Back to Journals » Clinical Ophthalmology » Volume 14
Choroidal Cavitary Disorders
Authors Nassar S , Tarbett AK, Browning DJ
Received 2 June 2020
Accepted for publication 7 August 2020
Published 8 September 2020 Volume 2020:14 Pages 2609—2623
DOI https://doi.org/10.2147/OPTH.S264731
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sandra Nassar,1 Aaron K Tarbett,2 David J Browning1
1Eye Department, Charlotte Eye, Ear, Nose, and Throat Associates, Charlotte, NC 28210, USA; 2Eye Department, WG Hefner VA Medical System, Salisbury, NC 28144, USA
Correspondence: Sandra Nassar
Eye Department, Charlotte Eye, Ear, Nose, and Throat Associates, 6035 Fairview Road, Charlotte, NC 28210, USA
Email [email protected]
Abstract: The structure and functions of the choroid have been long acknowledged but the pathophysiology behind various anomalies has been difficult to understand until the advent of optical coherence tomography (OCT). With OCT imaging, choroidal cavitations appear as optically empty spaces between the outer retinal and choroidal layers with attenuation or loss of outer retinal layers. Choroidal cavitations are found in the posterior pole and seen in conditions such as pathologic myopia, north carolina macular dystrophy (NCMD), focal choroidal excavation (FCE), and torpedo maculopathy (TM). To date, these disorders have not been linked. A commonality they all share is malformation of the RPE-photoreceptor-choroid complex. The following report describes the differences and similarities of choroidal cavitation amongst the different retinal disorders and emphasizes the importance of multimodal imaging in the detection and management of potential complications.
Keywords: peripapillary intrachoroidal cavitation, macular intrachoroidal cavitation, focal choroidal excavation, torpedo maculopathy, choroidal neovascularization
Key Points
Intrachoroidal cavitation secondary to pathologic myopia is associated with axial elongation and structural weakening of the involved sclera, RPE, and choroid, notably in the macula and peripapillary area near the myopic conus.
Macular intrachoroidal cavitation has also been reported in cases of north carolina macular dystrophy – in contrast, underlying mechanisms for ICC formation are thought to arise in utero.
Focal choroidal excavation, often an incidental finding on OCT, has gained attention due to its elusive pathophysiology and associated pachychoroid disorders.
Torpedo maculopathy, a congenital lesion that exhibits choroidal cavitation and thereby compromised RPE, can also exhibit vulnerability to the vascular effects of aging.
The relevance of these clinical findings becomes apparent when considering underlying pathophysiology, potential complications that may be vision threatening, and early detection/treatment of said complications.
Introduction
In primates, the choroid receives 85–90% of blood flow to the eye and delivers many times more blood to the eye than the retinal vasculature.1,2 The choroid functions to nourish the retina, influences scleral growth, and maintain the retina’s optical position by virtue of thickness changes.3 The uveal tract is the vascular tunic of the eye, positioned between the sclera and the neuroepithelium. This tract is made of the iris, ciliary body and the choroid. The choroid extends anteriorly from the ora serrata posteriorly to the optic nerve. Spanning from the posterior of the retina to the sclera, the choroid is made of five layers. The basement membrane of the retinal pigment epithelium is Bruch’s membrane. Posterior to this layer are the remaining choroidal layers, in order from nearest to the retina to the furthest: the choriocapillaris, Haller’s layer, Sattler’s layer, and the suprachoroid (Figure 1).3
The choroid receives its blood supply from the 2–3 ciliary branches of the ophthalmic artery which then further supply the 10–20 short posterior ciliary arteries.4,5 It is drained by the vortex veins. Two thirds of the oxygen consumed by the retina in the light-adapted state is delivered by the choroid.2 A high rate of choroidal blood flow is not only necessary for maintaining high oxygen tension but also maintaining homeostatic temperature in a highly metabolic tissue.6
The choroidal vasculature are innervated by sympathetic fibers emanating from the superior cervical ganglion.7 Parasympathetic innervation is achieved by the facial nerve and pterygopalatine ganglion.8 The choroid is typically thickest subfoveally with an average thickness of 272 microns in a healthy middle-aged adult and decreases gradually with age, reaching around 80um by age 90.9 The choroid plays a role in retinal homeostasis and secretes signal molecules and growth factors including vascular endothelial growth factor (VEGF), basic fibroblast growth factor and hepatocyte growth factor.3,10
Innovations in ophthalmic imaging including optical coherence tomography have vastly improved the visualization of pathology of the choroid. These innovations have allowed the further elucidation of known entities and led to the discovery of new choroidal conditions. In the text that follows, we review cavitary disorders of the choroid and the pathophysiologic mechanisms that underpin them. The connections made between the cavitary disorders in this review serve as one of the many of the initial building blocks in understanding common complications and treatment modalities.
