Back to Journals » OncoTargets and Therapy » Volume 15
Chronological Liquid Biopsy Reveals the Impact of Platinum-Based Chemotherapy on a Prostate Cancer Patient’s CDK12 Mutation: A Case Report
Authors Zhu S, Bao Y, Zheng L, Zhao J , Chen Y, Huang R, Sun G, Zhao F, Zhang X, Liang J, Chen J, Wang Z, Ni Y, Chen N, Shen P, Zeng H
Received 14 June 2022
Accepted for publication 18 August 2022
Published 2 September 2022 Volume 2022:15 Pages 947—952
DOI https://doi.org/10.2147/OTT.S377638
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr William C. Cho
Sha Zhu,1,* Yige Bao,1,* Linmao Zheng,2,* Jinge Zhao,1 Yuntian Chen,3 Rui Huang,4 Guangxi Sun,1 Fengnian Zhao,1 Xingming Zhang,1 Jiayu Liang,1 Junru Chen,1 Zhipeng Wang,1 Yuchao Ni,1 Ni Chen,2 Pengfei Shen,1 Hao Zeng1
1Department of Urology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 2Department of Pathology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 3Department of Radiology, West China Hospital, Sichuan University, Chengdu, People’s Republic of China; 4Department of Nuclear Medicine, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Pengfei Shen; Hao Zeng, Department of Urology, West China Hospital, Sichuan University, Chengdu, 610041, People’s Republic of China, Tel +86-18980602129, Fax +86-28-8542-2451, Email [email protected]; [email protected]; [email protected]
Abstract: CDK12 (Cyclin-Dependent Kinase 12)-mutated prostate cancer patients often respond badly to current therapies. Immunotherapy and platinum-based chemotherapy are recommended based on the molecular features of CDK12-mutated tumors, but the reported patient outcomes are still unsatisfying. Here we report a prostate cancer patient with CDK12 somatic mutation who received multiple therapy options, including platinum-based chemotherapy and immunotherapy. His sequential circulating tumor DNA (ctDNA) -based liquid biopsy tests showed that his original CDK12 mutation fell undetectable twice. This phenomenon was observed only when he was responding well to platinum-based chemotherapy. His responses to immunotherapy were not satisfying. This case indicates that platinum-based chemotherapy can be a good option for treating patients with CDK12 mutation. More importantly, dynamic ctDNA-based liquid biopsies to monitor patients’ CDK12 mutation status are critical in evaluating patients’ response and tolerance during platinum-based chemotherapy, therefore may lead to a better overall prognosis. In conclusion, CDK12-mutated prostate cancer patients are likely to benefit from platinum-based chemotherapy, especially with the help of dynamic ctDNA-based liquid biopsies to monitor their CDK12 mutation status.
Keywords: CDK12, liquid biopsy, platinum, prostate cancer, case report
Introduction
Androgen targeted medication (such as abiraterone1 and enzalutamide2) and cytotoxic drugs (such as docetaxel3 and cabazitaxel4) are the mainstream treatments in metastatic castration-resistant prostate cancer (mCRPC). Even though they demonstrate a strong effect, some mCRPC patients still have poor survival, and the lethal ones usually harbor certain genomic dysregulations. Therefore, emerging data focus on PARP inhibitors, immunotherapy, prostate-specific membrane antigen theranostics, etc.5
Prostate cancers with CDK12 (Cyclin-Dependent Kinase 12) mutation are aggressive and respond poorly to traditional therapies. CDK12-mutated prostate cancer delineates a distinct molecular phenotype. Although not directly participating in the homologous recombination (HR) repair machinery, CDK12 tightly regulates the transcription of several HR genes by phosphorylating RNA polymerase II.6,7 Thus, CDK12-mutated tumors harbor a characteristic genomically unstable phenotype. Since CDK12-mutated patients can also be considered HR-deficient, synthetic lethality, ie, PARP inhibitor and platinum chemotherapy, seems a compelling strategy for treating these patients. Nevertheless, PARP inhibitor did not perform well in CDK12-mutated prostate cancer patients.8–11 At the same time, platinum creates more potent double-strand breaks, while limited evidence supports its use in these patients (only one case report and a study in 2020 noted a platinum response but did not discuss it).7,12 Besides, due to the high neo-antigen burden, CDK12-mutated tumors are also speculated to benefit from immune checkpoint inhibitors (ICI).13,14 While currently, there has not been any clinical evidence for the rationality of this method. Therefore, to date, there are no treatment standards for CDK12-mutated prostate cancers.
