Back to Journals » Journal of Asthma and Allergy » Volume 16
Chronic Diarrhea with Villous Blunting of the Small Intestine Under Capsule Endoscopy in Common Variable Immunodeficiency and X-Linked Agammaglobulinemia: A Case Series
Authors Deng F, Wang H, Wang X
Received 4 May 2023
Accepted for publication 14 September 2023
Published 22 September 2023 Volume 2023:16 Pages 997—1006
DOI https://doi.org/10.2147/JAA.S418996
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Feihong Deng,1,2 Hanyu Wang,1,2 Xuehong Wang1,2
1Department of Gastroenterology, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, People’s Republic of China; 2Research Center of Digestive Disease, Central South University, Changsha, Hunan, 410011, People’s Republic of China
Correspondence: Xuehong Wang, Department of Gastroenterology, the Second Xiangya Hospital, Central South University; Research Center of Digestive Disease, Central South University, Changsha, Hunan, 410011, People’s Republic of China, Email [email protected]
Introduction: Primary immunodeficiencies (PIDs) are a heterogeneous group of disorders, common variable immunodeficiency disorder (CVID) and X-linked agammaglobulinemia (XLA) are PIDs related to B-cell defect, characterized by reduced levels of immunoglobulins and immune dysregulation. Infections are the most common clinical manifestations, while underlying autoimmune and inflammatory conditions are present in some patients with CVID and XLA, leading to clinical misdiagnosis and diagnostic delay. Chronic diarrhea in patients with CVID and XLA, particularly complicated malabsorption and protein-energy malnutrition, is responsible for poor prognostic outcomes.
Methods: In this study, we described three PID adult patients (two with CVID and one with XLA) who presented with varying degrees of chronic diarrhea, weight loss, and protein-energy malnutrition. We suggest that villous blunting of the small intestine under capsule endoscopy may be an endoscopic feature of PID-related enteropathy, thus highlighting the application of capsule endoscopy in patients with CVID and XLA presenting with chronic diarrhea.
Conclusion: We also summarize regular Ig supplementation is the basic treatment for CVID and XLA patients, proper enteral nutrition and probiotic therapy can be explored to use to alter gut microbiota and modulate intestinal immune response. However, vedolizumab is not helpful to PID-related enteropathy therapy, as it exacerbates the inflammatory response in extra-intestinal organs and ultimately causes poor clinical outcomes.
Keywords: chronic diarrhea, primary immunodeficiencies, villous blunting, capsule endoscopy, vedolizumab therapy
Introduction
Primary immunodeficiencies (PIDs) are a heterogeneous group of disorders comprising more than 300 diseases, and are characterized by varying degrees of immunodeficiency and immune dysregulation.1–3 PIDs have an increased susceptibility to infections, autoimmune diseases, and malignancies.4 Immune defects can affect B-cell immunity, such as common variable immunodeficiency disorder (CVID) and X-linked agammaglobulinemia (XLA), T-cell immunity, DiGeorge syndrome, and T- and B-cell immunity, such as Severe Combined Immunodeficiency (SCID).5 CVID is the most frequent symptomatic PIDs in adulthood, and is characterized by low levels of serum IgG, IgA, and/or IgM, with absent or impaired antibody production as poor antibody response to vaccines6,7 and the low relative values of switched memory B cells (<70% of age-related normal values).8–10 The incidence of CVID is approximately one in 25,000 inhabitants.11 Monogenic defects account for approximately 10% of all CVID.12,13 Genes including ICOS, TNFRSF13B (TACI), TNFRSF13C (BAFF-R), TNFSF12 (TWEAK), LRBA, CTLA4.12 XLA is generally caused by mutations in Bruton’s tyrosine kinase (BTK) gene, which is characterized by pan-hypogammaglobulinemia and absence of mature B-lymphocytes in peripheral blood.14–16 Because physiological immaturity can mimic PIDs and the history of several systemic symptoms in patients was neglected in their early years, the diagnostic delay was 4–8 years from symptom onset to PIDs diagnosis.17,18
The gastrointestinal (GI) tract is the largest lymphoid organ in the body, and it is not surprising that GI diseases are common among PIDs patients.5,19 Approximately 5–50% of PIDs patients have GI disease, with diarrhea and malabsorption being the most common manifestations.5 Infectious diarrhea accounts for 20–60% of GI symptoms related to CVID.5 However, a specific infectious agent was not identified in several of the CVIDs and XLAs, inflammatory or autoimmune complications is identified as an important phenotype for CVID, in gastrointestinal tract, including inflammatory bowel disease (IBD) and enteropathy.6,10 For XLA, the incidences of IBD and enteritis have increased in patients with XLA.20,21 More importantly, as a complex immune modulation mechanism, the risk of death was 11 times higher in CVID patients with one or more inflammatory or autoimmune complications than in patients who had infections only.22 Moreover, chronic diarrhea can cause malabsorption and protein-energy malnutrition, and failure of immunoglobulin replacement therapy is responsible for poorer prognostic outcomes.23,24 Thus, awareness of the presence of inflammatory diseases in PIDs and early diagnosis of PIDs can achieve a favorable prognosis and decrease morbidity and mortality. However, only a few studies have focused on improving the early diagnosis of PIDs. Here, we report three cases of PIDs that manifested with pathogen-negative chronic diarrhea as the primary symptom and showed small intestinal villous atrophy endoscopically and describe the diagnosis and treatment courses of these three cases to facilitate early identification of PIDs with enteropathy and optimize therapeutic management.
