Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Chronic Kidney Disease Management in the Middle East and Africa: Concerns, Challenges, and Novel Approaches
Authors Al-Ghamdi S, Abu-Alfa A, Alotaibi T, AlSaaidi A, AlSuwaida A, Arici M, Ecder T, El Koraie AF , Ghnaimat M, Hafez MH, Hassan M, Sqalli T
Received 16 March 2022
Accepted for publication 22 July 2022
Published 6 April 2023 Volume 2023:16 Pages 103—112
DOI https://doi.org/10.2147/IJNRD.S363133
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pravin Singhal
Saeed Al-Ghamdi,1 Ali Abu-Alfa,2 Turki Alotaibi,3 Ali AlSaaidi,4 Abdulkareem AlSuwaida,5 Mustafa Arici,6 Tevfik Ecder,7 Ahmed F El Koraie,8 Mohamed Ghnaimat,9 Mohamed H Hafez,10 Mohamed Hassan,11 Tarik Sqalli12
1Department of Medicine, Faculty of Medicine, King Abdulaziz University, Jeddah, Kingdom Saudi of Arabia; 2Department of Nephrology and Hypertension, American University of Beirut, Beirut, Lebanon; 3Department of Transplant Nephrology, Hamed Al-Essa Organ Transplant Center, Kuwait City, Kuwait; 4Department of Nephrology, College of Medicine, University of Baghdad, Nephrology and Transplantation Center, Medical City Complex, Baghdad, Iraq; 5Department of Medicine, King Saud University, Riyadh, Saudi Arabia; 6Department of Nephrology, Hacettepe University Faculty of Medicine, Ankara, Turkey; 7Department of Nephrology, Demiroglu Bilim University Faculty of Medicine, Istanbul, Turkey; 8Department of Internal Medicine and Nephrology, Alexandria Faculty of Medicine, Alexandria, Egypt; 9Department of Nephrology Specialty Hospital, Amman, Jordan; 10Department of Nephrology and Medicine, Cairo University, Cairo, Egypt; 11Department of Medical Affairs, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates; 12Department of Nephrology, Moroccan Society of Nephrology, Casablanca, Morocco
Correspondence: Mohamed Hassan, Department of Medical Affairs, Sheikh Khalifa Medical City, Abu Dhabi, United Arab Emirates, Tel +971 508187944, Email [email protected]
Abstract: The burden of chronic kidney disease (CKD) and other comorbidities, such as hypertension and diabetes, which increase the risk of developing CKD, is on the rise in the Middle East and Africa. The Middle East and Africa CKD (MEA-CKD) steering committee, comprising eminent healthcare specialists from the Middle East and Africa, was formed to identify and propose steps to address the gaps in the management of CKD in these regions. The current article lists the MEA-CKD steering committee meeting outcomes and evaluates the available evidence supporting the role of novel therapeutic options for patients with CKD. The need of the hour is to address the gaps in awareness and screening, early diagnosis, along with referral and management of patients at risk. Measures to bring about appropriate changes in healthcare policies to ensure access to all benefit-proven protective therapies, including novel ones, at community levels are also vital for reducing the overall burden of CKD on the healthcare system as well as governing bodies, especially in developing countries of the Middle East and Africa.
Keywords: chronic kidney disease, Middle East and Africa, CKD management, CKD management gaps, CKD concerns, novel therapies
Introduction
Chronic kidney disease (CKD) is a major cause of morbidity and mortality in developing as well as developed countries.1 CKD is associated with 35.8 million disability-adjusted life years (DALYs), one-third of which is attributed to diabetic kidney disease (DKD). The age-standardised DALY rates in North Africa and the Middle East are above 500 per 100,000 population.2 Between 2000 and 2016, the overall prevalence of CKD stages 1–5 was 15.8% and CKD stages 3–5 was 4.6% in Africa.1 The prevalence of end-stage kidney disease (ESKD) in the Middle East (including Iran, Egypt, Turkey, Tunisia, Yemen, Syria, Lebanon, Qatar, and Iraq) ranged between 55 and 818 per million population.3 The prevalence of CKD stages 3–5 in Abu Dhabi was 2.8% in females and 4.6% in males.4
The identification of risk factors for CKD is essential for improving personal and community health, considering some of these risk factors can be modified. Similarly, the identification of treatable targets can prevent or slow down the progression of CKD to ESKD.5 According to the Global Burden of Diseases, Injuries, and Risk Factors Study, some of the commonest risk factors for CKD include impaired fasting plasma glucose, hypertension, sodium-rich diet, high body mass index, and increased exposure to lead. Furthermore, CKD burden in Eastern Europe, East Asia, tropical Latin America, and Western sub-Saharan Africa is mainly attributed to high blood pressure, while high fasting plasma glucose level is the major risk factor in all other regions.2 DKD is considered a significant burden in the Gulf Cooperation Council (GCC), as more than one-third of individuals with diabetes eventually develop kidney disease.6 Diabetes was observed to be a cause of ESKD in 41% of patients undergoing haemodialysis in the GCC countries.7
Although the numbers are worrying, the steps taken to address these concerns are considerably minimal. Hence, there is a need to address these concerns at the earliest to reduce the morbidity and mortality associated with CKD in the Middle East and Africa.
