Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Chronic Atopic Dermatitis with Eosinophilia Improved by Daesiho-Tang: A Case Report
Authors Park MC, Lee JH , Seong EJ, Lee DS, Jo EH
Received 6 June 2023
Accepted for publication 3 September 2023
Published 20 September 2023 Volume 2023:16 Pages 2561—2572
DOI https://doi.org/10.2147/CCID.S424225
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Min-Cheol Park,1,2,* Ju-Hyun Lee,3,* Eun-Jin Seong,4 Dong-Sung Lee,5 Eun-Heui Jo2,6
1Department of Korean Medicine Ophthalmology and Otolaryngology and Dermatology, Wonkwang University Korean Medicine Hospital, Iksan, Jeollabuk-do, 54538, Republic of Korea; 2Research Center of Traditional Korean Medicine, Wonkwang University, Iksan, Jeollabuk-do, 54538, Republic of Korea; 3Department of Medical Support, Imsil-Gun Medical Center, Imsil, Jeollabuk-do, 55927, Republic of Korea; 4Seoul Clinic, Hanam, Gyeonggi-do, 12945, Republic of Korea; 5College of Pharmacy, Chosun University, Gwangju, Jeollanam-do, 61452, Republic of Korea; 6Department of Acupuncture and Moxibustion, Wonkwang University Korean Medicine Hospital, Jeonju, Jeollabuk-do, 54887, Republic of Korea
*These authors contributed equally to this work
Correspondence: Dong-Sung Lee, College of Pharmacy, Chosun University, 309, Pilmundae-ro, Dong-gu, Gwangju, Jeollanam-do, 61452, Republic of Korea, Tel +82-62-230-6386, Fax +82-62-232-8834, Email [email protected] Eun-Heui Jo, Department of Acupuncture and Moxibustion, Wonkwang University Korean Medicine Hospital, 99, Garyeonsan-ro, Deokjin-gu, Jeonju, Jeollabuk-do, 54887, Republic of Korea, Tel +82-63-270-1022, Fax +82-63-270-1199, Email [email protected]
Purpose: This study is to report a case of chronic atopic dermatitis (AD) with eosinophilia, which did not respond to conventional therapy and was improved by Daesiho-tang (DSHT).
Patients and Methods: The patient visited our clinic with symptoms of atopic dermatitis including skin lesions and pruritus. Based on her symptoms, DSHT was prescribed. At each visit, the Scoring Atopic Dermatitis (SCORAD), Dermatology Life Quality Index (DLQI), and accompanying systemic symptoms (ASS) were measured. Multiple Allergen Simultaneous Test (MAST) was initially performed for 108 allergens and analyzed by Western blotting using an Alternate Scoring Method (ASM) according to the specific IgE concentration. Also, peripheral blood laboratory (Lab) tests were performed three times during the patient’s visit.
Results: After taking DSHT, the total SCORAD score improved from 62.9 to 23.5, while the patient’s ASS also improved. The DLQI score improved from 19 to 5. The total number of eosinophils in the peripheral blood, which showed a mild increase, recovered from 17.2% (0.98 x103/μL) to 4.5% (0.24 x103/μL). The total IgE slightly decreased, while AST and ALT were also restored to normal ranges.
Conclusion: Based on this case, DSHT is considered a potential alternative treatment for AD.
