Back to Journals » Cancer Management and Research » Volume 11
Circ-ITGA7 sponges miR-3187-3p to upregulate ASXL1, suppressing colorectal cancer proliferation
Authors Yang G, Zhang T, Ye J, Yang J, Chen C, Cai S, Ma J
Received 27 January 2019
Accepted for publication 15 June 2019
Published 12 July 2019 Volume 2019:11 Pages 6499—6509
DOI https://doi.org/10.2147/CMAR.S203137
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Ahmet Emre Eşkazan
Guangpu Yang, Tianhao Zhang, Jinning Ye, Jie Yang, Chuangqi Chen, Shirong Cai, Jinping Ma
Department of Gastrointestinal Surgery, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou 510080, People’s Republic of China
Background: As a class of endogenous noncoding RNAs, some circular RNAs (circRNAs) have recently been reported to play a role in the regulation of tumorigenesis and progression in colorectal cancer (CRC). However, the mechanisms by which most these circRNAs function in CRC are still unclear.
Purpose: The objective of this study was to identify the role of circRNA-ITGA7 in CRC cell proliferation.
Patients and methods: Human genome-wide circRNA microarray v2 analysis was used for expression profile analysis. Target genes were predicted using online bioinformatics database, including TargetScan, miRDB, miRTarbase and miRMap. Gene overexpression and silencing cell models were built using cell transfection. qRT-PCR and Western blot were performed for gene and protein expression assessment. CCK8, colony formation and cell cycle analysis were used for proliferation testing. Annexin V-FITC analysis was performed for apoptosis detection.
Results: CircRNA sequencing analysis suggested that compared to that in adjacent normal control tissue, the expression of circ-ITGA7, a novel circRNA, is decreased significantly in CRC. Gain-of-function studies further demonstrated that circ-ITGA7 suppressed proliferation of CRC cells. Based on prediction and verification, we subsequently revealed that miR-3187-3p is a circ-ITGA7-associated miRNA. Furthermore, RNA sequencing and bioinformatics analyses showed that ASXL1-5ʹUTR, the target of miR-3187-3p, is upregulated in circ-ITGA7-overexpressed cells and mediates the circ-ITGA7-induced suppression of proliferation.
Conclusion: Circ-ITGA7 might suppress CRC proliferation by sponging miR-3187-3p and increasing ASXL1 expression. Thus, circ-ITGA7 might be a potential diagnostic biomarker and a therapeutic target for CRC.
Keywords: ASXL1, circ-ITGA7, colorectal cancer, miR-3187-3p, proliferation
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide.1 In China, the prevalence is growing dramatically in last decades due to dietary and lifestyle changes, and CRC is now the fifth leading cause of cancer-related death in China.2 Early diagnosis and treatment targeting tumorigenesis mechanisms have been considered to be the most promising solution to this problem. There are three major mechanisms of CRC development that have been generally recognized in recent research, including chromosomal instability, DNA repair defects or microsatellite instability (MSI), and the CpG island methylation phenotype.3 In addition, CRC suppressors, which comprise genetic and epigenetic mechanisms, prominently contribute to the development of novel CRC treatments.4
Circular RNAs (circRNAs), a novel class of endogenous noncoding RNAs, might have the ability to modulate gene expression and suppress tumorigenesis and progression of cancer. They have neither 5–3ʹ polarities nor polyadenylated tails5 but are instead three-dimensional covalently-closed loops. Exon-intron circular RNAs regulate transcription in the nucleus.6 They also act as microRNA (miRNA) sponges, protein decoys, regulators of splicing and transcription regulators,7,8 playing important roles in the physiological and pathophysiological processes such as tumorigenesis. Recently, some circRNAs had been shown to be relevant to the clinical progressions of CRC.9,10 However, the mechanisms by which circRNAs work in the proliferation and development of CRC remain underexplored.
In this study, we analyzed the expression profile of circRNAs in CRC tissues and cell lines. We hypothesis that circ-ITGA7 inhibits CRC cell proliferation by upregulating ASXL1 expression level and by acting as a sponge for miR-3187-3p. We believe that circ-ITGA7 might act as a cancer suppressor to inhibit CRC proliferation and also serve as a potential diagnostic biomarker and a therapeutic target for CRC.