Overview of CKD Management
Chronic kidney disease has been defined as “the presence and persistence of any of the following: estimated glomerular filtration rate (eGFR) of <60 mL/min/1.73 m2, albuminuria ≥30 mg/24 h, or markers of kidney damage (e.g. isolated proteinuria or isolated haematuria or structural abnormalities of the kidneys) present for more than 3 months”.8 Progression of CKD is influenced by numerous cardiovascular and noncardiovascular risk factors that are preventable, and it is accelerated in the presence of multiple risk factors.9,10 Noncardiovascular risk factors include urinary tract infections, renal stones, overuse of nonsteroidal anti-inflammatory drugs (NSAIDs), and history of polycystic kidney disease.10
The management of CKD needs a multipronged approach to control comorbidities, prevent the progression of CKD and its associated complications, as well as to address CKD complications.
While primary prevention aims at appropriate management of CKD risk factors, secondary prevention includes steps taken to diagnose CKD early, to refer timely, and to start measures for preventing the progression of CKD. Tertiary prevention aims at regulating CKD progression and providing better treatment of established CKD to prevent associated morbidity and mortality (Figure 1). These measures can, in turn, decrease the risk of cardiovascular disease (CVD) and associated mortality.11
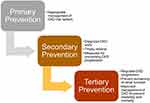 |
Figure 1 Primary, secondary, and tertiary prevention of CKD in LMICs. Data from Ameh et al.15 |
Gaps in the Management of CKD
The progression of CKD can often lead to numerous complications that are noted at lower levels of kidney function and can interact with each other. Some of these include hypertension, CVD, mineral bone disorder, anaemia, and volume overload, along with acid–base and electrolyte abnormalities. Some of these complications can, in turn, worsen kidney function, eventually resulting in morbidity, poor quality of life, and mortality.12 However, there are several gaps related to the appropriate identification and management of CKD reported in literature. These gaps are primarily related to awareness and screening, early diagnosis, referral and management.
Awareness and Screening
Emerging evidence suggests that the proper care of patients with CKD risk factors, such as diabetes, remains unaddressed despite the availability of consensus statements and guidelines. Studies from the Middle East have highlighted the lack of awareness among the medical community as well as the general public related to the recent preventive and therapeutic advancements.13 The prevalence of CKD risk factors, such as diabetes, obesity, and hypertension, is high in the Middle East countries. However, there is a lack of awareness about the influence of these risk factors on CKD in Arab countries. This lack of epidemiological data is considered a stumbling block for the effective implementation of preventive policies.14
While the role of screening for CKD among high-risk groups, such as the elderly and patients with comorbid conditions (diabetes, hypertension, and CVD) or a family history of CKD, has been noted to be efficient in developed countries, its efficacy in developing countries has not yet been evaluated in detail.15 The lack of a data registry system for recording the prevalence of disorders, such as ESKD, is also highlighted.3
Expert Opinion
There are considerable gaps in terms of awareness and screening of CKD, owing to its asymptomatic presentation until the advanced stage. Further, there is ambiguity about the role of primary care physicians and non-nephrology specialists in identifying patients at risk of CKD; underutilisation of urine albumin values for screening; and the lack of several aspects, including CKD registry, clear criteria for identifying high-risk patients, interaction between scientific societies related to kidney health, awareness about unregulated use of NSAIDs, and community education.