Keywords: case report, atopic dermatitis, eosinophilia, Daesiho-tang
Graphical Abstract:
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease caused by Th1-Th2 immune imbalance.1 In AD, Th2 cells mediate an allergic inflammatory response during the acute phase and Th1 cells medicate a response during the chronic phase.2 Activated Th2 cells are involved in allergic reactions by secreting cytokines, such as IL-4, IL-5, and IL-13. IL-4 and IL-13 stimulate B cells to induce IgE production,3 while IL-5 increases the activity and antioxidant efficacy of eosinophils4 and acts on the bone marrow to promote their generation and differentiation.5 Furthermore, eosinophils cause an allergic inflammatory reaction after being deposited on the skin, causing tissue damage5 and AD symptoms, such as edema, oozing, crusting, excoriation, dryness, and lichenification.6 Severe pruritus and changes in appearance accompanying the lesion may also cause to experience psychosocial changes, such as sleep disturbance and reduced quality of life.6,7 New treatments such as dupilumab are being developed to treat atopic dermatitis, which causes itching and reduces quality of life.8 In addition, researches to develop alternative medicine as a treatment for atopic dermatitis and itchiness are also being actively conducted.9
Daesiho-tang (DSHT) is an herbal medicine known to have antifungal,10 antioxidant,11 antidiabetic,12 anti-obesity,13 hepatoprotective,14 and lipid metabolism regulatory effects.15 DSHT has been used for treating various diseases such as cholecystitis and cholelithiasis.16 Qian et al14 reported that DSHT was effective in improving fatty liver and diabetes by reducing insulin resistance and inhibiting inflammation through SIRT1 and NF-kB regulatory mechanisms. Zhang et al12 reported that insulin resistance, glucose metabolism, and lipid metabolism were significantly improved after DSHT was administered to patients with diabetes who were receiving conventional diabetic treatment. There was a clinical report that showed the improvement of urticaria by DSHT.15 However, research on the use of DSHT in Dermatology is insufficient. This study aimed to report the clinical results of DSHT usage in a patient with chronic AD and mild eosinophilia.
Materials and Methods
Study Methods
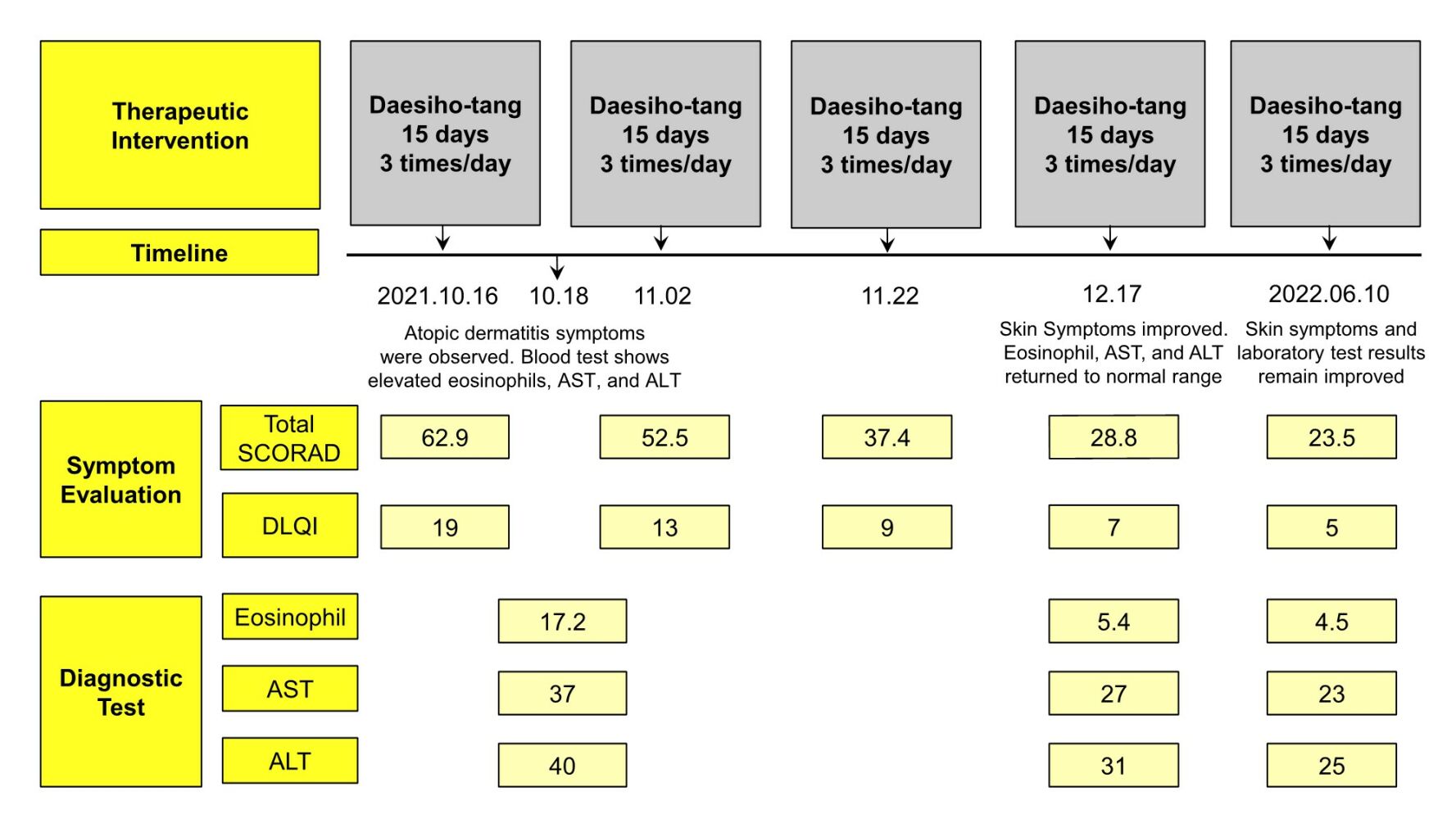
The patient was diagnosed with chronic atopic dermatitis and mild eosinophilia and treated at Wonkwang University Korean Medicine Hospital from October 16, 2021, to April 03, 2022 (Figure 1).