Case Presentation
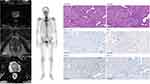
A 55-year-old man with a prostate-specific antigen (PSA) of 16.56 ng/mL was diagnosed with localized prostate cancer (NCCN high-risk group) at West China Hospital (Chengdu, China) in May 2018. He underwent laparoscopic radical prostatectomy soon after his diagnosis. His post-surgical pathological reports showed T-stage 3b (Figure 1), and his specimen was sent for whole-exome sequencing (2018/5/30), which showed a CDK12 mutation. Plus, his PSA decrease was not satisfying, so he received a total of 66 Gy’s adjuvant radiotherapy concomitant with ADT. Then his PSA remained undetectable for over a year until a drastic increase to 2.19 ng/mL. He was diagnosed with mCRPC in Nov. 2020, with metastases in lung, thoracic vertebra 6, neck, and mediastinum showing on PET/CT. First-line docetaxel barely led to a PSA decrease, which was maintained at around 6.00 ng/mL for a month. Thus, he was recommended to do a liquid biopsy-based genetic test (2021/2/5). The results showed a CDK12 mutation, which was also validated using the metastatic tissue in the lung. Due to the limited therapeutic efficacy of PARP inhibitors in patients with CDK12 mutation,8,9 we suggested adding cis-platinum to his chemotherapy regimen.7,12 He immediately displayed a PSA response to platinum-based chemotherapy, which dropped to 0.26 ng/mL within four cycles. At the same time, another liquid biopsy-based genetic test (2021/5/10) of him showed no CDK12 mutation anymore. However, the patients suffered from hematopoietic side effects (WBC decrease). We had to interrupt this treatment plan and change to abiraterone instead. The patient immediately started showing a constant PSA increase, even after adding apalutamide. And his liquid biopsy-based genetic test (2021/8/11) at this moment showed CDK12 mutation again. When he recovered from the chemotherapy-related side effects, his PSA increased to 4.19 ng/mL. We did a fully MDT panel discussion and decided to give him a platinum-based chemotherapy rechallenge under strict medical observation. However, although his PSA was dropping rapidly, the side effects were much more serious WBC decrease combined with severe edema; thus, we had to disrupt his platinum-based chemotherapy. Noticeably, right after the platinum treatment, his CDK12 mutation disappeared again (2021/10/4). Later, we changed to PD-1 inhibitor as recommended by literature.13,15 Unfortunately, the PD-1 inhibitor did not stop his PSA increase. During the PD-1 inhibitor treatment, the patient’s liquid biopsy genetic test (2021/11/29) showed CDK12 mutation again, and its mutation abundance significantly increased. The patients died at the end of 2021 due to cerebral infarction. His PSA results with important treatment annotations were displayed in Figure 2. All liquid biopsy test protocols in this study were described in our previous publication.16
 |
Figure 2 PSA change, medication strategy, disease conditions, and CDK12 mutation status of the patient during treatment. |
Discussion
Here we report a CDK12-mutated mCRPC patient who responded to second-line platinum-based treatment. His status of CDK12 mutation periodically changed along with the chemo-therapeutic periods. We performed immunohistochemistry for common biomarkers such as Ki-67, P53, PTEN, and RB1 on his primary tumor to understand more about its aggressiveness. The results showed that except for P53 loss, a common feature in human cancers, it displayed low cell proliferation, no loss of PTEN, and indeterminate RB1 level. Our patient’s treatment experience does not support the use of PD-1 inhibitors. Instead, this case demonstrates the efficacy of platinum in a CDK12-mutated patient. The patient achieved progression-free survival (PFS) of 8.3 months for platinum-based chemotherapy (even as a second-line therapy), which is much longer than the previously reported 3 months’ median PFS in patients with all HR mutations or CDK12 mutation specifically.17,18
Disease monitoring during the mCRPC phase has always been troublesome due to limited indicators. PSA, as an efficient and straightforward biomarker in localized prostate cancer, however, is insufficient to direct modern systemic therapeutic options. On one hand, a proportion of mCRPC tumors display low PSA while being highly aggressive (neuroendocrine prostate cancer, etc.).19 On the other hand, PSA change does not necessarily reflect the clinical benefit of treatment targeting pathways other than AR.20 Therefore, combining clinical symptoms and radiographic evidence with PSA change is usually required. Even so, both clinical manifestations and radiographic progression have inherent hysteretic nature. The recent decade has seen the development of various prostate cancer therapies targeting the DNA damage repair (DDR) pathway and tumor immune microenvironment. Thus, there is a great need for new methods to monitor these mCRPC patients’ responses and to provide comprehensive, sequential management. Blood-based liquid biopsies are an attractive tool to stratify prostate cancer patients and inform treatment guidance due to their minimally invasive nature, especially in the advanced metastatic setting when obtaining tissue biopsies samples is restricted by practical situations. Currently, ctDNA-based liquid biopsy is becoming the mainstream in precision oncology for selecting therapy, anticipating responses, developing novel biomarkers, monitoring tumor evolution, etc.21 As in our case, the reappearing CDK12 mutation was prior to his PSA increase. This suggests that our dynamic ctDNA-based monitoring approach is more timely than traditional PSA, which offers us a longer window of opportunity to alter treatment plans for aggressive patients with explicit pathogenic mutations.