Intrachoroidal Cavitation (ICC)
Peripapillary Intrachoroidal Cavitation
In 2003, Freund et al first published the report of a localized peripapillary retinal pigment epithelial detachment (PED) in patients with high myopia.11 Subsequent studies and more resolute time-domain OCT imaging by Toranzo et al12 revealed such lesions to be comprised of choroidal tissue cavitation and a flat retinal pigment epithelium (RPE) profile, not consistent with a dome-shaped elevations seen in a PED. The entity was accordingly renamed peripapillary intrachoroidal cavitation (pICC).11
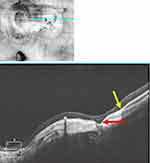
Further advancements in spectral-domain OCT (SD-OCT) imaging in 2008 demonstrated there to be an associated choroidal thickening, with or without cavitation, in these peripapillary lesions.13 Hence the name “peripapillary choroidal thickening and cavitation” was proposed by Freund et al in 2011 to be a more accurate description of the lesion.14 In 2012, Spaide et al, using SS-OCT, significantly improved visualization of the scleral-choroidal junction which demonstrated a characteristic bowing of the sclera beneath the choroidal cavitation. This implied that the cavitation is an effect of posterior excursion of the sclera rather than anterior displacement of the retina and RPE.15
With fundus examination, the characteristic finding of pICC is a well-circumscribed, peripapillary lobular, yellow-orange, elevation or thickening.11,15-17 These lesions are typically located inferior at the border of myopic conus but can occur less frequently temporal.14,18 In some cases pICC may be indistinguishable or resemble other retinal findings during fundus exam; therefore, OCT raster scans of the peripapillary region in highly myopic patients are advised so as to detect the presence of pICC in suspected cases.16,17
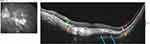
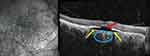
The appearance is similar to a PED and they were initially described as such.7,19 However, the lesion is not typically elevated as in a PED and does not display the fluorescein angiographic (FA) findings of early localized hyperfluorescence with progressive dye pooling.12 Fluorescein angiography of ICC lesions demonstrates early hypofluorescence with progressive staining of the peripapillary lesion without dye pooling. Indocyanine green angiography (ICGA) shows the area of peripapillary cavitation is hypofluorescent throughout the entire sequence.12,21 Literature on OCT-angiography (OCT-A) use in pICC is limited. A case report by Mazzaferro et al on a 67-year-old female with pathologic myopia and pICC demonstrated a hyporeflective area corresponding to the cavitation on OCTA which may indicate a sluggish choriocapillaris vasculature or a complete absence of choroid.21
Peripapillary ICC is predominantly found in pathologic or high myopia but may occur in lower degrees of myopia and rarely in hyperopia.11,16,17,19 Pathologic myopia is defined as a refractive error ≥-6.00 diopters and an axial length >26 mm.22 Complications arise secondary to excessive axial elongation of the globe and thinning of the sclera, choroid and RPE.23–25 These changes, along with advancing age, cause a weakening of the peripapillary tissue in the area of the myopic conus where choroid loss and cavitation occur.12,17,26
Peripapillary ICC is rare in younger patients. Shimada et al reported only 1 of 31 cases of pICC were younger than 30 years of age.26 Yeh et al found 79 of the 83 eyes with pICC were over thirty years of age.17 In addition, those patients with low refractive errors and pICC were significantly older than those with high myopia, underscoring the combined effects of time and structural weakness.17 Although pICC has been reported in patients with mild refractive error, these patients commonly exhibit findings typical of high myopia such as peripapillary atrophy, tilted disc and choroidal thinning.27,28
The mechanism of the actual formation of the cavity following structural weakening has been debated. Forte et al have suggested the posterior scleral bowing and deep excavation of the myopic conus create “the consequent impossibility for the retina-RPE to follow this steep fall,” thus detaching from the choroid and returning to a more natural anatomic level and in the process creates the area of cavitation.29 Toranzo hypothesized that staphyloma progression breaks the collagenous limiting tissue of Elschnig (and/or Jacoby15), which serves as the connection between the choroid and the optic nerve.30 The resultant retraction of the choroid from the nerve margin accounts for the gradual enlargement of the yellow-orange area of cavitation over time.12
Some studies have suggested that a cleft forms that connects the pICC and the vitreous cavity15,16,31,32 During peripapillary staphyloma progression and stretching of the retina, a cleft opens but the adherence of the retina and RPE at the conal margin prevents fluid from entering the subretinal space; instead, the fluid forms a cavity within the choroid.16 This is supported by observations of Spaide et al, who used SS-OCT to evaluate pICC.15 They conjectured that the sclera, thinned and weakened in the area of conus, bows over time in response to intraocular pressure creating space for cavitation to occur.15 Age is an associated risk factor for pICC and it has been suggested that poor fluid reabsorption associated with an aged retina, combined with gravity, produces the characteristic cavity at the inferior margin of the conus in pICC.17
Peripapillary ICC lesions can be stable and relatively benign without visual compromise.11,16 Complications that can arise include glaucomatous-like defects, macular detachment and retinoschisis17,19,20,26-29,32 Shimada et al found that 71% (22/31 eyes) of patients with pICC had glaucomatous visual field defects on Goldmann visual field perimetry.19 These defects, such as nasal step and arcuate defects, have been described and attributed in part to the RNFL damage occurring at the edge of conus excavation.29