Methods
Flow Cytometry Analysis
Fresh collected whole blood was obtained and processed within 24 h. Immunophenotyping of T cells and B cells were classified using conjugated anti-human murine monoclonal antibodies (mAbs) as follows: anti-CD3, anti-CD4, anti-CD8, anti-CD19, anti-CD27 and anti-IgD. All antibodies were purchased from BD Pharmingen. For cell staining, 8 mL whole blood was incubated with antibodies for 15 min at room temperature in the dark. Then, diluted FACS Lysing Solution was added for 10 min of erythrocyte lysis. Flow cytometry was performed on a FACS AriaIII (BD Biosciences) and captured data was subsequently analysed using FlowJo.
CE System, Examination Procedures
The OMOM CE was provided by Jin Shan Science and Technology Company (Chongqing, China). All patients were underwent bowel preparation by using polyethylene glycol electrolytes powder(II) before examination. The images were real-time obtained and recorded. The recorder was disconnected approximately 10 h after the start of the exploration. Then acquired diagnosis by the two independent senior endoscopists.
Case Presentation
Patient 1
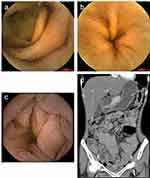
A 41-year-old female was admitted to our hospital in December 2019 with chronic diarrhea for 3 months, characterized by watery stools with undigested food residues and a stool frequency of 10–20 times a day, without fever, abdominal pain, or vomiting. Before the age of 10, the patient was susceptible to upper respiratory tract infections and recurrent skin lesions, presenting with erythema, desquamation, and pruritus. Upon admission, she was afebrile, with a pulse rate of 127 bpm, weight loss (approximately 15kg, body mass index [BMI]12kg/m2), hypoglobulinemia (albumin [ALB] 39.1 g/L, globulin [GLO] 15.6g/L), and electrolyte imbalance (K+2.99mmol/L). The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) were normal. A regular fecal test revealed that fecal calprotectin (45μg/g, normal range 0–50μg/g), culture, and Clostridium difficile were negative. Tests for HIV serology, viruses, autoimmune enteropathy, celiac disease (including anti-DGP, anti-EMA and anti-tTG), and immune-related antibodies yielded negative results. Computed tomography enterography (CTE) of the small intestine revealed multiple, slightly enlarged lymph nodes in the peritoneal and mesenteric regions. Gastroscopy revealed thinning of gastric mucosa. Colonoscopy showed congestion and swelling of the colorectal mucosa, small ulcers with a diameter of 3–8mm were scattered at transverse colon and rectum. Importantly, capsule endoscopy revealed that the mucosa of the jejunum and ileum was congested, edematous, and thinned out with blunting of the intestinal villi (Figure 1). The pathology of the terminal ileum was feathered, with massive intraepithelial lymphocytes and villous atrophy. This patient was suspected to have autoimmune enteropathy and received probiotics and enteral nutritional support; stool frequency was reduced to 6–8 stools per day. In May 2020, the patient was admitted with uncontrolled diarrhea. The serological test results for immunoglobulins, including IgG and IgA, were markedly reduced. Analysis of lymphocyte subsets showed a lack of B cells (1%), whereas the total T-cell counts were almost normal. Gene sequencing by Panel revealed a missense heterozyge mutation in CTLA-4, c.392T>C, p.Val131Ala. Histological examination showed infiltration of neutrophils and lymphocytes in the lamina propria of the gastric antrum and duodenum in the absence of plasma cells, and negative CD138 staining. A diagnosis of CVID was