Early Diagnosis
The lack of early diagnosis of CKD and its risk factors is another common concern. Chronic kidney disease is often underdiagnosed and undertreated in primary care settings. Kidney disease related to diabetes is underreported in the Middle East. Further, studies have also reported under-diagnosis (33%) and under-treatment (76%) of another risk factor, hypertension, in the United Arab Emirates.14 Accurate estimation of glomerular filtration rate (GFR), which is essential for the diagnosis of CKD, may be a challenge in resource-limited countries, as the population-wide eGFR validation studies to evaluate and suggest an appropriate equation for such population are generally lacking.15 Additionally, lack of reimbursement (for creatinine or albumin evaluations) and access to healthcare facilities prevent the early diagnosis of CKD.
Expert Opinion
High-risk groups are not adequately identified and treated. There is a lack of risk stratification at the primary care level and failure to record and report eGFR.
Referral
Patients with CKD often consult nephrologists during later stages (stages 4 and 5), owing to low level of awareness and delayed referrals from primary healthcare providers, low socioeconomic and educational status of the patients, and the parallel practice by complementary alternative medicine practitioners.15 Poor co-ordination and conflicting advice among specialists have also been cited as common barriers for the appropriate management of patients who are at risk of developing CKD.16
Expert Opinion
Late referral to nephrologists is common, and there is the need for a comprehensive model of care. The lack of enough nephrologists may have a role in inadequate care, while socioeconomic and educational status could influence patient choices.
Management
Inadequate management of patients at risk of CKD has also been reported across different studies. Although several evidence-based guidelines are available, the failure to implement these guidelines in clinical practice can lead to suboptimal management. Also, some of the up-to–date guidelines do not list all therapies despite the availability of robust evidence for such therapies. Further, the extent of evidence-practice gaps remains unknown in several countries, as the majority of such studies were conducted in high-income countries. One such study conducted in Australia has revealed that guideline-based management to address hyperlipidaemia and hypertension was not carried out in 64% and 59% of patients with CKD, respectively.17
Another issue of concern is the financial barriers in LMICs wherein access to healthcare is limited. This has also been reported in high-income countries, as the burden of CKD is higher among those with relatively fewer means.17
Other aspects that have been noted to hamper the appropriate management of CKD patients include shortage of kidney care specialists (including nephrologists, nurses specialised in CKD, dieticians, and community workers), along with poor healthcare delivery systems, and health infrastructure.15
Expert Opinion
Adaptation of novel therapies, appropriate management of hypertension and diabetes to avoid CKD, utilisation of nonpharmacological approaches, and management of acidosis and bone and mineral disorders are lacking.
Proposed Solutions to Address the Gaps in CKD Management
The proposed solutions that were discussed during the executive meeting of the MEA-CKD committee have been categorised into those related to awareness and screening, early diagnosis, and management.
Awareness and Screening
Numerous studies have suggested adopting several measures to improve the management of patients at risk of CKD and CVD. Among these, prime importance has been given to the efforts to increase awareness and better screening and management of patients at high risk of CKD and CVD.18 Although the cost-effectiveness of mass screening has not been assessed in LMICs, targeted screening may be a feasible option. This should involve the identification of the risk factors (such as hypertension and diabetes) followed by the screening of individuals with these risk factors to ascertain the presence of CKD through biochemical methods, including screening for albuminuria and eGFR. Commemorative days, such as World Diabetes Day or World Kidney Day, should also be effectively used to screen for CKD and improve awareness about the perils of this disorder among both patients and general practitioners. Funds for screening could be arranged by non-governmental organizations, societies in partnership with the government and multi-national cooperation of pharmaceutical companies.15
Expert Opinion
Utilise national renal societies, nephrology conferences, and commemorative days, such as World Kidney Day, to create awareness about novel therapies and the need for early identification and management of CKD among patients as well as primary healthcare professionals. Taking steps to create CKD registries and identifying high-risk groups may also be beneficial.
Early Diagnosis
The course of CKD is generally considered to be silent. However, contrary to this belief, several symptoms may point towards disease progression. A conscious enquiry of these symptoms is necessary for better management of patients at high risk of CKD. Some of these symptoms include nocturia, leg swelling, foaming in the urine, loss of appetite, chronic malaise, poor concentration, and poor sleep. The numbers and the severity of these symptoms may increase as the CKD stage worsens. Patients at high risk of CKD may need to be screened for albuminuria and eGFR.15 Therefore, measures to increase the awareness and screening of patients at risk of developing CKD should be implemented at the earliest, especially in the LMICs of the Middle East and Africa, where the burden of disorders, such as hypertension and diabetes, is on the rise.