Retinoschisis and macular detachment occur in patients with pICC and is thought to result when the choroidal cavity extends and connects to the vitreous. Cavitary fluid then gains access to the subretinal or intraretinal space.28,31,32 Vitrectomy has shown benefit when used in the treatment of myopic macular retinoschisis, with and without detachment.33 Employing pars plana vitrectomy in pICC has been shown to eliminate the choroidal-vitreal cavity communication and resolve the macular detachment and schisis.28,32
Macular Intrachoroidal Cavitation
Choroidal cavitation that occurs in the macula is typically associated with posterior staphyloma and pathologic myopia. Pathologic myopia is associated with many structural causes of macular visual impairment, most notably: myopic choroidal neovascularization (CNV), myopic macular retinoschisis, and myopic chorioretinal atrophy. The myopic chorioretinal atrophies can be subdivided into diffuse chorioretinal atrophy (DCA) and patchy chorioretinal atrophy (PCA).34–37
Diffuse chorioretinal atrophy is the milder form with a yellowish appearance and ill-defined borders. A thin choroid is interposed between the sclera and retina. DCA is distinguished from the more severe PCA, which shows sharply defined borders and white atrophy. Involved zones in PCA lack any choroid in front of the sclera accounting for the scleral coloration. Vision is severely impacted in PCA due to the absence of underlying choroid and, consequently, the degeneration of overlying RPE and photoreceptors.36 The progression and expansion of these lesions is the leading cause of central vision loss in pathologic myopia.37 Macular ICC has been shown to occur in 55.4% of patients with PCA. It occurs as an excavation zone of relatively thicker choroid immediately surrounding the PCA.34
The pathophysiology appears to be similar to pICC.36 Ohno-Matsui et al, in 2012, examined 56 eyes of 44 patients with PCA in pathologic myopia. Using SS-OCT, 31 of 56 eyes (55.4%) with PCA showed the characteristic posterior bowing of the sclera similar to pICC. This was not apparent in any of the 113 highly myopic eyes without PCA.34 The cavitary lesions tend to occur on the slope of the staphyloma. These focal areas, devoid of choriocapillaris and RPE (along with the loss of overlying photoreceptors), are thought to weaken the structural integrity of the eye wall.36 Intraocular pressure applied constantly and uniformly over time causes further weakening and thinning resulting cavitation. In addition, a direct communication between the mICC lesion and vitreal cavity was noted in 3 of 31 (9.7%) eyes with mICC.34
The characteristic posterior scleral bowing distinguishes PCA from focal choroidal cavitation in which scleral bowing is absent.36 We have also observed cases in which outward scleral bowing occurs in the absence of choroid (ie, within the patch of PCA) causing a cavitation separating the overlying retina from the underlying sclera (Figure 2). Not all outward bowing of the sclera in pathologic myopia is associated with mICC. For example, Figure 3 exhibits a case in which outward bowing of the sclera concomitant with scleral thinning, but instead of mICC, there is mild outer nuclear layer retinoschisis and localized choroidal thickening without mICC.
North Carolina macular dystrophy (NCMD) is an autosomal dominant macular dystrophy originally described in a multigenerational Irish family located in the mountains of North Carolina.35,38,39,48 Schoenberger and Agarwal published 2 cases of NCMD with mICC.35 Both patients had NCMD associated with posterior staphyloma and visual acuity in the 20/40–20/60 range. Imaging with EDI-OCT shows the characteristic bowing of the sclera that is associated with intrachoroidal cavitation. The cavitations appear nonreflective and, similar to mICC, tend to be along the slope of the focal posterior staphyloma in NCMD. The authors also describe a grayish-yellow color of NCMD-related mICC versus the yellow-orange color seen in myopic-related lesions.35
Mechanisms for ICC formation in NCMD are thought to arise in utero. NCMD lesions have been seen in patients as young as 2 months of age.40,47 In contrast, mICC development in pathologic myopia involves a progression in axial length and a weakening of the sclera over time.
Focal Choroidal Excavation
Focal choroidal excavation (FCE) is an area of focal choroidal thinning and concavity with near-normal overlying retina, in the absence of scleral ectasia, posterior staphyloma, and choroidal or PED.42–45 Wakabayashi et al described 2 variations of FCE, which were later termed the “conforming” type and the “nonconforming” type by Margolis et al.41 The determining factor was whether the outer retinal layers in FCE conformed to the choroidal depression without disruption. If the layers followed the depression without separation it was designated the conforming type (Figure 4). The nonconforming type demonstrated a localized neurosensory detachment or separation between the photoreceptor tips and underlying RPE with subretinal fluid (SRF) presumably occupying the space (Figure 5).43 While generally stable, FCE can change from conforming to nonconforming and vice versa over time.42,46 There are published reports of the transition of FCE in cases with47 and without48 central serous chorioretinopathy (CSC). Focal choroidal excavation lesions associated with serous retinal detachment in CSC or CNV have been reported to convert to conforming following resolution of SRF.42,49-51 Additionally, a nonconforming to conforming conversion occurred in FCE lesions with CNV treated with anti-VEGF therapy.52
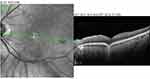 |
Figure 4 SD-OCT of conforming FCE in the macula shows the outer retinal layers in apposition to the area of focal choroidal concavity. |
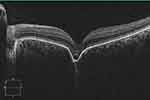 |
Figure 5 SD-OCT of non-conforming FCE in the macula. In contrast to the conforming type FCE in Figure 4, non-conforming shows a separation of photoreceptor tips from RPE. |