made, and 50 mg glucocorticoid was administered orally. However, diarrhea remained unrelieved, and she was hospitalized in September 2020 and received enteral and parenteral nutrition, electrolytes, vitamins, and probiotics replenished along with 15g of IV immunoglobulin (Ig); this time, the diarrhea symptom improved, with stool frequency reduced to 4–5 times per day. After discharge, the patient did not receive regular immunoglobulin therapy (every 2–3 months). Diarrhea was aggravated in January 2022, and 300mg of vedolizumab at 0, 2, 6, 14 and 22 weeks was initiated in this patient, with five infusions; however, immunoglobulin therapy was still irregularly used. During treatment with vedolizumab, the symptoms of diarrhea significantly improved, while the skin symptoms worsened concurrently with arthritis. This patient underwent hemorrhoidectomy in a local hospital in June 2022. The diarrhea worsened 2 weeks after surgery with severe perianal abscess, and the patient died 2 months later.
Patient 2
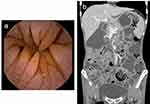
A 20-year-old male was admitted to the psychiatry department in December 2017 because of depression for more than 7 years and chronic diarrhea for more than 1 year. He had unshaped stools with a frequency–1–2 times a day, and no history of infection was reported. Upon admission, he had weight loss (approximately 25kg, BMI 15.5 kg/m2), blood exams showed mild anemia (hemoglobin 94 g/l), hypoglobulinemia (ALB 36.7 g/L, GLO 8.1 g/L), and electrolyte imbalance (K+2.26mmol/L). The stool test results for regular fecal tests, occult blood tests, and culture tests were negative. The ESR and CRP levels were normal. Tests for HIV serology, viruses, thyroid function, and immune-related antibodies yielded negative results. Immunoglobulin levels, including IgA, IgM, and IgG levels, were significantly reduced. Colonoscopy did not indicate any abnormalities, and abdominal CT just showed a slightly enlarged spleen. The psychiatrist diagnosed the patient with depression, and he was transferred to the Department of Gastroenterology for further treatment because of unresolved diarrhea. Tests for celiac disease-related antibodies (anti-DGP, anti-EMA and anti-tTG) were negative. Gastroscopy showed biliary reflux gastritis. Capsule endoscopy revealed extensive blunting of the intestinal villi (Figure 2). Small bowel endoscopy also showed that the mucosa of the duodenum, jejunum, and ileum was congested with villi blunting. The patient received enteral nutrition and probiotics during hospitalization while without any significant improvement in diarrhea, probiotics were discontinued after discharge. He continued to have chronic diarrhea and intermittent fever and was admitted to a local hospital twice between May 2019 and was treated for irritable bowel syndrome (IBS). The patient was hospitalized again in September 2021 with watery stools five to 5–6 times per day. Immunoglobulin levels, including IgA, IgM, and IgG, decreased. The lymphocyte subset test showed decreased B cells (4%) and switched memory B cell (5.4%) with normal T-cell counts. In addition, multiple enlarged lymph nodes were detected in the mesentery on the abdominal CT. However, the results of the whole exome sequencing (WES) were negative. Pathology of the terminal ileum showed villous atrophy and prominent infiltration of lymphocytes and eosinophils into the lamina propria with a lack of mucosal plasma cells. The diagnosis of CVID was made. The patient obtained monthly 15g of IV Ig therapy monthly. To date, no infectious symptoms or complications have been reported.