 |
Figure 1 Treatment timeline. |
Symptom Assessment
At each visit, the Scoring Atopic Dermatitis (SCORAD) Index17 and Dermatology Life Quality Index (DLQI)18 were used to evaluate the symptoms. Photographs of the affected sites were also taken (IXUS 500HS, Canon Korea, Seoul, Korea). The Numerical Rating Scale (NRS) was used to evaluate changes in the accompanying systemic symptoms (ASS) found in the systematic interview process.
Diagnostic Tests
The patient underwent the Multiple Allergen Simultaneous Test (MAST) and the peripheral laboratory (Lab) tests. The specimens were collected from Wonkwang University Korean Medicine Hospital (Iksan, Korea) and sent to the Samkwang Medical Foundation (Seoul, Korea) for analysis. MAST was classified into 7 levels (0–6) using an Alternate Scoring Method (ASM) according to the specific IgE concentration19 and analyzed by Western blotting only once initially for 108 allergens. Peripheral blood was collected three times during the patient’s hospital visit and Lab test results, including complete blood cell count and differential count (CBC&D/C), liver function test (LFT), renal function test (RFT), total cholesterol, glucose, and total IgE were obtained.
Preparation of the Intervention
The quality and safety of herbal medicines were recognized by the Korea Food and Drug Administration and supplied by pharmaceutical companies. The residual content of pesticides and heavy metals in herbal medicine was tested by the Drug Committee of Wonkwang University Korean Hospital (Iksan, Korea). To obtain a water extract of DSHT on a 15-day basis, herbal medicines and approximately 7700 cc of water were placed in a water heater (Kyungseo E&P, Incheon, Korea) and eluted at a water temperature of 95 °C for 2 h to extract 5400 cc. The DSHT water extract was sealed and packaged individually into 120 cc aliquots (total 45 packs) and provided to the patient. The daily amount of DSHT and suppliers of the herbal medicines used in DSHT are presented in Table 1.
 |
Table 1 Composition and Daily Dosage of Herbal Medicine, Daesiho-Tang |
Components of Daesiho-Tang
Orbitrap-HRMS analysis was performed on DSHT for component analysis. 14 compounds were profiled using Orbitrap-HRMS chromatogram analysis of the Daesiho-tang. The main peaks attributed to the chromatogram were observed in both the positive and negative modes using electrospray ionization–MS. Moreover, to identify the compound, the ion peak was compared with those found in literature, along with the MS information of the compound. As a result of the analysis, DSHT contains about 14 components, including Paeoniflorin, Naringin, Didymin, 8-Angeloylegelolide, and Oroxindin (Table 2 and Figure 2).
 |
Table 2 Compounds Identified in Daesiho-Tang Using Orbitrap-HRMS |
 |
Figure 2 Total chromatograms of Daesiho-tang using Orbitrap-HRMS analysis. |
Duration of Treatment
From October 16, 2021, to April 03, 2022, DSHT was prescribed and the patient was provided with a 15-day supply each time of her hospital visit. In total, 75-day of DSHT was prescribed and supplied to the patient as treatment. The patient was instructed to consume the DSHT water extract (120 cc/pack/dose) 30 min after meals three times a day. Medication adherence was calculated using the Medication Possession Ratio (MPR)20 and determined to be 44.12%.