Despite typically occurring in the fovea or perifovea, FCE is associated with good visual acuity and a stable clinical course. The finding may be incidental on routine examination.46,53,54 Subjective disturbances such as metamorphopsia have been reported even in eyes with normal vision.43 On examination of the fundus, small FCE lesions may not be apparent. Larger lesions show non-specific pigmentary disruption and with choroidal vasculature intact the lesion may appear reddish, yellowish, or orange.55
Focal choroidal excavation is easily recognized on OCT imaging with a characteristic focal intrachoroidal concavity or downward deflection of the Bruch’s membrane-RPE-choriocapillaris complex line.44 The inner retinal layers appear relatively intact despite deformation.42,43,54 There is a thickening of the outer nuclear layer which is thought to be from the traction between the excavation dip and vascular system of the inner retina; creating strain on the RPE-photoreceptor interface.54
Subretinal fluid is thought to occupy the subretinal space in nonconforming FCE but vitelliform material occasionally accumulates.56 The presence of an unusually high reflective choroidal material beneath the FCE is thought to represent scar formation and this scarring subsequently contracts leading to traction on RPE and FCE formation.49,51 Reduced vision and symptoms of metamorphopsia are more commonly associated with nonconforming FCE.49,57,58 The SRF in nonconforming FCE has been hypothesized to arise from traction on the photoreceptors, but OCT evidence of traction is found in a minority of cases.53
The choroid is thin in the area of FCE, but the scleral contour and thickness are intact and normal.49 A staphylomatous excavation of the sclerochoroidal junction needs to be excluded via OCT to diagnose FCE and avoid confusion with other choroidal cavitary disorders.45,61 Immediately beneath FCE, the choroid may be hyperreflective on OCT, with a paucity of vessels compared to the adjacent choroid.59
Although initially described as a finding in otherwise healthy eyes, FCE has been shown to accompany a host of choroidal pathologies including CSC,51,60,61 CNV,52,62 polypoidal choroidal vasculopathy,63–66 choroidal osteoma,67 multiple evanescent white dot syndrome (MEWDS),68–70 multifocal choroiditis,71 Epstein Barr virus infection,69 and Vogt-Koyanagi-Harada disease.72 Retinal disorders have also been associated with FCE such as age-related macular degeneration (AMD),63,73 Stargardt Disease,74 Best Disease,75,76 pattern dystrophy,74 cone dystrophy,77 foveoschisis,78 retinal hamartoma,79,80 vitreomacular traction and retinal hole,81,82 and torpedo maculopathy.42 Speculation remains on the exact relationship of FCE to the above findings but associated weakening or mechanical changes in the RPE-Bruch’s membrane complex and consequences of disrupted choroidal vasculature are thought to be predisposing factors (Figure 6).53
A large retrospective study examined 4436 SD-OCT patient scans in an 18-month period and found 16 patients with FCE ranging from 31–78 years.53 There was no gender predilection and the refraction of both the involved eye and fellow eye was approximately −3 diopters (D). Macular pathology was present in four cases (25.0%) of eyes with FCE: one patient with macular pucker, one with parafoveal scarring, one associated with PCV and one with macular hemorrhage from lacquer crack formation in high myopia. The remaining 12 cases (75%) were not associated with concurrent macular pathology in the FCE eye. Among those 12 eyes with FCE but without macular pathology, fundus examination showed half of them had visible RPE changes while the rest were not discernible clinically and only seen by OCT imaging.53
The size of the lesion is useful clinically as it has been found to have bearing on the risk of concurrent macular pathology. A greater length, area and volume of an FCE lesion impart greater risk of associated macular pathology.53,54 Chen et al found that in multimodal imaging of FCE, the most sensitive modality was near-infrared autofluorescence (NIA), with the ability to better image subretinal and choroidal melanin loss in the form of hypoautofluorescence. A greater area of FCE on autofluorescent imaging was shown to be associated with maculopathy such as CNV and CSC. Greater volume (NIA area) x (Depth measured by SD-OCT) of FCE was associated with more subjective distortion.54
Fluorescein angiographic findings include varying degrees of hyper and hypo fluorescence associated with RPE window defects.55 Late phase hypofluorescence and abnormal staining on ICGA has been described, as well as, choroidal vascular hyperpermeability.51,55 These findings suggest a correlation of FCE and pachychoroid spectrum disease.83 Pachychoroid disease and its encompassing maladies of PCV, CSC and CNV have been related to FCE.59,63 The prevalence of FCE in eyes with CSC has been documented to be between 4.8% and 7.8%.47,51,60 However, the prevalence of CSC in eyes with FCE has been reported up to 24.4%, much higher than that of normal eyes.47
The pathologic mechanism in FCE remains unclear. Inflammatory,68,71 infectious,84 vascular,51,60 developmental79 and congenital42,57,85 processes have all been suggested. Age varies significantly in patients that have FCE lesions with mean ages falling in the 45–55 range.42,46,49 Park et al found a relatively low prevalence in FCE among children and adolescents with only 3 of 1697 eyes with FCE.86 Many studies have shown an association of myopia with FCE.50,51,63 It has been suggested that eyes with longer axial lengths have thinning of the choroid and weakening of RPE-Bruch’s complex resulting in FCE formation.63 Congenital or embryological developmental disorders have been proposed in light of the relatively intact outer retinal layers and choriocapillaris which are absent in chorioretinal scars.42,85 Kumano et al speculated an outward traction created by choroidal vascular abnormalities from embryonic developmental failure of the choroid.57 Other clinicians have theorized that choroidal abnormalities may lead to ischemic changes causing RPE atrophy.61