Patient 3
A 27-year-old male was admitted in April 2020 with recurrent diarrhea and intermittent abdominal pain for one year, characterized by a stool frequency of 4–5 times a day. The patient had a history of tuberculous pleurisy, bronchiectasis with an infection, and chronic mastoiditis. The patient was diagnosed with IBS at a local hospital due to the absence of abnormalities in colonoscopy, serology, and fecal tests. Upon admission, he experienced weight loss (approximately 10kg) and blood tests indicated hypoglobulinaemia (17.3 g/l). Levels of CRP level and ESR were normal. Tests for HIV serology, parasites, viruses, tuberculosis, and immune-related antibodies yielded negative results. The stool tests of regular fecal samples, OB, fecal culture, and Clostridium difficile were negative. Gastroscopy and colonoscopy revealed no abnormalities or remarkable pathological changes upon biopsy. The patient was unresponsive to conventional antidiarrheal treatment and discharged after 8 days. In May 2020, he was admitted for the second time due to aggravated diarrhea, with a stool frequency of up to 6–8 times a day. All the celiac disease-related antibodies (including anti-DGP, anti-EMA and anti-tTG) tested negative. Diarrhea was not relieved after a gluten-free diet for nearly 4 weeks. Capsule endoscopy revealed that the mucosa of the duodenum and jejunum was congested and thinned out, with blunting of the intestinal villi (Figure 3). Gastroscopic biopsy of the duodenum showed villous atrophy, lack of mucosal plasma cells, and massive lymphocytic infiltration in the lamina propria. The patient was started on 40 mg of intravenous injection (IV) steroids, and the diarrhea was greatly improved; the stool frequency was reduced to 1 to 2 times a day. Subsequently, immunoglobulin (Ig) analysis showed that the levels of IgG, IgA, and IgM were almost undetectable. The lymphocyte subset classification showed a complete absence of B cells (0%) despite normal T cell counts. Gene sequencing by Sanger indicated a nonsense hemizygote mutation in Bruton’s tyrosine kinase (BTK) gene c.1558C>T,p.Arg520*. Therefore, the diagnosis of XLA was made. 20 g of intravenous immunoglobulin (IVIG, 400 mg/kg) was administered to the patient before discharge. Thereafter, regular IV Ig therapy was administered every 4 weeks. In March 2022, the patient was admitted for intermittent fever (up to 38.3°), diarrhea (stool frequency 4–8 times a day), abdominal pain, and thrombocytopenia (platelets 38 x109/L), and CT showed thickening and edema of the small intestine and colorectum, multiple enlarged lymph nodes in the mesentery, mild inflammation, and bronchiectasis in the lungs. The patient was unresponsive to antibacterial therapy. A small dose of steroids (5mg daily, orally) was administered, and the diarrhea moderately improved. However, the patient experienced gastrointestinal bleeding one month after steroid therapy, accompanied by aggravated edema of the intestinal wall with incomplete intestinal obstruction. 300mg vedolizumab was initiated with three infusions at 0, 2, 6 weeks, and the GI symptoms clearly improved. However, the patient continued to have an intermittent fever, and the CT showed new inflammatory lesions in the lungs. He died in November 2022 due to fungal infection of the lungs.
Discussion
Primary immunodeficiencies (PIDs) are characterized by immune defects in circulating B and/or T cells, which cause agammaglobulinemia and recurrent infections.25 CVID and XLA are B-cell immunodeficiency.19 Chronic diarrhea is the most frequent gastrointestinal symptom related to CVID and XLA, especially non-infectious diarrhea, which causes diagnostic delays and poor prognostic outcomes and remains a difficult clinical problem.5,7 We describe three adult PID patients (two with CVID and one with XLA) with varying degrees of diarrhea, weight loss, and protein-energy malnutrition. The clinical characteristics of the patients are summarized in Table 1. These patients presented with similar clinical symptoms with different underlying pathogeneses and were hospitalized at least twice before a diagnosis was made. Through these three cases, we demonstrated that patients with chronic diarrhea and malabsorption with a history of numerous infections in their early years, combined with significantly decreased globulin levels in the blood and extensive villous blunting of the small intestine under capsule endoscopy, should highly suspect PID-related enteropathy. Further analysis of Ig, lymphocyte subsets, and gene sequencing is needed for ultimate diagnosis.