Expert Opinion
Innovative steps, such as automated reporting of eGFR, could be highly beneficial in addressing the gaps related to early diagnosis. Testing for albuminuria and evaluating urinary albumin-to–creatinine ratio (UACR) on a routine basis among patients at risk of developing CKD could also help identify CKD patients and should be implemented. Primary care physicians are to be updated about the risks for CKD progression and need for early diagnosis and referral.
Management
Addressing the evidence-practice gap among patients who are at risk can potentially decrease the progression of CKD over a period. The use of decision support tools that can automatically generate guideline-based advice based on the clinical and laboratory data should be promoted. These tools can be helpful for early diagnosis as well as appropriate management with newer therapies with more effective outcomes.17
Several novel therapeutic approaches have now been suggested by evidence-based guidelines, such as that from the Kidney Disease: Improving Global Outcomes (KDIGO). Steps should be taken to increase the awareness and implementation of these guidelines. Another issue that needs to be addressed is the variability in the availability and cost of treatments recommended in the guidelines. Many of the guideline-endorsed therapies are not equally accessible across different healthcare settings.17 Therefore, steps should be taken to address these issues so that the adaptation and implementation can be adequately addressed in LMICs. Real-world studies that evaluate the cost-effectiveness of novel therapies can also be beneficial in this regard.
The lack of functional health insurance systems that deter the procurement of medications as a part of secondary or tertiary prevention strategies is also a concern in the LMICs.15 Appropriate changes in the healthcare policies issued by the local and national governing bodies are vital to ensure adequate and appropriate care for those at a high risk of developing CKD, especially among those with pre-existing disorders, such as diabetes and hypertension.
The adoption of an integrated approach, as in developed countries, may be beneficial in the management of chronic disorders. Approaches such as the Innovative Care for Chronic Conditions (ICCC) by the World Health Organization (WHO) suggest the use of a unique platform between families/patients, healthcare professionals, and communities to exchange and provide information related to the management of chronic disorders. This will also aid in motivation and improving preparedness for managing these disorders. A platform to ensure continuity of care is also suggested in this model. Further, engaging policymakers and legislation for providing leadership and advocacy to improve the access of novel medications to the community is the key aspect of the WHO model.15
Outsourcing of certain aspects of management has been adopted as a solution to lessen the burden on the government healthcare facilities. One such example is the outsourcing of haemodialysis procedures in Saudi Arabia. The Ministry of Health, along with outsourced dialysis centres, cater to 62% of the haemodialysis patients in Saudi Arabia.20 In any such arrangement, quality of care and maintenance of standards need to be assured.
Expert Opinion
Both general practitioners and specialists should be made aware of the need for nephroprotection along with early identification and management of patients at risk of CKD. Better management of comorbidities is also the need of the hour along with efforts to slow the progression of CKD. Steps should also be taken to increase patients’ access to novel therapies through changes in government policies to include these medications in various coverage plans. Further, trials to prove the cost-effectiveness of the novel therapies was also suggested.
Novel and Upcoming Therapies in CKD
The implementation of therapies with proven benefits is the fastest and most efficient method to improve kidney outcomes among patients at risk and/or diagnosed with CKD. Some of the strategies that have been proven to be useful in improving CKD outcomes include controlling blood pressure, using angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), reducing proteinuria, and using statins.17 The blockage of the renin–angiotensin system (RAS) with either ACE inhibitors or ARBs can slow down disease progression in individuals with DKD.19 However, the use of ACE inhibitors or ARBs may be associated with hyperkalaemia, especially in advanced CKD, while a reduction in GFR is expected based on the mechanism of action with longer preservation of GFR.21
The use of glycaemic control agents, such as sodium-glucose transport protein 2 (SGLT2) inhibitors, has been observed to have several additional benefits, including reduction of albuminuria, improved cardiovascular outcomes, and decreased progression of CKD among patients with diabetes.19 Other novel therapies being evaluated include mineralocorticoid receptor antagonists (MRAs), anti-inflammatory drugs, and drugs that mitigate oxidative injury.22 Among these, the SGLT2 inhibitors have been reported to be associated with reductions in cardiovascular event rates, blood pressure, and weight, apart from slowing nephropathy progression.