Viral-related inflammation such as that seen in MEWDS has been associated with the development of FCE.68,69 Hashimoto et al suggested that the pathophysiology is related to inflammation at the level of the outer retina causing disruption of the RPE-Bruch membrane complex. Following this disruption, there is an adherence of the outer retinal and choroidal tissues to each other through the ruptured RPE-Bruch membrane complex. Intraocular pressure then causes the adherent complex to protrude outward through the Bruch membrane gap creating a choroidal excavation.68 Similarly, the associated RPE and inner choroidal inflammation in multifocal choroiditis and panuveitis/punctate inner choroidopathy are associated with FCE formation.71
Vision is typically unaffected in FCE unless it is located near the fovea. Management involves excluding associated CNV, PCV and CSC. Choroidal neovascularization in FCE has shown to be amenable to anti-VEGF treatment.52,62,73,74 Both PCV and CSC with associated FCE have shown to respond to anti-VEGF injections and photodynamic therapy (PDT).58,64 Restoration of vision varies depending on residual retinal damage.58
Torpedo Maculopathy
Torpedo maculopathy (TM) is a congenital, solitary, spindle-shaped chorioretinal lesion in the temporal macula.87 It was originally classified as congenital hypertrophy of the RPE (CHRPE) by Gass in 198988 and revised to a “solitary hypopigmented nevus of the RPE” by Roseman and Gass in 1992.89 It was later given the name Torpedo Maculopathy by Daily in 1993 due to its torpedo-like appearance.90
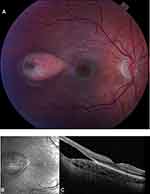
Fundus exam shows a hypopigmented, horizontally oriented, oval-shaped lesion in the temporal macula (Figure 7).91,92 Although typically unilateral, bilateral cases have been reported.93,94 TM is non-progressive and spares the fovea.91 There have been reports of growth and change in shape.95,96 While usually isolated, occasionally small, round satellite lesions temporal to the main lesion have been noted.88,92,97,98 Choroidal cavitation in TM differs from FCE in which lesions are not usually oval in shape, typically involve the fovea, and are associated with less outer retinal thinning and degeneration.42
Since TM lesions are rarely located in the fovea, the patient is asymptomatic and the condition is found incidentally on exam.87,99-101 Visual field defects are present and correlate with the size, shape and location of the lesion.87,92,102 Shirley et al estimated the prevalence of TM as 2 per 100,000 population, but suggested that this is an underestimate due to lack of symptoms.92 There are no known associated systemic conditions with TM.87,92
The TM lesion may have varying degrees of hyperpigmentation most commonly located at the temporal edge of the lesion.94,101 Geographic regions of hyperpigmentation within the central hypopigmented area are also possible.103 The orientation of the lesion is horizontal with a comparatively sharper pointed “head” directed toward the center of the macula.87,90 Two different variations have been described for the tail: rounded or frayed.101 The tails with the “rounded” configuration tend to be smooth while the “frayed” tail may be associated with hyper or hypopigmentation. Typical TM lesions measure around 2 disc diameters horizontally and 1 disc diameter vertically.101
Spectral domain OCT demonstrates a thin RPE layer with increased signal transmission to the choroid, occasional loss of the ellipsoid zone line, and no overlying neuroretinal changes.80,85,104,105 Wong et al classified two types of TM lesions based on the presence of outer retinal and/or choroidal cavitation. Type 1 was defined by having attenuation of the outer retinal structures without retinal or inner choroidal cavitation; Type 2 featured attenuation of the outer retinal structures with subretinal cavitation.97 Torpedo lesions that contain choroidal excavation were recently classified as type 3 TM by Tripathy et al and studied by Venkatesh et al.105–107
Cavitation appears as an optically empty space on OCT as a result of thinning or loss of the outer retinal layers or choroidal layers. The distinction between cavitation and a neurosensory detachment that occurs in some cases can be made on the basis of preservation of retinal layer thickness in a detachment and attenuation or loss in cavitation.97 It is speculated that the outer retinal excavation in the type 2 variant is created by loss of photoreceptors and/or RPE.87,103,105 However, thinning and disruption of the retinal layers is variable and may be due to the chronicity of the lesion or phenotypic differences.104 The cavitation can be distinguished from the SRF found in other causes such as CSC by the absence of ragged photoreceptors.96 Similarly, it is unclear if the choroidal excavation seen in type 3 TM is acquired or part of the underlying mechanism pathophysiology.107
While some authors have reported these lesions to be nonprogressive,87 others have shown mild progression over time.97 Wong et al hypothesized that the Type 1 subtype progressed to type 2 over time but reports of type 2 TM in patients as young as 5 years old cast doubt on that hypothesis.92
Fundus autofluorescence can vary in appearance but generally shows the large central area of the TM lesion to be hypoautofluorescent with surrounding edges demonstrating hyperautofluorescence.87,97,108,109 While some type 1 lesions demonstrate hypoautofluorescence, suggesting hypofunctioning RPE, all type 2 lesions are hypoautofluorescent. Wong et al have suggested that the hypoautofluorescence points to reduced interaction between RPE and outer segments that lead to a progressive loss of photoreceptor cells and outer retinal cavitation.97 Adaptive optics imaging has been used to evaluate the photoreceptor density in TM. Venkatesh et al studied a type 2 lesion that had decreased cone density. However, because the existing photoreceptors and external limiting membrane were intact, visual acuity was not compromised.110