 |
Table 1 Characteristics of 3 PIDs Patients with Chronic Diarrhea |
CVID and XLA are PIDs related to B cell immunodeficiency. CVID is characterized by a defect in the differentiation to plasma and memory B cells.26 XLA arrests pro-B-cell to pre-B-cell differentiation and partially blocks the pre-B-cell to immature B-cell transition.27 These three patients all showed decreased peripheral B-cells and immunoglobulins. Numerous T-cell abnormalities have been found in both CVID and XLA, including a decreased T-cell count and proliferative response, increased T-cell activation, and abnormal cytokine production.28 Specifically, the numbers of CD4+ and Treg cells decreased, whereas CD8+ T cells increased in CVID.28 From these cases, T cell counts were almost normal in three patients, while the activation of T cells were unclear without the deeper analysis. Gut microbial dysbiosis and mucosal IgA deficiency are associated with CVID enteropathy and systemic inflammation in CVID.29 Mutually, IgA deficiency can lead to microbial dysbiosis and subsequently increased T-cell activation, which cause aggravated gut immune dysregulation in CVID.30 For XLA, enteropathy is less common since the T-cell dysfunction is less present than CVID. BTK was a critical mediator of B cell receptor signaling in the functioning of adaptive immunity, it also modulated the innate immune system in mononuclear cells.31 Dysfunctional BTK expression increased NLRP3 inflammasome activation in bone marrow–derived macrophages (BMDMs) that gives rise to increased proinflammatory responses, may contribute to the pathogenesis of XLA enteropathy.32 The pathology of CVID-enteropathy includes villous blunting, massive intraepithelial lymphocytes, and a lack of plasma cells in the lamina propria of the gastrointestinal mucosa, which is only present in approximately two-thirds of CVID patients.33,34 In addition, the histology of several CVID-related enteropathies presents celiac disease or Crohn's disease-like, which are difficult to identify.34 No study has focused on the histological manifestations of XLA, because its incidence is lower than that of CVID. Importantly, these three patients all underwent capsule endoscopy and showed extensive thinning of the small intestinal villi without remarkable ulcers.
Chronic diarrhea can cause a wide spectrum of potentially life-threatening complications including malabsorption, protein-energy malnutrition, and electrolyte imbalance. Ig supplementation is the most important treatment for PIDs.13,26 The survival of patients with CVID has improved in recent years, largely due to the use of Ig, which has greatly decreased the frequency of recurrent and severe infections and hospitalizations.35 The recommended dosage of Ig is 400–600mg/kg administered intravenously every 3–4 weeks.36 In addition, IgG levels should be tested during Ig treatment.37 In these three cases, patients were obtained Ig therapy was administered at the beginning of the disease course; however, Patient 1 discontinued regular use and had numerous hospitalizations with poorly controlled diarrhea. Patients 2 and 3 received regular Ig supplementation, while patient 3 had deteriorated inflammation of the gut and failed Ig therapy. It should be noted that intravenous Ig therapy is less effective in patients with enteropathy-related symptoms in CVID,38 which may be because mucosal IgA levels remain diminished over time after intravenous Ig replacement therapy.39 Long-term effects of Ig therapy in patients with XLA and enteropathy have not been reported. Corticosteroids or immunomodulators can be administered; lower doses and shorter treatment periods are often required in cases of autoimmune diseases.26,40,41 Additionally, patients with CVID have microbial dysbiosis with reduced α-diversity compared with patients with inflammatory bowel disease (IBD).13 Potentially pathogenic microbes Streptococcus parasanguinis and Erysipelatoclostridium ramosum was enhanced in the gut microbiome of CVID patients with non-infectious complications.42 Thus, improving the structure of the gut microbiota can partly control intestinal inflammation in PID-related enteropathy, and administration of enteral nutrition and probiotics may exert a therapeutic effect in these patients. Patient 1 achieved partial relief from diarrhea after enteral nutrition and probiotic replenishment. However, patient 2 did not receive improvement in diarrhea after probiotic treatment. Further, the effect of probiotic and fecal microbiota transplantation (FMT) therapy can be explored for the enteropathy of IgA-deficient PIDs. Based on the immune response dysfunction in CVID and XLA, gut-selective treatments that suppress T-cell activation are used in patients with CVID- and XLA-related enteropathy.43–45 Vedolizumab has been reported to be useful in inducing GI symptom remission, as it blocks the ingress of regulatory T cells into the mucosa.44,46 However, Sifers et al reported that vedolizumab was not effective in 86% of CVID patients.47 In our cases, patients 1 and 3, who had been diagnosed with CVID and XLA, respectively, achieved vedolizumab therapy due to uncontrolled diarrhea and aggravated intestinal inflammation, and the GI symptoms were greatly improved during treatment with vedolizumab; however, they experienced severe inflammatory spread in extra-intestinal organs, such as the skin and lungs, and died due to progressive inflammation or infections. Since vedolizumab may be more likely to develop extra-intestinal manifestations,48 it may potentially worsen clinical outcomes, indicating that vedolizumab was not helpful in patients with CVID- and XLA-related enteropathy.