Materials and methods
Cell culture and transfection
HCT116 and SW480 cells, which were obtained from the cell bank of Chinese Academy of Sciences (Shanghai, China), were cultured under ATCC recommended conditions. For cell transfection, cells were seeded in 6-well plates and cultured to 60–70% confluence before transfection. miRNA mimics, including inhibitors or corresponding controls and siRNAs (RiboBio, Guangzhou, China), were transiently transfected using Lipofectamine 2,000 (Invitrogen, Carlabad, CA, USA) all according to the manufacturer’s instructions. Plasmids were transiently transfected with Lipofectamine 3,000 (Invitrogen, Carlabad, CA, USA) according to the manufacturer’s protocol.
Tissue specimens
This study was carried out in accordance with The Code of Ethics of the World Medical Association, and was supervised by Medical Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. Informed consent was obtained from included patients for experimentation with tissue specimens. Fresh-frozen primary tumor and paired normal colorectal tissues were obtained from individuals with colorectal cancer, which was diagnosed in the first affiliated hospital of Sun Yat-sen university. The clinical and pathological features of all tissue specimens are listed in Table S1. All tissue samples were collected in compliance with consent policy and the patient consent was written informed consent.
Microarray analysis and RNA sequencing
RNA was subjected to human genome-wide circRNA microarray v2 analysis at Arraystar Inc. The sample preparation and microarray hybridization were performed based on the Arraystar’s standard protocols. CircRNAs with fold changes ≥3.0 and p-values ≤0.05 were identified as significantly differentially expressed. The gene expression profiles of circ-ITGA7-overexpressed HCT116 and SW480 cells and corresponding control cells were determined by RNA sequencing. The libraries were prepared using the Agilent SureSelect stranded RNAseq protocol, which allows polyA selection but is modified to work with ribodepletion. The prepared libraries were sequenced using an Illumina HiSeq 4,000 system.
siRNA and plasmid construction
siRNAs targeting ASXL1 and miRNA mimics or inhibitors were designed and synthesized by RiboBio (Guangzhou, China) as follows: ASXL1 si-1: GCTGATGGTGAATTTACTCAT; si-2: GCTTCTGTAAGTATGCTCTAT. The circ-ITGA7-overexpressed plasmid was synthesized by RiboBio (Guangzhou, China).
RNA preparation and quantitative real-time RT-PCR (qRT-PCR)
Total RNA from whole-cell lysates or the nuclear and cytoplasmic fractions were isolated using TRNzol reagent (Invitrogen, Carlabad, CA, USA) for cultured cells or using the GeneJET RNA Purification Kit (Invitrogen, Carlabad, CA, USA) for fresh-frozen tissues. For RNase R treatment, 2 μg of total RNA was incubated for 20 min at 37 °C with or without 3 U/μg of RNase R (Epicentre Technologies, Madison, WI) in 1× RNase R reaction buffer, and the resulting RNA was purified using RNeasy MinElute cleaning Kit (Qiagen, Duesseldorf, Germany); this was transcribed into cDNA (Vazyme Biotech, Nanjing, China). Real-time PCR was performed in accordance with the manufacturer’s protocol, using the 2× SYBR Green qPCR Master Mix (Selleck, Shanghai, China). Before calculation using the 2−ΔΔCt method, the GAPDH levels were used to normalize the relative expression levels of circRNA and linear mRNA, and the levels of small nuclear U6 were used to normalize the miRNA expression level. Primers used are as follows:
ASXL1: F: TCAGAGCAATGTTACAGGCCA, R: CTGTTCTCGGCATTTGCCTT;
Circ-ITGA7: F: GTGTGCACAGGTCCTTCCAA, R: TGGAAGTTCTGTGAGGGACG.
CCK8, colony formation
Cell proliferation was assessed using the Cell Counting Kit (CCK)-8 assay (Dojindo Laboratories, Kumamoto, Japan). Five thousand cells were added to each well of 96-well plates. 10 μl of CCK-8 solution was subsequently added to each well at four time points. After 2 h of incubation at 37 °C, the absorbance at 450 nM was measured using an automatic microplate reader (BioTek, Winooski, VT, USA). The experiments were repeated three times.