Data Arrangement
Patient information recorded in the electronic medical record system was collected, and changes in each observation item according to the treatment progress were retrospectively analyzed.
Case and Results
Patient Characteristics and Medical History
Our patient was a 28-year-old woman who complained of objective skin symptoms including erythema, papules, edema, abrasions, exudates, dryness, scales, and lichenification at the initial consultation. The patient also experienced subjective symptoms of NRS 8 pruritus, which occurred mainly under the joints of both limbs since 2018. During the systemic interview, the patient reported frequent discomfort due to constipation, bloating, chest tightness, and dyspepsia. Past medical history included appendectomy approximately 13 years ago. The patient’s family history was nonspecific. The patient had been treated and managed with topical steroid therapy (Lidomex ointment, Sama Pharmaceutical, Wonju, Korea) and BepanthenTM ointment (Bayer Korea, Seoul, Korea) based on a diagnosis of AD with symptoms of eczema at the local dermatology clinic. Despite treatment, the symptoms worsened (about a year ago) and systemic steroid therapy (Solondo Tablet, Yuhan Corporation, Seoul, Korea) was administered for 2 weeks a month ago. Even with systemic steroid therapy, aggravated skin symptoms persisted. Therefore, the patient voluntarily discontinued all prescribed treatments.
Treatment Progress
October 16, 2021
Erythema, papules, edema, exudation, dryness, scale, and lichenification were observed in the distal area below both limb joints (Objective SCORAD 49.9; Figures 3 and 4). The patient’s symptoms were severe in the distal joints of the fingers, accompanied by scratches and bleeding around the affected areas due to itching and scratching (Subjective SCORAD 13; Figures 3 and 4). The patient was diagnosed with chronic AD based on the criteria described by Hanifin and Rajka.21 The patient complained of suffering in daily life due to symptom aggravation when she was stressed or in the evening, and especially when she washed her hands with soap. The affected areas were sore and painful (DLQI 19; Figure 4). DSHT was prescribed as a treatment intervention considering the total SCORAD, DLQI, and ASS.
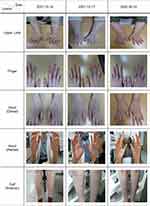 |
Figure 3 Clinical progress of atopic dermatitis. |
 |
Figure 4 Progress score of the Scoring Atopic Dermatitis and the Dermatology Life Quality Index. |
October 18, 2021
According to the Lab test results, the white blood cell (WBC) count was 5.70 x103/μL and within the normal range, the eosinophil count showed a mild increase at 17.2% (980/μL), and the total IgE showed a severe increase at 13,419 IU/mL. Moreover, AST and ALT were 37 U/L and 40 U/L, respectively, which were higher than the normal values (Table 3). MAST results showed strong allergic reactions to horses, pork, dogs, guinea pigs, rabbits, hamsters, house dust, cats, and Alternaria alternata (Table 4). The patient was living with a dog as a companion animal at the time. The patient was recommended to live apart from the dog as avoidance therapy. However, the patient declined due to affection for the companion animal.
 |
Table 3 Peripheral Blood Test Results |
 |
Table 4 Multiple Allergen Simultaneous Test Results |
December 17, 2021
Overall skin symptoms improved from 49.9 to 23.8 on the objective SCORAD index, except for light scratches and pigmentation (Figures 3 and 4). The subjective SCORAD index improved from 13 to 5, which was less than half of the first diagnosis, and the patient’s quality of life improved significantly from a DLQI score of 19 to 7 (Figure 4). Eosinophils improved to the normal range of 5.4% (310/μL) and AST and ALT also returned to normal at 27 U/L and 31 U/L, respectively (Table 3). The patient’s ASS also improved from an NRS score of 25 to 8, compared to the first visit (Figure 5).
 |
Figure 5 Progress of the accompanying systemic symptoms. |
June 10, 2022
The patient visited the hospital after six months for a follow-up. The total SCORAD index score improved to 23.5, and the DLQI score also improved to 5 (Figure 4). Additional Lab test results showed that AST, ALT, and eosinophil levels were all maintained within the normal ranges. Moreover, the total IgE level was slightly improved (Table 3).