In summary, chronic diarrhea is a common clinical manifestation in patients with CVID and XLA and is responsible for poorer prognostic outcomes. Early recognition and appropriate clinical management are required to improve clinical outcomes. In this study, we demonstrated that extensive villous blunting of the small intestine under capsule endoscopy may be an endoscopic feature of PID-related enteropathy, and capsule endoscopy is recommended for each CVID and XLA patient presenting with chronic diarrhea in order to not delay the specific treatment and to not worsen the prognostic outcome. Regular Ig supplementation is the basic treatment for PIDs, combined with proper enteral nutrition and probiotic therapy to supplement serum immunoglobulins and regulate local and systemic immunity and the gut microbiota. Vedolizumab is not helpful to PIDs PID-related enteropathy therapy, as it exacerbates the inflammatory response in extra-intestinal organs and ultimately causes poor clinical outcomes. We suspected that therapies that control immune dysregulation, such as monoclonal IL-12/23 antibody Ustekinumab24, Janus kinase inhibitors49 and FMT, could be used in the future for PID-related enteropathy.
Data Sharing Statement
The data underlying this article are available in the article.
Consent Statement
This study obtained written patient consent for potential publication of their case and images, and institutional approval was not required to publish the case details.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was sponsored by the National Natural Science Foundation of China (No. 81900478).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Arunachalam AK, Maddali M, Aboobacker FN, et al. Primary immunodeficiencies in India: molecular diagnosis and the role of next-generation sequencing. J Clin Immunol. 2021;41(2):393–413. doi:10.1007/s10875-020-00923-2
2. Picard C, Bobby Gaspar H, Al-Herz W, et al. International Union Of Immunological Societies: 2017 primary immunodeficiency diseases committee report on inborn errors of immunity. J Clin Immunol. 2018;38(1):96–128. doi:10.1007/s10875-017-0464-9
3. Demirdag Y, Fuleihan R, Orange JS, et al. New primary immunodeficiencies 2021 context and future. Curr Opin Pediatr. 2021;33(6):657–675. doi:10.1097/MOP.0000000000001075
4. Bousfiha A, Jeddane L, Picard C, et al. The 2017 IUIS phenotypic classification for primary immunodeficiencies. J Clin Immunol. 2018;38(1):129–143. doi:10.1007/s10875-017-0465-8
5. Agarwal S, Cunningham-Rundles C. Gastrointestinal manifestations and complications of primary immunodeficiency disorders. Immunol Allergy Clin North Am. 2019;39(1):81–94. doi:10.1016/j.iac.2018.08.006
6. Cunningham-Rundles C. The many faces of common variable immunodeficiency. Hematology Am Soc Hematol Educ Program. 2012;2012(1):301–305. doi:10.1182/asheducation.V2012.1.301.3798316
7. Pecoraro A, Nappi L, Crescenzi L, et al. Chronic diarrhea in common variable immunodeficiency: a case series and review of the literature. J Clin Immunol. 2018;38(1):67–76. doi:10.1007/s10875-017-0461-z
8. Szczawinska-Poplonyk A, Schwartzmann E, Bukowska-Olech E, et al. The pediatric common variable immunodeficiency - from genetics to therapy: a review. Eur J Pediatr. 2022;181(4):1371–1383. doi:10.1007/s00431-021-04287-6
9. Seidel MG, Kindle G, Gathmann B, et al. The European Society for Immunodeficiencies (ESID) Registry working definitions for the clinical diagnosis of inborn errors of immunity. J Allergy Clin Immunol Pract. 2019;7(6):1763–1770. doi:10.1016/j.jaip.2019.02.004