For the colony formation assays, cells were seeded in 6-well plates (300 cells per well) and incubated at 37 °C for 14 days. For colony scoring, the cells were fixed in 0.5 ml of methanol for 30 min and were stained with crystal violet (Beyotime Biotechnology, Nantong, China) for 15 min. The numbers of colonies were counted and expressed as means ± SEM of at least three independent experiments.
Apoptosis analysis and cell cycle analysis
Cells were washed twice with PBS buffer and digested by trypsin without EDTA after each different treatment. Subsequently, they were centrifuged at 1,500 rpm to collect the cells. For apoptosis analysis, a rapid Annexin V-FITC apoptosis detection kit (KeyGEN Biotech, Nanjing, China) was used according to the manufacturer’s instructions. Then analysis was performed using a FACS Calibur flow cytometer (Beckman Coulter, USA). For cell cycle analysis, the collected cells were washed with 4 °C PBS buffer for 3 min and then were incubated in −20 °C 75% ethanol overnight. Then the cells were resuspended with 100 ml PBS. 1 mg/ml RNase A (Invitrogen, USA) was added into the tube in 37 °C for 40 min. Subsequently, 100 µg/ml PI agent (Invitrogen, USA) was added for 20 min in dark. The cells were assessed in 585 nm and analyzed with Modfit LT 5.0 (Verity Software House, USA).
Western blotting
Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes (Merck KGaA, Darmstadt, Germany). The membranes were blocked for 2 h at room temperature (RT) with 5% non-fat milk in Trisbuffered saline (TBS) plus 0.1% Tween-20 (TBST), which was followed by an overnight incubation at 4 °C with antibodies diluted in the blocking buffer. After three 10-min washes with TBST, blots were incubated at RT for 1 h with the appropriate secondary antibody conjugated to horseradish peroxidase, and protein expression was detected with an enhanced chemiluminescent reagent (Beijing 4A Biotech, Beijing, China). The following antibodies were used: monoclonal anti-GAPDH (AG019, 1:1000) and HRP-labeled Goat Anti-Rabbit IgG (A0208, 1:500) were from Beyotime Biotechnology (Nantong, China); polyclonal anti-AXSL1 (Catalogue #52519, 1:1000) and Anti-cyclin D1 (Catalogue #2922, 1:1000) were from Cell Signaling Technology (Massachusetts, USA).
Statistical analysis
The results were reported as the means ± SEM of at least three independent experiments. Each exact n value is indicated in the corresponding figure legend. Comparisons were performed using Student’s t-test or one-way ANOVA as indicated in individual figures. All statistical analyses were performed using SPSS software, version 16.0 (SPSS Inc., USA). Correlation between the expressions of circ-ITGA7 and ASXL1 was established by Pearson’s correlation analysis.
Results
Circ-ITGA7 is expressed at low levels in CRC cells
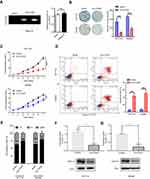
The expression intensity estimation on patients’ cancer tissues and adjacent normal tissues found several decreased circRNAs in CRC, where the 6 most decreased circRNAs are shown in Figure 1A. The expression of the circ-ITGA7 (circRNA_0026782) in CRC cells was dramatically lower than that in normal colonic epithelial cells (Figure 1B). Based on aforementioned information, among these circRNAs, this research focused on circ-ITGA7. Subsequently by comparing the expected sequences of this circRNA using CircBase with the mRNA sequence of their parental gene ITGA7, we noted that circ-ITGA7 is a transcript of the ITGA7 gene exon 4 (Figure 1C). In turn, we then analyzed gene expression microarray data from the Oncomine Database and identified a differentially expressed ITGA7 gene that was low-expressed in colon adenocarcinoma samples (T=39) compared with colon samples (T=22) (p<0.001). Outward-facing primers were subsequently designed to target this circRNA to examine its expression. We then examined the expression of circ-ITGA7 in cancerous and matched non-tumoral tissues from 20 CRC patients. The result showed that circ-ITGA7 expression was significantly decreased in CRC tissues (Figure 1D).
