation: potential role in atherosclerosis. Diabetes. 2015;64:2028–2041. doi:10.2337/db14-1225
55. Min X, Ungureanu D, Maxwell S, et al. Structural and functional characterization of the JH2 pseudokinase domain of JAK family tyrosine kinase 2 (TYK2). J Biol Chem. 2015;290:27261–27270. doi:10.1074/jbc.M115.672048
56. Ceolotto G, Papparella I, Bortoluzzi A, et al. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am J Hypertens. 2011;24(2):241–246. doi:10.1038/ajh.2010.211
57. Johnson AW, Kinzenbaw DA, Modrick ML, Farac FM. Small-molecule inhibitors of signal transducer and activator of transcription 3 protect against angiotensin II–induced vascular dysfunction and hypertension. Hypertension. 2013;61:437–442. doi:10.1161/HYPERTENSIONAHA.111.00299
58. Huang Y, Chen J, Zhou Y, et al. Circulating miR155 expression level is positive with blood pressure parameters: potential markers of target-organ damage. Clin Exp Hypertens. 2016;38:331–336. doi:10.3109/10641963.2015.1116551
59. Huang Y-Q, Huang C, Zhang B, Feng Y-Q. Association of circulating miR-155 expression level and inflammatory markers with white coat hypertension. Hum Hypertens. 2020;34:397–403. doi:10.1038/s41371-019-0250-7
60. Faccini J, Ruidavets J-B, Cordelier P, et al. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Sci Rep. 2017;7:42916. doi:10.1038/srep42916
61. Yang X, Jia J, Yu Z, et al. Inhibition of JAK2/STAT3/SOCS3 signaling attenuates atherosclerosis in rabbit. BMC Cardiovasc Disord. 2020;20:133. doi:10.1186/s12872-020-01391-7
62. Tran KV, Majka J, Sanghai S, et al. Micro-RNAs are related to epicardial adipose tissue in participants with atrial fibrillation: data from the MiRhythm study. Front Cardiovasc Med. 2019;6:115. doi:10.3389/fcvm.2019.00115
63. Komal S, Yin -J-J, Wang S-H, Huang C-Z, Tao H-L, Dong J-Z. MicroRNAs: emerging biomarkers for atrial fibrillation. J Cardiol. 2019;74(6):475–482. doi:10.1016/j.jjcc.2019.05.018
64. McManus DD, Tanriverdi K, Lin H, et al. Plasma microRNAs are associated with atrial fibrillation and change after catheter ablation (the miRhythm study). Heart Rhythm. 2015;12(1):3–10. doi:10.1016/j.hrthm.2014.09.050
65. Xue XD, Huang JH, Wang HS. Angiotensin II activates signal transducers and activators of transcription 3 via Rac1 in the atrial tissue in permanent atrial fibrillation patients with rheumatic heart disease. Cell Biochem Biophys. 2015;71:205–213. doi:10.1007/s12013-014-0186-z
66. Chen Y, Surinkaew S, Naud P. JAK-STAT signalling and the atrial fibrillation promoting fibrotic substrate. Cardiovasc Res. 2017;113:310–320. doi:10.1093/cvr/cvx004
67. Yang S, Zhao J, Chen Y, Lei M. Biomarkers associated with ischemic stroke in diabetes mellitus patients. Cardiovasc Toxicol. 2016;16:213–222. doi:10.1007/s12012-015-9329-8
68. Fichtlscherer S, Zeiher AM, Dimmeler S. Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol. 2011;31:2383–2390. doi:10.1161/ATVBAHA.111.226696
69. Yang M, Tian M, Zhang X. Role of the JAK2/STAT3 signaling pathway in the pathogenesis of type 2 diabetes mellitus with macrovascular complications. Oncotarget. 2017;8:96958–96969. doi:10.18632/oncotarget.18555
70. Pan B, Jin X, Jun L, Qiu S, Zheng Q, Pan M. The relationship between smoking and stroke. Medicine. 2019;98(12):e14872. doi:10.1097/MD.0000000000014872
71. Frati G, Forte M, Di Nonno F, Bordin A, Chimenti I, Picchio V. inhibition of miR‐155 attenuates detrimental vascular effects of tobacco cigarette smoking. J Am Heart Assoc. 2020;9:e017000. doi:10.1161/JAHA.120.017000
72. Zhang Y, Xie Y, Zhang L, Zhao H. MicroRNA-155 participates in smoke-inhalation-induced acute lung injury through inhibition of SOCS-1. Molecules. 2020;25(5):1022. doi:10.3390/molecules25051022
73. De Smet EG, Van Eeckhoutte HP, Avila Cobos F, et al. The role of miR-155 in cigarette smoke-induced pulmonary inflammation and COPD. Mucosal Immunol. 2020;13(3):423–436.
74. Xu S, Ni H, Chen H, Dai Q. The interaction between STAT3 and nAChRα1 interferes with nicotine-induced atherosclerosis via Akt/mTOR signaling cascade. AGING. 2019;11(19):8120. doi:10.18632/aging.102296
75. Kim JM, Jung KH, Chu K, et al. Atherosclerosis-related circulating microRNAs as a predictor of stroke recurrence. Transl Stroke Res. 2015;6:191–197. doi:10.1007/s12975-015-0390-1
76. Zhang Z, Xu G, Cai B, Zhang H, Zhu W, Liu X. Genetic variants in microRNAs predict recurrence of ischemic stroke. Mol Neurobiol. 2017;54(4):2776–2780. doi:10.1007/s12035-016-9865-7
77. Ryu CS, Oh SH, Lee KO, et al. MiR-10a, 27a, 34b/c, and 300 polymorphisms are associated with ischemic stroke susceptibility and post-stroke mortality. Life. 2020;10(12):309. doi:10.3390/life10120309
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.