10. Ameratunga R, Allan C, Woon ST. Defining common variable immunodeficiency disorders in 2020. Immunol Allergy Clin North Am. 2020;40(3):403–420. doi:10.1016/j.iac.2020.03.001
11. Cunningham-Rundles C. Common variable immune deficiency: dissection of the variable. Immunol Rev. 2019;287(1):145–161. doi:10.1111/imr.12728
12. Bogaert DJ, Dullaers M, Lambrecht BN, et al. Genes associated with common variable immunodeficiency: one diagnosis to rule them all? J Med Genet. 2016;53(9):575–590. doi:10.1136/jmedgenet-2015-103690
13. Yazdani R, Habibi S, Sharifi L, et al. Common variable immunodeficiency: epidemiology, pathogenesis, clinical manifestations, diagnosis, classification, and management. J Investig Allergol Clin Immunol. 2020;30(1):14–34. doi:10.18176/jiaci.0388
14. Park SE, Neaves BI, Adams K. A case of rare splice-site Bruton’s tyrosine kinase mutation with atypical X-linked agammaglobulinemia. Ann Allergy Asthma Immunol. 2022;130(3):364–365. doi:10.1016/j.anai.2022.12.004
15. Cinicola B, Uva A, Leonardi L, et al. Case report: a case of X-linked agammaglobulinemia with high serum IgE levels and allergic rhinitis. Front Immunol. 2020;11:582376. doi:10.3389/fimmu.2020.582376
16. Taneja A, Muco E, Chhabra A. Bruton Agammaglobulinemia. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC; 2023.
17. Ilkjaer FV, Rasmussen LD, Martin-Iguacel R, et al. How to identify common variable immunodeficiency patients earlier: general practice patterns. J Clin Immunol. 2019;39(7):641–652. doi:10.1007/s10875-019-00666-9
18. Dong J, Liang H, Wen D, et al. Adult common variable immunodeficiency. Am J Med Sci. 2016;351(3):239–243. doi:10.1016/j.amjms.2015.12.010
19. Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol. 2013;11(9):1050–1063. doi:10.1016/j.cgh.2013.02.024
20. Hernandez-Trujillo VP, Scalchunes C, Cunningham-Rundles C, et al. Autoimmunity and inflammation in X-linked agammaglobulinemia. J Clin Immunol. 2014;34(6):627–632. doi:10.1007/s10875-014-0056-x
21. Barmettler S, Otani IM, Minhas J, et al. Gastrointestinal manifestations in X-linked agammaglobulinemia. J Clin Immunol. 2017;37(3):287–294. doi:10.1007/s10875-017-0374-x
22. Stockman JA. Morbidity and mortality in common variable immune deficiency over 4 decades. Yearbook Pediatr. 2013;2013:321–325. doi:10.1016/j.yped.2012.05.023
23. Jørgensen SF, Reims HM, Frydenlund D, et al. A cross-sectional study of the prevalence of gastrointestinal symptoms and pathology in patients with common variable immunodeficiency. Am J Gastroenterol. 2016;111(10):1467–1475. doi:10.1038/ajg.2016.329
24. Fevang B. Treatment of inflammatory complications in common variable immunodeficiency (CVID): current concepts and future perspectives. Expert Rev Clin Immunol. 2023;19(6):1–12.
25. Parvaneh L, Sharifi N, Azizi G, et al. Infectious etiology of chronic diarrhea in patients with primary immunodeficiency diseases. Eur Ann Allergy Clin Immunol. 2019;51(01):32–37. doi:10.23822/EurAnnACI.1764-1489.77
26. Song J, Lleo A, Yang GX, et al. Common Variable Immunodeficiency and Liver Involvement. Clin Rev Allergy Immunol. 2018;55(3):340–351. doi:10.1007/s12016-017-8638-z
27. Lackey AE, Ahmad F. X-linked Agammaglobulinemia. In: StatPearls. Treasure Island (FL): StatPearls Publishing. Copyright © 2023, StatPearls Publishing LLC; 2023.