Scant information exists on perimetric function over TM. Available literature suggests a present, yet inconsistent, correlation of perimetry with lesion type.97 Five of eight patients with a type 1 lesion were tested on a Humphrey field analyzer or microperimeter with only 1 patient demonstrating a scotoma.100–112 All patients with type 2 lesions had documented scotomas.96,101,113 The variability between visual field defect and lesion may be due to the size of the TM lesions falling beneath the detection sensitivity of Humphrey field testing.97 Although photoreceptor degeneration does not correlate with resulting acuity, it does seem to correlate with visual field defect.110
Wong et al proposed a hypothesis for TM formation in relation to the two types.97 Type 1 forms during a lack of melanin production within the torpedo lesion leading to an altered interaction between the RPE and photoreceptor outer segments. This disruption leads to attenuation of the ellipsoid zone and interdigitation zone. Because the RPE continues to metabolize the outer segments, retinal sensitivity is preserved as demonstrated on perimetry testing. However, photoreceptor loss over time leads to loss of the outer nuclear layer thickness and cavitation. They noted these cavitations can appear similar to outer retinal cavitations in macular telangiectasia type2111 and cone dystrophy or achromatopsia.97 Choroidal cavitation occurring in TM, Wong surmised, was a result of reduced VEGF production from compromised RPE leading to a thinning and loss of inner choroidal vessels identified in type 2 lesions.97
Shirley et al hypothesize that satellite lesions, which appear to be an extension of the main lesion, support the theory of a congenital defect of the RPE during fetal development. The phenotypic variation seen in TM, uniformity of location, orientation and general stability, adds weight to a congenital etiology.92,114,115 Shields et al also endorse an abnormality in fetal development (particularly the RPE) that occurs at a precise time.101 The fetal histology studies of Streeten116 showed that during early development there is a cone-shaped bulge temporal to the fovea in the vicinity of TM lesions. This bulge that occurs at 4–6 months of gestation lessens as development occurs between 6 and 8.5 months. A developmental disruption is speculated to occur at some point during the last 3 months and the variation in the time of occurrence accounts for different clinical TM phenotypes.92,101
Alternatively, disruption of the choroidal vasculature in fetal development has been put forth as a possible etiology. In 1997, Teitelbaum et al suggested an abnormal event in the embryologic development of the choroidal vasculature underlying the macula could cause a disruption of the RPE and give rise to the torpedo-like lesion.115 With the advent of OCT-A, the hypothesis focuses on a malformation of the RPE-choriocapillaris complex.91,108,114,117,118 Using OCT-A, Giannakaki-Zimmermann et al evaluated 4 patients with TM lesions. In this study, all lesions demonstrated normal superficial and deep vascular capillary beds within the TM lesion, and choriocapillaris with on average 26% less signal when compared with healthy choriocapillaris in the same region.114 Additional studies have revealed a reduced capillary density within the choriocapillaris using OCT-A.101,105 Some reports have shown normal91,118 large caliber vessels of Sattler’s and Haller’s layer while others show them to be mildly attenuated.117 Giannakaki-Zimmermann et al suggested since the TM lesion extends into the choriocapillaris, congenital choroidal malformation is involved. However, it remains speculative whether the choroid is the primary site of malformation or related to a defect in the development of the RPE in view of the fact that the choroid needs an intact RPE to develop.105,114
Further ideas on TM development have been proposed. Pian et al suggested that the lesion is a paramacular coloboma with the defect at the horizontal raphe of the nerve fiber layer.99 Trevino et al also endorse the idea of a colobomatous etiology. He cites the diagnostic criteria of Mann for macular coloboma and TM intersect when there is associated choroidal excavation in TM.102 Golchet et al suggest a malformation of the emissary canal of the long posterior ciliary artery and nerve given the anatomical proximity with TM lesions.87 The temporal long posterior ciliary artery joins the long posterior ciliary nerve, which together penetrates through the sclera and runs temporarily towards the anterior of the eye through the suprachoroidal space and choroid. They hypothesize that the modification of the sclera during the embryological process allowing the nerve and artery to pass may have an effect on the developing choroid and RPE; thus leading to TM formation.87
Choroidal neovascularization has been shown to occur in TM. Shirley et al documented a case of a patient with TM-related CNV which responded well to a single injection of anti-VEGF (Ranibizumab).92 However, the authors stated that given the structural changes present, CNV remains an ongoing concern and should be monitored accordingly.
The choroidal cavitary disorders discussed in this review are summarized in Table 1.
 |
Table 1 Choroidal Cavitary Disorders Summary |
Conclusion
A true visualization and a better understanding of choroidal cavitary disorders have eluded the medical community until recently with the introduction of optical coherence tomography. Over time as these conditions are encountered, tracked, and imaged with more sophisticated techniques we will gain much more knowledge about the potential impact on the patient and strategies to mitigate associated pathology. The connection between these choroidal cavitary disorders is difficult to establish as most hypotheses to explain pathogenesis cite poorly documented embryological mechanisms and will be difficult to test in their present level of articulation. Although these disorders can be asymptomatic, and at times even non-progressive, the possible complications are sight threatening and patients benefit from prompt diagnosis and treatment. To conclude, we have highlighted the known similarities and differences in an attempt to establish a preliminary connection to help aid in the future strategies to treat, and perhaps prevent, sight threatening complications from seemingly benign findings.