HR-related genes usually remain stable during prostate cancer progression.22 Nevertheless, in this case, sequential ctDNA-based liquid biopsy results clearly show that besides the good response, his periodical CDK12 mutation status directly reflects his reactions to this therapy. The CDK12 mutation in his ctDNA was undetectable each time he reached PSA nadir during platinum-based chemotherapy treatment. No similar phenomenon has been reported before, and we suppose that strong selection pressure caused by platinum can diminish the corresponding CDK12-mutated tumor clones. This implies platinum could indeed be a strong candidate for treating tumors with CDK12 mutation as the driver event. Also, dynamic liquid biopsy for the CDK12 mutation status is a valuable tool to monitor treatment response and stratify patients by selecting out those more likely to show a satisfying response to platinum. We hypothesize that for patients with CDK12 mutation, their ctDNA CDK12 mutation status at the PSA nadir timepoint when receiving platinum-based chemotherapy is probably crucial for adjusting the treatment regime accordingly. In addition, the long-term use of many therapies is currently restricted by side effects and the emergence of resistance. With the help of dynamic liquid biopsy tests, clinicians may be more confident in providing intermittent therapy plans according to concrete molecular evidence of the patient rather than his/her own clinical experience. Furthermore, this case also gives us insight into the use of dynamic liquid biopsy monitoring in other targeted drugs or synthetic lethal agents.
Conclusions
Our report shows the potential of platinum-based chemotherapy in CDK12-mutated advanced prostate cancer patients and underscores the significance of dynamic ctDNA-based liquid biopsies for CDK12 mutation status in monitoring these patients’ responses. Further prospective studies of platinum-based regimens in CDK12-mutant prostate cancer patients, with at least another liquid biopsy test at PSA nadir timepoint during therapy, are warranted.
Abbreviations
ctDNA, circulating tumor DNA; PSA, prostate-specific antigen; mCRPC, metastatic castration-resistant prostate cancer; HR, homologous recombination; ICI, immune checkpoint inhibitors; PFS, progression-free survival; DDR, DNA damage repair.
Availability of Supporting Data
Data in this study is available upon reasonable request to the contact author.
Ethical Approval and Consent to Participate
Protocols in this study obtained West China Hospital institutional review board approval. The patient provided written consent to participate in this study and to publish his data.
Consent for Publication
All authors give their consent for publication.
Acknowledgments
The authors thank the patient involved in this study, as well as his families/caregivers.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The Science and Technology Support Program of Sichuan Province (2021YFS0119); The Natural Science Foundation of China (NSFC 81902577); The Research Foundation for the Postdoctoral Program of Sichuan University (2021SCU12014).
Disclosure
The authors declare no competing interests.