28. Azizi G, Rezaei N, Kiaee F, et al. T-cell abnormalities in common variable immunodeficiency. J Investigat Allergol Clin Immunol. 2016;26(4):233–243. doi:10.18176/jiaci.0069
29. Andersen IM, Jorgensen SF. Gut inflammation in CVID: causes and consequences. Expert Rev Clin Immunol. 2022;18(1):31–45. doi:10.1080/1744666X.2021.2008241
30. Berbers RM, Nierkens S, van Laar JM, et al. Microbial dysbiosis in common variable immune deficiencies: evidence, causes, and consequences. Trends Immunol. 2017;38(3):206–216. doi:10.1016/j.it.2016.11.008
31. Weber ANR, Bittner Z, Liu X, et al. Bruton’s tyrosine kinase: an emerging key player in innate immunity. Front Immunol. 2017;8:1454. doi:10.3389/fimmu.2017.01454
32. Mao L, Kitani A, Hiejima E, et al. Bruton tyrosine kinase deficiency augments NLRP3 inflammasome activation and causes IL-1beta-mediated colitis. J Clin Invest. 2020;130(4):1793–1807. doi:10.1172/JCI128322
33. Bonilla FA, Barlan I, Chapel H, et al. International Consensus Document (ICON): common variable immunodeficiency disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. doi:10.1016/j.jaip.2015.07.025
34. Daniels JA, Lederman HM, Maitra A, et al. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31(12):1800–1812. doi:10.1097/PAS.0b013e3180cab60c
35. Salehzadeh M, Aghamohammadi A, Rezaei N. Evaluation of immunoglobulin levels and infection rate in patients with common variable immunodeficiency after immunoglobulin replacement therapy. J Microbiol Immunol Infect. 2010;43(1):11–17. doi:10.1016/S1684-1182(10)60002-3
36. Cunningham-Rundles C, Maglione PJ. Common variable immunodeficiency. J Allergy Clin Immunol. 2012;129(5):1425–1426.e3. doi:10.1016/j.jaci.2012.03.025
37. Gathmann B, Mahlaoui N, Gérard L, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134(1):116–126. doi:10.1016/j.jaci.2013.12.1077
38. Malamut G, Verkarre V, Suarez F, et al. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol. 2010;105(10):2262–2275. doi:10.1038/ajg.2010.214
39. Oksenhendler E, Gérard L, Fieschi C, et al. Infections in 252 patients with common variable immunodeficiency. Clin Infect Dis. 2008;46(10):1547–1554. doi:10.1086/587669
40. Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92(1):34–48. doi:10.1006/clim.1999.4725
41. Cunningham-Rundles C. Autoimmune manifestations in common variable immunodeficiency. J Clin Immunol. 2008;28(Suppl 1):S42–S45. doi:10.1007/s10875-008-9182-7
42. Hajjar J. Common variable immunodeficiency patient fecal microbiota transplant recapitulates gut dysbiosis; 2023.
43. Çekiç Ş, Özgür T, Karalı Y, et al. Vedolizumab treatment in a patient with X-linked agammaglobulinemia, is it safe and efficient? Turk J Pediatr. 2019;61(6):937–940. doi:10.24953/turkjped.2019.06.016
44. Johnson D, Lee G, Weber F. Vedolizumab Therapy in Refractory Enteropathy Associated With CVID. ACG Case Rep J. 2022;9(1):e00721. doi:10.14309/crj.0000000000000721
45. Albshesh A, Eder P, Ribaldone DG. Primary hypogammaglobulinaemia with inflammatory bowel disease-like features: an ECCO CONFER multicentre case series. J Crohn’s Colitis. 2022;16(1):91–97. doi:10.1093/ecco-jcc/jjab124
46. Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710. doi:10.1056/NEJMoa1215734
47. Sifers T, Hirten R, Mehandru S, et al. Vedolizumab therapy in common variable immune deficiency associated enteropathy: a case series. Clin Immunol. 2020;212:108362. doi:10.1016/j.clim.2020.108362
48. Dubinsky MC, Cross RK, Sandborn WJ, et al. Extraintestinal manifestations in vedolizumab and anti-TNF-treated patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018;24(9):1876–1882. doi:10.1093/ibd/izy065
49. Franzblau LE, Fuleihan RL, Cunningham-Rundles C, et al. CVID-associated intestinal disorders in the USIDNET registry: an analysis of disease manifestations, functional status, comorbidities, and treatment. Res Sq. 2023. doi:10.21203/rs.3.rs-2838051/v1
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.