Discussion and Conclusions
AD is a chronic inflammatory skin disease characterized by an increased IgE level and eosinophilia, in addition to characteristic skin symptoms.5,21 Eosinophils are distributed in the gastrointestinal tract, thymus, and uterus to defend against viruses and parasites.22,23 When suffering from an allergic disease, such as AD, the secretion of IL-4, IL-5, IL-13, etc., is promoted by Th2 cell activation, and the eosinophil precursor cell receptor is stimulated by IL-5, resulting in increased eosinophils.2,3,5,22,24 When eosinophils are excessively increased in the peripheral blood, especially when eosinophils of 0.351 x103/μL or more are observed, this is defined as eosinophilia. Eosinophilia is classified into mild (0.351–1.5 x103/μL), moderate (1.51–5 x103/μL), and severe (>5 x103/μL). Although the total eosinophil count in the peripheral blood is an adjunctive assessment tool with limited clinical usefulness, it is elevated to 60% in many allergic diseases.25,26 Importantly, the correlation between an increase in eosinophils and total IgE is an important feature of AD.27 Therefore, when treating patients with AD patients who have eosinophilia, the patient’s clinical symptoms must be managed along with strict control of the eosinophil level.5
Moisturizers, antibiotics, antihistamines, steroids, and immunosuppressants are commonly used to treat AD. The most important and frequently used treatment options include avoidance therapy, which involves guided avoidance to contact with allergens, and topical steroid therapy.28,29 Topical steroid therapy has significant side effects including rosacea, acne, perioral dermatitis, and telangiectasia, with chronic use.30 Therefore, interest in complementary and alternative medicine, including herbal medicines for the management of AD has been gradually increasing.
DSHT, which has been used mainly in East Asia for approximately 2000 years, is a traditional herbal medicine used to treat diseases, such as hepatitis, cholecystitis, cholelithiasis, nausea, vomiting, and urticaria.15,31 Research on herbal medicines related to DSHT includes studies by Zhou32 and Lee et al,33 who reported the anti-inflammatory and anti-allergic efficacy of Poncirus trifoliata (L.) Raf. through inhibition of iNOS and COX-2 expression and stabilization of mast cells. Park et al34 reported the anti-inflammatory effect of psycosaponin contained in Bupleurum falcatum L. through the regulation of the NF-κB pathway. The anti-allergic35,36 and anti-inflammatory37,38 effects of Ziziphus jujuba Mill. and Zingiber officinale Roscoe have also been reported in several previous studies.
Moreover, paeoniflorin, naringin, and didymin, which are components of DSHT, also exhibited anti-inflammatory and immunomodulatory effects. In the previous study, paeoniflorin and naringin have anti-inflammatory, antioxidant, and immunomodulatory effect by regulating immune substances such as G-protein-coupled receptors, nuclear factor erythroid 2-related factor 2, Interleukin-4, 6, 10, and inducible nitric oxide synthase.39,40 In addition, it also reported that didymin showed anti-inflammatory and antioxidant effects by regulating immune factors such as tumor necrosis factor-α and Interleukin-6.41 Although the various biological effects of DSHT are known, but there is only few research on the regulation of skin disease by DSHT.
Our patient had skin symptoms that met the AD diagnosis criteria of Hanifin and Rajka,21 and her Lab tests showed a mild increase in eosinophils to 17.2% (980/μL) and a severe increase in total IgE to 13,419 IU/mL. Hence, chronic AD with eosinophilia was diagnosed. Since the MAST showed strong allergic reactions to horses, pork, dogs, guinea pigs, rabbits, hamsters, house dust, cats, and Alternaria alternata (Table 4), it was assumed that the dog, the patient’s companion animal, was one of the main allergens responsible for the occurrence of symptoms.28,29 Therefore, the patient was advised to stay away from the dog as avoidance therapy. However, the proposal was rejected because of the patient’s affection for her dog.