Circ-ITGA7 inhibits the proliferation of CRC cells in vitro
To explore whether circ-ITGA7 exerts anti-tumor effects on CRC, we synthesized an expression construct, using the empty vector as a control. After transfecting this plasmid into HCT116 cells, a CRC cell line, circ-ITGA7 levels increased significantly with the expression of parental ITGA7 mRNA remaining unchanged (data not shown). To confirm circularization of the product, we then treated RNAs, which were extracted from circ-ITGA7- and vector-transfected cells, with RNase R. Results of RT-PCR confirmed that cells transfected with the construct expressed higher levels of circ-ITGA7 than those receiving the vector control. The data also suggested that RNase R treatment did not affect circ-ITGA7 levels (Figure 2A) because RNase R is an exoribonuclease that can degrade RNA from its 3–5ʹ end but does not act on circRNAs.
The phenotypic function of circ-ITGA7 expression was analyzed. Based on single cell proliferation (colony formation) assay, we estimated the effect of circ-ITGA7 on cell proliferation using HCT116 and SW480 cells. Circ-ITGA7-transfected and vector-transfected cells were cultured in 6-well plates (500 cells/well) and quantification was performed for 14 days. The data suggested that ectopic circ-ITGA7 diminished the proliferative capacity of CRC cells, relative to that of vector-transfected cells, within 7 days (Figure 2B and C). Additionally, we incubated CRC cells with serum-free medium for 4 days and estimated the apoptosis by flow cytometry. This confirmed that circ-ITGA7 significantly enhances apoptosis based on annexin V/propidium iodide double staining (Figure 2D). Furthermore, we performed analysis of cell cycle and cell cycle related proteins. Results showed that the percentages of cells at G1 stage in circ-ITGA7 over-expressed group were significantly higher than the ones in control groups in both HCT 116 and SW 480 cells (Figure 2E). In addition, the expression levels of cyclin D1 were significant lower in in circ-ITGA7 over-expressed groups in both HCT 116 and SW 480 cells (Figure 2F and G).
Circ-ITGA7 acts as a miRNA sponge to regulate ASXL1 expression
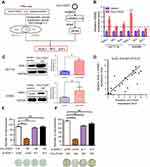
As described above, circRNAs were stable and mostly localized to the cytoplasm, where they were thought to function as miRNA sponges to post-transcriptionally regulate gene translation and expression. To explore this, we assessed binding between circ-ITGA7 and miRNAs and explored target genes using online bioinformatics databases (TargetScan, miRDB, miRTarbase and miRMap). We determined that circ-ITGA7 potentially targets 13 miRNAs (miR-1225b-5p, miR-4776-5p, miR-3187-3p, miR-152-5p, miR-6716-5p, miR-874-5p, miR-3661, miR-4677-3p, miR-15a-3p, miR-491-5p, miR-3741, miR-1914-5p, and miR-92a-2-5p). Meanwhile, genes targeted by these 13 miRNAs were predicted as well (Figure 3A). Circ-ITGA7-regulated genes were then confirmed by RNA-seq (cancer suppressor genes) in circ-ITGA7- transfected and corresponding control cells. As a result, 64 and 51 genes, respectively, were found to be upregulated in HCT116 and SW480 cells (ratio ≥2.0). Among these, three tumor suppressor candidates that were in the miRNA-targeting gene set were involved in our further investigation (Figure 3A).
The expressions of these 3 gene candidates were examined by qRT-PCR analysis. Remarkably, the expression of ASXL1 at the mRNA and protein level were higher in circ-ITGA7-overexpressing CRC cells (Figure 3B and C). Circ-ITGA7 expression was also positively correlated with ASXL1 mRNA levels in adjacent non-cancerous tissues (n=20, p<0.01) (Figure 3D). Moreover, knockdown of ASXL1 suppressed circ-ITGA7-induced proliferation in CRC cells (Figure 3E and F). These results suggested that circ-ITGA7 might inhibit the down-regulation of ASXL1 mRNA.
miR-3187-3p mediates Circ-ITGA7-dependent ASXL1 expression and cell proliferation
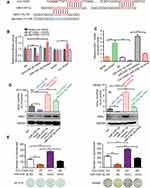
Among the 13 potential circ-ITGA7-binding miRNAs, miR-3187-3p was initially predicted to bind to the 5ʹ-UTR of ASXL1 (Figure 4A). To verify this, we inserted the wild-type AXSL1 5ʹ-UTR, or that with specific site mutations, into a luciferase reporter vector and measured luminescence intensity. Transfection of the miR-3187-3p mimic significantly inhibited luciferase activity compared to that in the negative control group. However, this effect was not significant when the corresponding binding sites (5′-UTR of ASXL1) were randomly mutated (Figure 4B). Transfection of a miR-3187-3p inhibitor had the opposite effect compared to that with transfection of the wild-type sequence. These results suggested that the predicted elements were essential for miR-3187-3p binding to the ASXL1-5′-UTR.