The nonsteroidal MRA, finerenone, has been reported to have beneficial effects in slowing down nephropathy progression.23,24 Further, glucagon-like peptide-1 receptor agonists (GLP-1RA) have shown cardiovascular and renal benefits among patients with diabetes and CKD.25
SGLT2 Inhibitors in CKD Management
The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial has been driving the emergence of the SGLT2 inhibitor class into the treatment landscape of CKD, irrespective of diabetes presence. This trial assessed the efficacy and safety of dapagliflozin among patients with CKD, with or without type 2 diabetes (T2D). Dapagliflozin was associated with a significant reduction in the risk of ESKD, sustained ≥50% eGFR decline, and death due to renal or cardiovascular causes compared to placebo (197 vs 312 events; hazard ratio (HR), 0.61; 95% confidence interval (CI), 0.51–0.72; p<0.001). Notably, this effect was consistent across prespecified subgroups, including patients with or without T2D, those with eGFR <45 mL/min/1.73 m2 or ≥45 mL/min/1.73 m2, and those with UACR ≤1000 mg/g or >1000 mg/g at baseline.26
Further, the risk of ESKD, sustained ≥50% eGFR decline, or death due to renal causes was also reduced significantly in the dapagliflozin group compared to placebo group (142 vs 243 events; HR 0.56; 95% CI 0.45–0.68; p<0.001). All-cause mortality was also lower with dapagliflozin compared to placebo (101 vs 146 deaths; HR, 0.69; 95% CI, 0.53 to 0.88; p=0.004).26
This trial was stopped early as per the recommendations of the independent data monitoring committee because of the overwhelming benefit in the intervention group.26 Findings of the DAPA-CKD trial build upon the evidence for dapagliflozin in the prevention of hospitalisation for heart failure (HF) and worsening of renal disease in DECLARE27 and reduction in the risk of worsening HF and CV death in DAPA-HF studies.28 Notably, the DAPA-CKD trial outcomes suggested that the kidney-protective effects of SGLT2 inhibitors extend to a broader cohort of patients with CKD without T2D, for whom ACE inhibitors/or ARBs are the only therapeutic options available for slowing progression to ESKD or kidney failure. Further, dapagliflozin was observed to be safe for individuals with an eGFR as low as 25 mL/minute/1.73 m2.26
A class effect in reducing the risk of composite kidney disease endpoints in T2D was noted with SGLT2 inhibitors as per the outcomes reported in EMPAREG-OUTCOME, CANVAS, DECLARE, and CREDENCE trials (Figure 2).29–32 A significant reduction in the risk of DKD progression and HF was noted with all classes of SGLT2 inhibitors.33
 |
Figure 2 Class effect of SGLT2 inhibitors (A) EMPA REG outcome; from N Engl J Med, Wanner C, Inzucchi SE, Lachin JM et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. 375: 323-334, Copyright © (2016) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.29 (B) CANVAS; From N Engl J, MedNeal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Event in Type 2 Diabetes. 377: 644-657, Copyright © (2017) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.;30 (C) DECLARE-TIMI 58 outcome; from Mosenzon31 (D) CREDENCE; From N Engl J Med.Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. 380:2295–2306, Copyright ©(2019) Massachusetts Medical Society. Reprinted with permission from Massachusetts Medical Society.32 |
Large-scale studies to evaluate the cost-effectiveness of SGLT2 inhibitors are required to substantiate the overall benefits of this class of drugs in the prevention or regulation of CKD among patients with diabetes.