Considering the AD skin symptoms and the patient’s frequent discomfort due to constipation, bloating, chest tightness, and dyspepsia in the systematic symptoms interview, DSHT was prescribed as a treatment intervention. The elevation of AST and ALT also served as a basis for prescribing DSHT to the patient for hepatoprotective14 and cholesterol absorption inhibitory effects.15 This study showed that the patient’s total SCORAD score (Figures 3 and 4), DLQI score (Figure 4), and ASS (Figure 5) were significantly improved after the use of DSHT water extract, although avoidance therapy and conventional therapy were both rejected. Moreover, AST, ALT, and eosinophil levels were restored to the normal ranges (Table 3). A biopsy of the skin deposition of eosinophils was not performed. The slight improvement in total IgE level was likely related to continuous exposure to the main allergen, namely, the dog. The patient’s medication adherence was low at 44.12% due to time constraints related to her occupation. Once the symptoms improved and the patient discomfort in daily life was greatly reduced, DSHT treatment was terminated because the patient did not want any further treatment.
For our patient, the DSHT played an important role in alleviating her allergic reactions and reducing eosinophils. In this case report, the improvement of chronic AD with mild eosinophilia despite her rejection of avoidance therapy and conventional treatment seems to be due to the multisynergistic effects of DSHT. The improvement of AST and ALT also may be attributed to the hepatoprotective effect of Bupleurum falcatum L, the main component of DSHT.42 However, since this is a case report, and the findings were from one patient, it is difficult to generalize the results. In the future, further researches on DSHT as a complementary and alternative treatment method for AD are warranted.
Data Sharing Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to the patient’s privacy.
Ethics Approval
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Wonkwang University (date of approval: November, 11th, 2022; approval number: WKIRB-202211-BM-107).
Informed Consent for Publication
Informed consent was obtained from all subjects involved in the study. Written Informed consent has been obtained from the patient to publish this paper. Before starting treatment, the patient was informed of the academic use of photographs and medical records, and consent was obtained.
Acknowledgments
Min-Cheol Park and Ju-Hyun Lee are co-first authors for this study. We would like to thank Editage for editing and reviewing this manuscript for English language.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was carried out by funding from Wonkwang University in 2023. The sponsors had no role in the study design, study execution, data collection, data analysis, data interpretation, or manuscript writing and preparation of the study.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Wollenberg A, Kraft S, Oppel T, Bieber T. Atopic dermatitis: pathogenetic mechanisms. Clin Exp Dermatol. 2008;25(7):530–534. doi:10.1046/j.1365-2230.2000.00699.x
2. Grewe M, Bruijnzeel-Koomen CAFM, Schöpf E, et al. A role for Th1 and Th2 cells in the immunopathogenesis of atopic dermatitis. Immunol Today. 1998;19(8):359–361. doi:10.1016/S0167-5699(98)01285-7
3. Furue M, Ulzii D, Vu Y, Tsuji G, Kido-Nakahara M, Nakahara T. Pathogenesis of atopic dermatitis: current Paradigm. Iran J Immunol. 2019;16(2):97–107. doi:10.22034/IJI.2019.80253
4. Mita S, Tominaga A, Takatsu K, Suda T. Highly purified murine interleukin 5 (IL-5) stimulates eosinophil function and prolongs in vitro survival. IL-5 as an eosinophil chemotactic factor. J Exp Med. 1988;167(5):1737–1742. doi:10.1084/jem.167.5.1737
5. Simon D, Braathen LR, Simon HU. Eosinophils and atopic dermatitis. Allergy. 2004;59(6):561–570. doi:10.1111/j.1398-9995.2004.00476.x
6. Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. Diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2015;70(2):338–351. doi:10.1016/j.jaad.2013.10.010
7. Carroll CL, Balkrishnan R, Feldman SR, Fleischer AB Jr, Manuel JC. The burden of atopic dermatitis: impact on the patient, family, and society. Pediatr Dermatol. 2005;22(3):192–199. doi:10.1111/j.1525-1470.2005.22303.x
8. Mastorino L, Viola R, Panzone M, et al. Dupilumab induces a rapid decrease of pruritus in adolescents: a pilot real-life study. Dermatol Ther. 2021;34(6):e15115. doi:10.1111/dth.15115
9. Hussain Z, Thu HE, Shuid AN, Kesharwani P, Khan S, Hussain F. Phytotherapeutic potential of natural herbal medicines for the treatment of mild-to-severe atopic dermatitis: a review of human clinical studies. Bio Pharmaco. 2017;93:596–608. doi:10.1016/j.biopha.2017.06.087
10. Ikegami F, Sumino M, Fujii Y, Akiba T, Satoh T. Pharmacology and toxicology of Bupleurum root-containing Kampo medicines in clinical use. Hum Exp Toxicol. 2006;25(8):481–494. doi:10.1191/0960327106het654oa
11. Da X, Talahasjo H, Hein KZ, Morita E. In vitro antifungal activity of kampo medicine water extracts against Trichophyton rubrum. Nat Prod Commun. 2016;11(6):763–766. doi:10.1177/1934578X1601100616
12. Sato N, Li W, Takemoto H, et al. Comprehensive evaluation of antioxidant effects of Japanese Kampo medicines led to identification of Tsudosan formulation as a potent antioxidant agent. J Nat Med. 2019;73:163–172. doi:10.1007/s11418-018-1259-x
13. Zhang Z, Leng Y, Fu X, et al. The efficacy and safety of dachaihu decoction in the treatment of type 2 diabetes mellitus: a systematic review and meta-analysis. Front Pharmacol. 2022;13:918681. doi:10.3389/fphar.2022.918681
14. Hussain A, Yadav MK, Bose S, et al. Daesiho-Tang is an effective herbal formulation in attenuation of obesity in mice through alteration of gene expression and modulation of intestinal microbiota. PLoS One. 2016;11:e0165483. doi:10.1371/journal.pone.0165483
15. Qian W, Cai X, Zhang X, Wang Y, Qian Q, Hasegawa J. Effect of Daisaikoto on expressions of SIRT1 and NF-kappaB of diabetic fatty liver rats induced by high-fat diet and streptozotocin. Yonago Acta Med. 2016;59(2):149–158.
16. Umeda M, Amagaya S, Ogihara Y. Effect of shosaikoto, daisaikoto and sannoshashinto (traditional Japanese and Chinese medicines) on experimental hyperlipidemia in rats. J Ethnopharmacol. 1989;26(3):255–269. doi:10.1016/0378-8741(89)90098-6
17. European Task Force on Atopic Dermatitis. Severity scoring of atopic dermatitis: the SCORAD index. Dermatology. 1993;186:23–31. doi:10.1159/000247298
18. Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) - a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19(3):210–216. doi:10.1111/j.1365-2230.1994.tb01167.x
19. Wickman M, Ahlstedt S, Lilja G, van Hage Hamsten M. Quantification of IgE antibodies simplifies the classification of allergic diseases in 4-year-old children. A report from the prospective birth cohort study--BAMSE. Pediatr Allergy Immunol. 2003;14:441–447. doi:10.1046/j.0905-6157.2003.00079.x
20. Sperber CM, Samarasinghe SR, Lomax GP. An upper and lower bound of the medication possession ratio. Patient Prefer Adherence. 2017;11:1469–1478. doi:10.2147/PPA.S136890
21. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl. 1980;59:44–47. doi:10.2340/00015555924447
22. Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2004;24:147–174. doi:10.1146/annurev.immunol.24.021605.090720
23. Blanchard C, Rotehnberg ME. Biology of the eosinophil. Adv Immunol. 2009;101:81–121.
24. Takatsu K. Interleukin-5 and IL-5 receptor in health and diseases. Proc Jpn Acad Ser B Phys Biol Sci. 2011;87(8):463–485. doi:10.2183/pjab.87.463
25. Rothenberg ME. Eosinophilia. N Engl J Med. 1998;338(22):1592–1600. doi:10.1056/NEJM199805283382206
26. Lee HK, Pyun BY, Lee SJ. Etiologic allergens, blood eosinophil count and total IgE level according to age and site of skin lesion in atopic dermatitis. J Asthma Allergy Clin Immunol. 1992;12:70–77.