Western blot and qRT-PCR (Figure 4C and D) confirmed the result of luciferase activity analysis. Co-transfection of the miR-3187-3p mimic and the circ-ITGA7-encoding plasmid effectively ablated the mimic-induced augmentation. We further tested the term of proliferation (Figure 4E). Circ-ITGA7 effectively inhibited the miR-3187-3p-induced proliferation of CRC cells. These results suggested that circ-ITGA7 inhibited cell proliferation by serving as a miR-3187-3p sponge to protect ASXL1 degradation.
Discussion
The identification of predictive and reliable biomarkers for CRC is urgently needed. Some circRNAs were previously reported to be relevant to the development and clinical progression of CRC, including processes such as proliferation, metastasis and prognosis.11 However, the expression profile and function of important circRNAs in CRC occurrence and progression remain unknown. In our research, we identified a novel circRNA, circ-ITGA7, which was significantly downregulated in CRC tissues compared to expression in adjacent normal areas. ITGA7 is located on the human chromosome 12q13 and its downregulation increases migration of malignant pleural mesothelioma.12 Adequate ITGA7 expression has been believed to suppress migration and invasion in prostate cancer and melanoma.13,14 This study indicates that circ-ITGA7 is decreased in CRC tissue and can inhibit the proliferation of CRC cells. CircRNAs feature prominently as stable covalently closed loops and are abundant in specific cell types.11 Besides, circRNAs have been reported to be transferred by exosomes into body fluids.15,16 These characteristics imply that the changes in circRNAs during tumorigenesis might be utilized as potential serum biomarkers and therapeutic targets.
As promising as these results have been, studies focusing on the mechanisms through which these cancer-associated circRNAs function in the pathological process of CRC are far from adequate. CircRNAs have been demonstrated to participate in biological functions by acting as sponges for miRNAs and RNA-binding proteins, translating peptides, and through alternatively slicing. Among these mechanisms, their function as sponges has been the predominant mechanism identified in cancer.11 Recently emerging evidence indicated that some circRNAs exhibited aberrant expression in CRC and regulate proliferation and development in CRC. They include ciRS7 and circ_HIPK3 both targeting miR-7,17 circ_0026344 targeting miR-12,18 circ_001569 targeting miR-145,19 hsa_circ_000984 targeting miR-106,19 hsa_circ_0020397 targeting miR-138,20 and hsa_circ_007158921 targeting miR-600. In this study, we identified that circ-ITGA7 acts as a sponge for miR-3187-3p. To our knowledge, few previous studies focused on the function of miR-3187-3p, and we are the first to report its role in tumorigenesis. One genome-wide microRNA analysis reported that serum miR-3187-3p is one of the six-miRNA panels for the diagnosis of bladder cancer (BC).22 The corresponding sensitivities of this panel for Ta, T1 and T2–T4 in BC were 90.00, 84.85 and 89.36% respectively, which were significantly higher than those of urine cytology. Serum miR-3187-3p was significantly corelated with advanced tumor stage. In Kaplan–Meier analysis, patients with non-muscle-invasive BC with low miR-3187-3p level had worse recurrence-free survival. These results indicated that serum miR-3187-3p might be associated to malignant disease. However, the authors did not report any evidence about the miR-3187-3p in tumor tissue, and about the role it plays in oncogenesis. Our result suggests that miR-3187-3p is sponged by circ-ITGA7 and targets ASXL-1, playing a significant role in proliferation inhibition induced by circ-ITGA7.