Finerenone in Patients with T2D and CKD
Overactivation of the mineralocorticoid receptor has been attributed to the progression of renal and cardiovascular dysfunction among those with CKD and diabetes. Finerenone, a nonsteroidal selective MRA, reportedly had better anti-inflammatory and antifibrotic effects compared to steroidal MRAs in preclinical models. The Finerenone in Reducing Kidney Failure and Disease Progression in Diabetic Kidney Disease (FIDELIO-DKD) trial evaluated the beneficial effects of finerenone among patients with advanced CKD and T2D in terms of prevention of CKD progression and reduction in cardiovascular morbidity and mortality.24
A significant reduction in the risk of sustained ≥40% eGFR decline, kidney failure, or death from renal causes was noted with finerenone compared to placebo in the FIDELIO-DKD trial. Further, finerenone therapy was also associated with a better reduction of UACR compared to placebo. However, higher rate of hyperkalaemia-related discontinuation was noted in the finerenone group compared to placebo group (2.3% and 0.9%, respectively).24
GLP-1RAs in T2D and CKD
The renal benefits of glucagon-like peptide-1 (GLP-1) receptor agonists (GLP-1RAs) are indirectly attributed to the potential of reducing blood glucose, insulin level, weight, and blood pressure. Further, these may also influence endothelial dysfunction and inflammation, thus conferring a direct cardio-renal protective benefit in patients with diabetes and CKD. The use of human GLP-1 analogues is approved for use in patients with T2D with eGFR up to 15 mL/min/1.73 m2. However, the use of GLP-1RAs among patients with eGFR below 30 mL/min/1.73 m2 is contraindicated owing to the risk of accumulation and toxicity. Further, the exact renoprotective mechanisms and the cardio-renal protective effect of GLP-1RA in moderate-advanced DKD are unknown.25
Role of Diet Optimisation
The kidney has a unique role in nutrient metabolism and adequate nutrient homeostasis is often lacking in patients with CKD. Alterations in the nutrient homeostasis can lead to further worsening of the condition. Monitoring nutritional status and modification of the nutrient intake is hence considered to have a vital role in the management of patients with CKD.34 Low-protein diet and plant-based dietary regimens have been recommended in the KDIGO guidelines for patients with CKD stages 3 to 5. Low-protein diets are reportedly associated with reduced urinary protein excretion and single nephron hyperfiltration, thereby exerting a renoprotective effect. These effects are similar to SGLT2 inhibitors and hence, low-protein diet is considered to work in synergy with SGLT2 inhibitors to reduce the progression of CKD.35 Further evaluation of these aspects in the Middle East and African population would be beneficial in planning the therapeutic strategy for patients at risk of CKD.
Implications of Novel Therapies in CKD Management
The positive outcomes of the key trials with novel therapies, such as SGLT2 inhibitors, for patients with T2D have been acknowledged by several guidelines that suggest a re-look into the management of individuals at risk of CKD. The 2020 KDIGO Diabetes Management in CKD guideline has evaluated the current advances in monitoring and treatment of patients with diabetes and CKD who are at a high risk of poor health outcomes.36
According to the KDIGO 2020 guidelines, preventive strategies, such as glycaemic and blood pressure control, lipid management, regular exercise, optimal nutrition, and cessation of smoking, have been recommended for all patients with diabetes (Figure 3). The combination of metformin and SGLT2 inhibitor has been recommended for most of the patients with T2D when the eGFR is ≥30 mL/min/1.73 m2. The use of SGLT2 inhibitors is recommended for patients with T2D and CKD while addition of RAS inhibitor is suggested for patients with comorbidities such as albuminuria and hypertension.36 However, MRAs should be used with caution, owing to the risk of hyperkalaemia or decline in GFR (reversible); especially among those with a low eGFR.37
 |
Figure 3 KDIGO 2020 clinical practice guidelines for diabetes management in CKD. Notes: Adapted from de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO Diabetes Management in CKD Guideline: Evidence-based advances in monitoring and treatment. Kidney Int. 2020;98:839–848.35 Copyright © 2020, KDIGO. Published by Elsevier on behalf of the International Society of Nephrology. CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). |
Conclusion
There are several gaps in the patient care and management of CKD in the Middle Eastern and African regions. When addressing CKD-related gaps, it is important to appraise the designated regional administrative decision-makers about the findings and proposed solutions to develop a proper and comprehensive implementation strategy. These strategies are parallel to the establishment of patient data registries and augmented epidemiological studies. International guidelines related to CKD management should be implemented while considering the outcomes of recent trials highlighting the benefits of novel approaches in the management of CKD or at risk for CKD patients. This includes adopting novel treatment strategies, such as SGLT2 inhibitors and mineralocorticoid receptor antagonists. Novel treatment strategies might necessitate financial support or subsidised programmes to make it available in LMICs. Customised local guidelines may be needed in situations where international guidelines are inapplicable.
Data Sharing Statement
Data available on request. The data that support the findings of this study are openly available. If required, readers can connect with the corresponding author for the details using the email id [email protected].
Acknowledgments
We thank BioQuest Solutions Pte Ltd for providing editorial support. We also thank AstraZeneca, Middle East Africa, for funding the expert panel meeting, which helped to identify the gaps in the management of CKD in the region and propose steps to address these gaps. The panel included eminent healthcare specialists from the Middle East and Africa, who came together to identify and address the gaps in CKD management and shape practices that can support the Ministries of Health and patient communities in the region.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work. All authors are eminent healthcare specialists from the Middle East and Africa who have been extensively involved academically and clinically in the field of nephrology and have published several articles related to the diagnosis, prevention, and management of CKD.