Acknowledgment
We acknowledge Dr. Douglas Adams for the image courtesy of Figure 5 from his clinic.
Disclosure
David J. Browning, MD, PhD, reports DCRC network, Regeneron, Novartis, Alcon – grant support, Zeiss – stock ownership, and Springer Inc. - book royalties. The authors report no other potential conflicts of interest for this work.
References
1. Hill DW. Measurement of retinal blood flow. Trans Ophthalmol Soc U K. 1976;96:199–201.
2. Alm A, Bill A. Ocular and optic nerve blood flow at normal and increased intraocular pressures in monkeys (Macaca irus): a study with radioactively labelled microspheres including flow determinations in brain and some other tissues. Exp Eye Res. 1973;15:15–29. doi:10.1016/0014-4835(73)90185-1
3. Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29:144–168. doi:10.1016/j.preteyeres.2009.12.002
4. Weiter JJ, Ernest JT. Anatomy of the choroidal vasculature. Am J Ophthalmol. 1974;78:583–590. doi:10.1016/S0002-9394(14)76294-4
5. Hayreh SS. Segmental nature of the choroidal vasculature. Br J Ophthalmol. 1975;59:631–648. doi:10.1136/bjo.59.11.631
6. Parver LM, Auker C, Carpenter DO. Choroidal blood flow as a heat dissipating mechanism in the macula. Am J Ophthalmol. 1980;89:641–646. doi:10.1016/0002-9394(80)90280-9
7. Ehinger B. Adrenergic nerves to the eye and to related structures in man and in the cynomolgus monkey (Macaca irus). Invest Ophthalmol. 1966;5:42–52.
8. Ruskell GL. Facial parasympathetic innervation of the choroidal blood vessels in monkeys. Exp Eye Res. 1971;12:166–172. doi:10.1016/0014-4835(71)90085-6
9. Manjunath V, Taha M, Fujimoto JG, Duker JS. Choroidal thickness in normal eyes measured using Cirrus HD optical coherence tomography. Am J Ophthalmol. 2010;150:325–329 e321. doi:10.1016/j.ajo.2010.04.018
10. Hu W, Criswell MH, Fong SL, et al. Differences in the temporal expression of regulatory growth factors during choroidal neovascular development. Exp Eye Res. 2009;88:79–91. doi:10.1016/j.exer.2008.10.014
11. Freund KB, Ciardella AP, Yannuzzi LA, et al. Peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2003;121:197–204. doi:10.1001/archopht.121.2.197
12. Toranzo J, Cohen SY, Erginay A, Gaudric A. Peripapillary intrachoroidal cavitation in myopia. Am J Ophthalmol. 2005;140:731–732. doi:10.1016/j.ajo.2005.03.063
13. Spaide RF, Koizumi H, Pozzoni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146:496–500. doi:10.1016/j.ajo.2008.05.032
14. Freund KB, Mukkamala SK, Cooney MJ. Peripapillary choroidal thickening and cavitation. Arch Ophthalmol. 2011;129:1096–1097. doi:10.1001/archophthalmol.2011.208
15. Spaide RF, Akiba M, Ohno-Matsui K. Evaluation of peripapillary intrachoroidal cavitation with swept source and enhanced depth imaging optical coherence tomography. Retina. 2012;32:1037–1044. doi:10.1097/IAE.0b013e318242b9c0
16. Wei YH, Yang CM, Chen MS, Shih YF, Ho TC. Peripapillary intrachoroidal cavitation in high myopia: reappraisal. Eye (Lond). 2009;23:141–144. doi:10.1038/sj.eye.6702961
17. Yeh SI, Chang WC, Wu CH, et al. Characteristics of peripapillary choroidal cavitation detected by optical coherence tomography. Ophthalmology. 2013;120:544–552. doi:10.1016/j.ophtha.2012.08.028
18. Ohno-Matsui K, Shimada N, Akiba M, Moriyama M, Ishibashi T, Tokoro T. Characteristics of intrachoroidal cavitation located temporal to optic disc in highly myopic eyes. Eye (Lond). 2013;27:630–638. doi:10.1038/eye.2013.16
19. Shimada N, Ohno-Matsui K, Yoshida T, et al. Characteristics of peripapillary detachment in pathologic myopia. Arch Ophthalmol. 2006;124:46–52. doi:10.1001/archopht.124.1.46
20. Azar G, Leze R, Affortit-Demoge A, Faure C. Peripapillary intrachoroidal cavitation in myopia evaluated with multimodal imaging comprising “en-face” technique. Case Rep Ophthalmol Med. 2015;2015:890876.