Circular RNAs possess an abundant number of miRNA target sites for one or more specific miRNAs. These miRNAs might act in different ways. Li et al reports that circ-ITGA7 acts by modulating the Ras pathway and its host gene ITGA7.23 Our study demonstrates that circ-ITGA7 sponges miR-3187-3p and upregulates Additional sex combs like 1 (ASXL1). ASXL1 gene is a human homologue of the Drosophila Asx gene.24 To our knowledge, despite the known functions of ASXL-1 in myelopoiesis and hematological malignant diseases, its effect in CRC has been poorly studied. ASXL1 is assumed to be tumor suppressive or oncogenic in a context-dependent manner.24,25 In some conditions, ASXL1 activates host cell factor-1 (HCF-1) by deubiquitinating. In turn, the activated HCF-1 regulates the G1-to-S-phase transition. In other conditions, ASXL1 induces transcriptional repression by affecting polycomb repressor complex 1 (PRC1) and PRC2.24 In myeloid malignancies, ASXL1 truncation mutations are believed to be gain-of-function mutations and tumor promoters.26 Our study reports that low-level expression of circ-ITGA7 in CRC down-regulates ASXL-1, whereby it might act as a tumor suppressor. Although much more evidence is required, our work partly depicts a potential way of how ASXL-1 acts in colorectal cancer, which expands our understanding about the cancer-associated role of ASXL-1 and offers a promising therapeutic target.
In conclusion, our findings demonstrate that circ-ITGA7 sponges miR-3187-3p thereby upregulating ASXL1 expression level. The circ-ITGA7/miR-3187-3p/ASXL1 signaling axis inhibits CRC cell proliferation. We provide a new competing endogenous RNA regulatory mechanism in CRC proliferation. Advanced research and techniques need to be developed to create an effective diagnostic biomarker or plasmid-molecule treatment into CRC for future therapy.
Ethics approval and informed consent
This study was carried out in accordance with The Code of Ethics of the World Medical Association, and was supervised by Medical Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University. All tissue samples were collected in compliance with consent policy and the patient consent was written informed consent.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
We thank Xiujian Lan and Xuanhong Zhang (Department of Medical Science Experimentation Center, SunYat-sen University) for technical support. We would like to thank Jiabo Zheng for his contribution to language editing and polishing. This work was supported by Guangzhou Science Technology and Innovation Commission (grant number: 201709010062); Traditional Chinese Medicine Bureau of Guangdong province (grant number: 20131144).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. doi:10.3322/caac.20107
2. Yang L, Parkin DM, Li LD, Chen YD, Bray F. Estimation and projection of the national profile of cancer mortality in China: 1991-2005. Br J Cancer. 2004;90(11):2157–2166. doi:10.1038/sj.bjc.6601813
3. Kouzminova N, Lu T, Lin AY. Molecular basis of colorectal cancer. N Engl J Med. 2010;362(13):1246–1247.
4. Schaeybroeck SV, Allen WL, Turkington RC, Johnston PG. Implementing prognostic and predictive biomarkers in CRC clinical trials. Nat Rev Clin Oncol. 2011;8(4):222–232. doi:10.1038/nrclinonc.2011.15
5. Zhang Y, Zhang XO, Chen T, et al. Circular intronic long noncoding RNAs. Mol Cell. 2013;51(6):792–806. doi:10.1016/j.molcel.2013.08.017
6. Li Z, Huang C, Bao C, et al. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22(3):256–264. doi:10.1038/nsmb.2959
7. Barrett SP, Salzman J. Circular RNAs: analysis, expression and potential functions. Development. 2016;143(11):1838. doi:10.1242/dev.128074
8. Daniel C, Lagergren J, Ohman M. RNA editing of non-coding RNA and its role in gene regulation. Biochimie. 2015;117:22–27. doi:10.1016/j.biochi.2015.05.020
9. Bachmayrheyda A, Reiner AT, Auer K, et al. Correlation of circular RNA abundance with proliferation – exemplified with colorectal and ovarian cancer, idiopathic lung fibrosis, and normal human tissues. Sci Rep. 2015;5(8057):8057. doi:10.1038/srep08057
10. Shang Q, Yang Z, Jia R, Ge S. The novel roles of circRNAs in human cancer. Mol Cancer. 2019;18(1):6. doi:10.1186/s12943-019-1010-6
11. Wang P, He X. Current research on circular RNAs associated with colorectal cancer. Scand J Gastroenterol. 2017;52(11):1203–1210. doi:10.1080/00365521.2017.1365168
12. Laszlo V, Hoda MA, Garay T, et al. Epigenetic down-regulation of integrin alpha7 increases migratory potential and confers poor prognosis in malignant pleural mesothelioma. J Pathol. 2015;237(2):203–214. doi:10.1002/path.4567
13. Ren B, Yu YP, Tseng GC, et al. Analysis of integrin alpha7 mutations in prostate cancer, liver cancer, glioblastoma multiforme, and leiomyosarcoma. J Natl Cancer Inst. 2007;99(11):868–880. doi:10.1093/jnci/djk199
14. Ziober BL, Chen YQ, Ramos DM, Waleh N, Kramer RH. Expression of the alpha7beta1 laminin receptor suppresses melanoma growth and metastatic potential. Cell Growth Differ. 1999;10(7):479–490.