Funding
Funded by AstraZeneca Middle East & Africa; The editorial and writing support has been given by BioQuest Solutions Pte Ltd.
Disclosure
Saeed Al-Ghamdi reports personal fees from AstraZeneca, Bayer, Viforpharma, and Astellas, outside the submitted work. Ali Abu-Alfa reports personal fees from Astra-Zeneca, during the conduct of the study; personal fees from Boehringer Ingelheim, Sanofi-Aventis, Astellas, Baxter Healthcare, and Novartis, outside the submitted work; and received honoraria for presentations, educational materials, expert panels, advisory boards, or educational events from Astra Zeneca, Baxter, Boehringer Ingelheim, and Sanofi. Mustafa Arici reports personal fees from Astra Zeneca, during the conduct of the study; personal fees from Amgen, Astellas, Astra Zeneca, Bayer, Boehringer Ingelheim, Menarini, MSD, Novo Nordisk, Sandoz, and Sanofi, outside the submitted work; and has received payment or honoraria for lectures, presentations, speaker bureaus, manuscript writing, or educational events from Amgen, Astellas, AstraZeneca, Bayer, Baxter, Boehringer Ingelheim, Menarini, MSD, Novo Nordisk, Sandoz, and Sanofi. El Koraei Ahmed F and Hafez Mohamed H have received honoraria for participation in the expert panel meetings with AstraZeneca. The authors report no other potential conflicts of interest in relation to this work.
References
1. Kaze AD, Ilori T, Jaar BG, et al. Burden of chronic kidney disease on the African continent: a systematic review and meta-analysis. BMC Nephrol. 2018;19:125. doi:10.1186/s12882-018-0930-5
2. Bikbov B, Purcell CA, Levey AS; GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi:10.1016/S0140-6736(20)30045-3
3. Malekmakan L, Tadayon T, Roozbeh J, et al. End-stage renal disease in the middle east: a systematic review and meta-analysis. Iran J Kidney Dis. 2018;12:195–203.
4. Richards N, Hassan M, Saleh AK, et al. Epidemiology and referral patterns of patients with chronic kidney disease in the Emirate of Abu Dhabi. Saudi J Kidney Dis Transpl. 2015;26:1028–1034. doi:10.4103/1319-2442.164600
5. Kazancioglu R. Risk factors for chronic kidney disease: an update. Kidney Int Suppl. 2013;3:368–371. doi:10.1038/kisup.2013.79
6. Farag YM, Al Wakeel JS. Diabetic nephropathy in the Arab Gulf countries. Nephron Clin Pract. 2011;119:c317–c322. doi:10.1159/000328909
7. AlSahow A. Demographics and key clinical characteristics of hemodialysis patients from the gulf cooperation council (GCC) participating in DOPPS. Nephrol Dialysis Transplant. 2016;31:i279–i297. doi:10.1093/ndt/gfw175.29
8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. 2013;3:1–150.