References
1. Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled Phase 3 study. Lancet Oncol. 2015;16(2):152–160. doi:10.1016/S1470-2045(14)71205-7
2. Penson DF, Armstrong AJ, Concepcion R, et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J Clin Oncol. 2016;34(18):2098–2106. doi:10.1200/JCO.2015.64.9285
3. Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512. doi:10.1056/NEJMoa040720
4. de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus abiraterone or enzalutamide in metastatic prostate cancer. N Engl J Med. 2019;381(26):2506–2518. doi:10.1056/NEJMoa1911206
5. Sandhu S, Moore CM, Chiong E, et al. Prostate cancer. Lancet. 2021;398(10305):1075–1090. doi:10.1016/S0140-6736(21)00950-8
6. Dubbury SJ, Boutz PL, Sharp PA. CDK12 regulates DNA repair genes by suppressing intronic polyadenylation. Nature. 2018;564(7734):141–145. doi:10.1038/s41586-018-0758-y
7. Krajewska M, Dries R, Grassetti AV, et al. CDK12 loss in cancer cells affects DNA damage response genes through premature cleavage and polyadenylation. Nat Commun. 2019;10(1):1757. doi:10.1038/s41467-019-09703-y
8. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091–2102. doi:10.1056/NEJMoa1911440
9. Abida W, Patnaik A, Campbell D, et al. Rucaparib in men with metastatic castration-resistant prostate cancer harboring a BRCA1 or BRCA2 gene alteration. J Clin Oncol. 2020;38(32):3763–3772. doi:10.1200/JCO.20.01035
10. Antonarakis ES, Isaacsson Velho P, Fu W, et al. CDK12 -altered prostate cancer: clinical features and therapeutic outcomes to standard systemic therapies, poly (ADP-Ribose) polymerase inhibitors, and PD-1 inhibitors. JCO Precis Oncol. 2020;4:370–381. doi:10.1200/PO.19.00399
11. Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, Phase 2 trial. Lancet Oncol. 2020;21(1):162–174. doi:10.1016/S1470-2045(19)30684-9
12. Reimers MA, Yip SM, Zhang L, et al. Clinical outcomes in cyclin-dependent kinase 12 mutant advanced prostate cancer. Eur Urol. 2020;77(3):333–341. doi:10.1016/j.eururo.2019.09.036
13. Wu YM, Cieślik M, Lonigro RJ, et al. Inactivation of CDK12 delineates a distinct immunogenic class of advanced prostate cancer. Cell. 2018;173(7):1770–1782.e14. doi:10.1016/j.cell.2018.04.034
14. Rescigno P, Gurel B, Pereira R, et al. Characterizing CDK12-mutated prostate cancers. Clin Cancer Res. 2021;27(2):566–574. doi:10.1158/1078-0432.CCR-20-2371
15. Lotan TL, Antonarakis ES. CDK12 deficiency and the immune microenvironment in prostate cancer. Clin Cancer Res. 2021;27(2):380–382. doi:10.1158/1078-0432.CCR-20-3877
16. Zhao J, Sun G, Zhu S, et al. Circulating tumour DNA reveals genetic traits of patients with intraductal carcinoma of the prostate. BJU Int. 2022;129(3):345–355. doi:10.1111/bju.15530
17. Fan L, Fei X, Zhu Y, et al. Distinct response to platinum-based chemotherapy among patients with metastatic castration-resistant prostate cancer harboring alterations in genes involved in homologous recombination. J Urol. 2021;206(3):630–637. doi:10.1097/JU.0000000000001819
18. Schmid S, Omlin A, Higano C, et al. Activity of platinum-based chemotherapy in patients with advanced prostate cancer with and without DNA repair gene aberrations. JAMA Netw Open. 2020;3(10):e2021692. doi:10.1001/jamanetworkopen.2020.21692
19. Aggarwal R, Huang J, Alumkal JJ, et al. Clinical and genomic characterization of treatment-emergent small-cell neuroendocrine prostate cancer: a multi-institutional prospective study. J Clin Oncol. 2018;36(24):2492–2503. doi:10.1200/JCO.2017.77.6880
20. Pezaro C, Woo HH, Davis ID. Prostate cancer: measuring PSA. Intern Med J. 2014;44(5):433–440. doi:10.1111/imj.12407
21. Crocetto F, Russo G, Di Zazzo E, et al. Liquid biopsy in prostate cancer management-current challenges and future perspectives. Cancers. 2022;14(13):3272. doi:10.3390/cancers14133272
22. Zurita AJ, Graf R, Villacampa G, et al. Genomic evolution from hormonal therapies and suitability of prostate cancer diagnostic specimens for metastatic prostate cancer (mPC) genomic stratification. J Clin Oncol. 2022;40(6_suppl):143. doi:10.1200/JCO.2022.40.6_suppl.143
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.