15. Madhavan B, Yue S, Galli U, et al. Combined evaluation of a panel of protein and miRNA serum-exosome biomarkers for pancreatic cancer diagnosis increases sensitivity and specificity. Int J Cancer. 2015;136(11):2616–2627. doi:10.1002/ijc.29324
16. Dou Y, Cha DJ, Franklin JL, et al. Circular RNAs are down-regulated in KRAS mutant colon cancer cells and can be transferred to exosomes. Sci Rep. 2016;6:37982. doi:10.1038/srep37982
17. Weng W, Wei Q, Toden S, et al. Circular RNA ciRS-7 - A promising prognostic biomarker and a potential therapeutic target in colorectal cancer. Clin Cancer Res. 2017;23(14):3918. doi:10.1158/1078-0432.CCR-16-2541
18. Yuan Y, Liu W, Zhang Y, Zhang Y, Sun S. CircRNA circ_0026344 as a prognostic biomarker suppresses colorectal cancer progression via microRNA-21 and microRNA-31. Biochem Biophys Res Commun. 2018;503:870–875. doi:10.1016/j.bbrc.2018.06.089
19. Xie H, Ren X, Xin S, et al. Emerging roles of circRNA_001569 targeting miR-145 in the proliferation and invasion of colorectal cancer. Oncotarget. 2016;7(18):26680.
20. Zhang XL, Xu LL, Wang F. Hsa_circ_0020397 regulates colorectal cancer cell viability, apoptosis, and invasion by promoting the expression of the miR-138 targets TERT and PD-L1. Cell Biol Int. 2017;41(9). doi:10.1002/cbin.10826
21. Yong W, Zhuoqi X, Baocheng W, Dongsheng Z, Chuan Z, Yueming S. Hsa_circ_0071589 promotes carcinogenesis via the miR-600/EZH2 axis in colorectal cancer. Biomed Pharmacother. 2018;102:1188–1194. doi:10.1016/j.biopha.2018.03.085
22. Jiang X, Du L, Wang L, et al. Serum microRNA expression signatures identified from genome-wide microRNA profiling serve as novel noninvasive biomarkers for diagnosis and recurrence of bladder cancer. Int J Cancer. 2015;136(4):854. doi:10.1002/ijc.29041
23. Li X, Wang J, Zhang C, et al. Circular RNA circITGA7 inhibits colorectal cancer growth and metastasis by modulating the Ras pathway and upregulating transcription of its host gene ITGA7. J Pathol. 2018;246(2):166–179. doi:10.1002/path.5125
24. Katoh M. Functional and cancer genomics of ASXL family members. Br J Cancer. 2013;109(2):299. doi:10.1038/bjc.2013.623
25. Micol JB, Abdel-Wahab O. The role of additional sex combs-like proteins in cancer. Cold Spring Harb Perspect Med. 2016;6(10):a026526. doi:10.1101/cshperspect.a026526
26. Balasubramani A, Larjo A, Bassein JA, et al. Cancer-associated ASXL1 mutations may act as gain-of-function mutations of the ASXL1-BAP1 complex. Nat Commun. 2015;6:7307. doi:10.1038/ncomms8307
Supplementary material
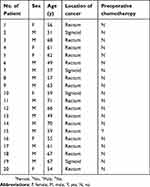 |
Table S1 Characteristics of patients |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.