9. Al-Shamsi S, Regmi D, Govender RD, Cheng X. Chronic kidney disease in patients at high risk of cardiovascular disease in the United Arab Emirates: a population-based study. PLoS One. 2018;13:e0199920. doi:10.1371/journal.pone.0199920
10. Feehally J, Khosravi M. Effects of acute and chronic hypohydration on kidney health and function. Nutr Rev. 2015;73:110–119. doi:10.1093/nutrit/nuv046
11. Ameh OI, Ekrikpo UE, Kengne AP. Preventing CKD in low- and middle-income countries: a call for urgent action. Kidney Int Rep. 2019;5:255–262. doi:10.1016/j.ekir.2019.12.013
12. Bello AK, Alrukhaimi M, Ashuntantang GE, et al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl. 2017;7:122–129. doi:10.1016/j.kisu.2017.07.007
13. Shaheen FA, Souqiyyeh MZ. Kidney health in the Middle East. Clin Nephrol. 2010;74:S85–S88. doi:10.5414/cnp74s085
14. Farag YMK, Kari JA, Singh AK. Chronic kidney disease in the Arab world: a call for action. Nephron Clin Pract. 2013;121:c120–c123. doi:10.1159/000345149
15. Ameh OI, Ekrikpo U, Bello A, et al. Current management strategies of chronic kidney disease in resource-limited countries. Int J Nephrol Renovasc Dis. 2020;13:239–251. doi:10.2147/IJNRD.S242235
16. Lo C, Teede H, Fulcher G, et al. Gaps and barriers in health-care provision for co-morbid diabetes and chronic kidney disease: a cross-sectional study. BMC Nephrol. 2017;18:80. doi:10.1186/s12882-017-0493-x
17. Jardine MJ, Kasiske B, Adu D, et al. Closing the gap between evidence and practice in chronic kidney disease. Kidney Int Suppl. 2017;7:114–121. doi:10.1016/j.kisu.2017.07.006
18. Razavian M, Heeley EL, Perkovic V, et al. Cardiovascular risk management in chronic kidney disease in general practice (the AusHEART study). Nephrol Dial Transplant. 2012;27:1396–1402. doi:10.1093/ndt/gfr599
19. Yacoub R, Campbell KN. Inhibition of RAS in diabetic nephropathy. Int J Nephrol Renovasc Dis. 2015;8:29–40. doi:10.2147/IJNRD.S37893
20. Al Attar B. Renal replacement therapy in the Kingdom of Saudi Arabia. Saudi J Kidney Dis Transpl. 2020;31:1458–1469. doi:10.4103/1319-2442.308375
21. Nagata D, Hishida E, Masuda T. Practical strategy for treating chronic kidney disease (CKD)-associated with hypertension. Int J Nephrol Renovasc Dis. 2020;13:171–178. doi:10.2147/IJNRD.S259931
22. Breyer MD, Susztak K. Developing treatments for chronic kidney disease in the 21st century. Semin Nephrol. 2016;36:436–447. doi:10.1016/j.semnephrol.2016.08.001
23. Cherney DZI, Bakris GL. Novel therapies for diabetic kidney disease. Kidney Int Suppl. 2018;8:18–25. doi:10.1016/j.kisu.2017.10.005
24. Bakris GL, Agarwal R, Anker SD, et al.; FIDELIO-DKD Investigators. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–2229. doi:10.1056/NEJMoa2025845
25. Górriz JL, Soler MJ, Navarro-González JF, et al. GLP-1 receptor agonists and diabetic kidney disease: a call of attention to nephrologists. J Clin Med. 2020;9:947. doi:10.3390/jcm9040947
26. Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al.; DAPA-CKD Trial Committees and Investigators. Dapagliflozin in patients with chronic kidney disease. N Engl J Med. 2020;383:1436–1446. doi:10.1056/NEJMoa2024816
27. Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380:347–357. doi:10.1056/NEJMoa1812389
28. McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995. doi:10.1056/NEJMoa1911303
29. Wanner C, Inzucchi SE, Lachin JM, et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016; 375: 323334.; 375: 323334.
30. Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and Cardiovascular and Renal Event in Type 2 Diabetes. N Engl J Med 2017; 377: 644657.; 377: 644657.
31. Mosenzon O, Wiviott SD, Cahn A. et al. Effect of dapagliflozin on development and progression of kidney disease in patients with type 2 diabetes. Lancet Diabetes Endocrinol 2019; 7: 60617.; 7: 60617.
32. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380:22952306. doi: 2019;380:22952306. doi: 10.1056/NEJMoa1811744
33. Giugliano D, Esposito K. Class effect for SGLT-2 inhibitors: a tale of 9 drugs. Cardiovasc Diabetol. 2019;18:94. doi:10.1186/s12933-019-0899-9
34. Molina P, Gavela E, Vizcaíno B, Huarte E, Carrero JJ. Optimizing Diet to Slow CKD Progression. Front Med. 2021;8:654250. doi:10.3389/fmed.2021.654250
35. Cupisti A, Giannese D, Moriconi D, D’Alessandro C, Torreggiani M, Piccoli GB. Nephroprotection by SGLT2i in CKD patients: may it be modulated by low-protein plant-based diets? Front Med. 2020;7:622593. doi:10.3389/fmed.2020.622593
36. de Boer IH, Caramori ML, Chan JCN, et al. Executive summary of the 2020 KDIGO diabetes management in CKD guideline: evidence-based advances in monitoring and treatment. Kidney Int. 2020;98:839–848. doi:10.1016/j.kint.2020.06.024
37. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98:S1–S15. doi:10.1016/j.kint.2020.06.019
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