27. Sacco O, Sale R, Silvestri M, et al. Total and allergen-specific IgE levels in serum reflect blood eosinophilia and fractional exhaled nitric oxide concentrations but not pulmonary functions in allergic asthmatic children sensitized to house dust mites. Pediatr Allergy Immunol. 2003;14:475–481. doi:10.1046/j.0905-6157.2003.00092.x
28. Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part I. J Eur Acad Dermatol Venereol. 2018;32:657–682. doi:10.1111/jdv.14891
29. Wollenberg A, Barbarot S, Bieber T, et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: part II. J Eur Acad Dermatol Venereol. 2018;32:850–878. doi:10.1111/jdv.14888
30. Abrahan A, Roga G. Topical steroid-damaged skin. Indian J Dermatol. 2014;59(5):456–459. doi:10.4103/0019-5154.139872
31. Mao C, Zhou Y, Ji D, et al. Chemical fingerprint of Dachaihu granule and its chemical correlation between raw herbs. J Chromatogr Sci. 2017;55(4):405–410.
32. Zhou HY, Shin EM, Guo LY, et al. Anti-inflammatory activity of 21(α, β)-methylmelianodiols, novel compounds from Poncirus trifoliata Rafinesque. Eur J Pharmacol. 2007;572(2–3):239–248. doi:10.1016/j.ejphar.2007.07.005
33. Lee YM, Kim DK, Kim SH, Shin TY, Kim HM. Antianaphylactic activity of Poncirus trifoliata fruit extract. J Ethnopharmacol. 1996;54(2–3):77–84. doi:10.1016/S0378-8741(96)01451-1
34. Park WH, Kang S, Piao Y, et al. Ethanol extract of Bupleurum falcatum and saikosaponins inhibit neuroinflammation via inhibition of NF-κB. J Ethnopharmacol. 2015;174(4):37–44. doi:10.1016/j.jep.2015.07.039
35. Naik SR, Bhagat S, Shah PD, Tare AA, Ingawale D, Wadekar RR. Evaluation of anti-allergic and anti-anaphylactic activity of ethanolic extract of Zizyphus jujuba fruits in rodents. Rev Bras Farmacogn. 2013;23(5):811–818. doi:10.1590/S0102-695X2013000500014
36. Chen BH, Wu PY, Chen KM, Fu TF, Wang HM, Chen CY. Antiallergic potential on RBL-2H3 cells of some phenolic constituents of Zingiber officinale (Ginger). J Nat Prod. 2009;72(5):950–953. doi:10.1021/np800555y
37. Goyal R, Sharma PL, Singh M. Possible attenuation of nitric oxide expression in anti-inflammatory effect of Ziziphus jujuba in rat. J Nat Med. 2011;65:514–518. doi:10.1007/s11418-011-0531-0
38. Mahluji S, Ostadrahimi A, Mobasseri M, Attari VE, Payahoo L. Anti-Inflammatory effects of Zingiber Officinale in type 2 diabetic patients. Adv Pharm Bull. 2013;3(2):273–276. doi:10.5681/apb.2013.044
39. Zhou YX, Gong XH, Zhang H, Peng C. A review on the pharmacokinetics of paeoniflorin and its anti-inflammatory and immunomodulatory effects. Bio Pharmaco. 2020;130:110505. doi:10.1016/j.biopha.2020.110505
40. Chen R, Qi QL, Wang MT, Li QY. Therapeutic potential of naringin: an overview. Pharm Biol. 2016;54(12):3203–3210. doi:10.1080/13880209.2016.1216131
41. Yao Q, Lin MT, Zhu YD, Xu HL, Zhao YZ. Recent trends in potential therapeutic applications of the dietary flavonoid Didymin. Molecules. 2018;23(10):2547. doi:10.3390/molecules23102547
42. Abe H, Sakaguchi M, Odashima S, Arichi S. Protective effect of saikosaponin-d isolated from Bupleurum falcatum L. on CCl4-induced liver injury in the rat. Naunyn Schmiedebergs. Arch Pharmacol. 1982;320:266–271. doi:10.1007/BF00510139
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