21. Mazzaferro A, Carnevali A, Zucchiatti I, Querques L, Bandello F, Querques G. Optical coherence tomography angiography features of intrachoroidal peripapillary cavitation. Eur J Ophthalmol. 2017;27:e32–e34. doi:10.5301/ejo.5000901
22. Curtin BJ. Physiologic vs pathologic myopia: genetics vs environment. Ophthalmology. 1979;86:681–691. doi:10.1016/S0161-6420(79)35466-5
23. Jonas JB, Xu L. Histological changes of high axial myopia. Eye (Lond). 2014;28:113–117. doi:10.1038/eye.2013.223
24. Grossniklaus HE, Green WR. Pathologic findings in pathologic myopia. Retina. 1992;12:127–133. doi:10.1097/00006982-199212020-00009
25. Noble KG, Carr RE. Pathologic myopia. Ophthalmology. 1982;89:1099–1100. doi:10.1016/S0161-6420(82)34677-1
26. Shimada N, Ohno-Matsui K, Nishimuta A, Tokoro T, Mochizuki M. Peripapillary changes detected by optical coherence tomography in eyes with high myopia. Ophthalmology. 2007;114:2070–2076. doi:10.1016/j.ophtha.2007.01.016
27. Akimoto M, Akagi T, Okazaki K, Chihara E. Recurrent macular detachment and retinoschisis associated with intrachoroidal cavitation in a normal eye. Case Rep Ophthalmol. 2012;3:169–174. doi:10.1159/000339292
28. Ando Y, Inoue M, Ohno-Matsui K, Kusumi Y, Iida T, Hirakata A. Macular detachment associated with intrachoroidal cavitation in nonpathological myopic eyes. Retina. 2015;35:1943–1950. doi:10.1097/IAE.0000000000000575
29. Forte R, Pascotto F, Cennamo G, de Crecchio G. Evaluation of peripapillary detachment in pathologic myopia with en face optical coherence tomography. Eye (Lond). 2008;22:158–161. doi:10.1038/sj.eye.6702666
30. Anderson DR. Ultrastructure of human and monkey lamina cribrosa and optic nerve head. Arch Ophthalmol. 1969;82:800–814. doi:10.1001/archopht.1969.00990020792015
31. Shimada N, Ohno-Matsui K, Iwanaga Y, Tokoro T, Mochizuki M. Macular retinal detachment associated with peripapillary detachment in pathologic myopia. Int Ophthalmol. 2009;29:99–102. doi:10.1007/s10792-007-9174-2
32. Yoshizawa C, Saito W, Noda K, Ishida S. Pars plana vitrectomy for macular schisis associated with peripapillary intrachoroidal cavitation. Ophthalmic Surg Lasers Imaging Retina. 2014;45:350–353. doi:10.3928/23258160-20140617-03
33. Gaucher D, Haouchine B, Tadayoni R, et al. Long-term follow-up of high myopic foveoschisis: natural course and surgical outcome. Am J Ophthalmol. 2007;143:455–462. doi:10.1016/j.ajo.2006.10.053
34. Ohno-Matsui K, Akiba M, Moriyama M, Ishibashi T, Hirakata A, Tokoro T. Intrachoroidal cavitation in macular area of eyes with pathologic myopia. Am J Ophthalmol. 2012;154:382–393. doi:10.1016/j.ajo.2012.02.010
35. Schoenberger SD, Agarwal A. Intrachoroidal cavitation in North Carolina macular dystrophy. JAMA Ophthalmol. 2013;131:1073–1076. doi:10.1001/jamaophthalmol.2013.1598
36. Venkatesh R, Jain K, Aseem A, Kumar S, Yadav NK. Intrachoroidal cavitation in myopic eyes. Int Ophthalmol. 2020;40:31–41. doi:10.1007/s10792-019-01146-0
37. Hayashi K, Ohno-Matsui K, Shimada N, et al. Long-term pattern of progression of myopic maculopathy: a natural history study. Ophthalmology. 2010;117:1595. doi:10.1016/j.ophtha.2009.11.003
38. Lefler WH, Wadsworth JA, Sidbury JB. Hereditary macular degeneration and amino-aciduria. Am J Ophthalmol. 1971;71:224–230. doi:10.1016/0002-9394(71)90394-1
39. Frank HR, Landers MB, Williams RJ, Sidbury JB. A new dominant progressive foveal dystrophy. Am J Ophthalmol. 1974;78:903–916. doi:10.1016/0002-9394(74)90800-9
40. Khurana RN, Sun X, Pearson E, et al. A reappraisal of the clinical spectrum of North Carolina macular dystrophy. Ophthalmology. 2009;116:1976–1983. doi:10.1016/j.ophtha.2009.03.028
41. Kiernan DF, Shah RJ, Hariprasad SM, et al. Thirty-Year follow-up of an African American family with macular dystrophy of the retina, locus 1 (North Carolina macular dystrophy). Ophthalmology. 2011;118:1435–1443. doi:10.1016/j.ophtha.2010.10.041
42. Margolis R, Mukkamala SK, Jampol LM, et al. The expanded spectrum of focal choroidal excavation. Arch Ophthalmol. 2011;129:1320–1325. doi:10.1001/archophthalmol.2011.148
43. Wakabayashi Y, Nishimura A, Higashide T, Ijiri S, Sugiyama K. Unilateral choroidal excavation in the macula detected by spectral-domain optical coherence tomography. Acta Ophthalmol. 2010;88:e87–91. doi:10.1111/j.1755-3768.2010.01895.x
44. Turgut B. The frequency of focal choroidal excavation detected by optical coherence tomography. Int J Ophthal Res. 2017;3:220–225. doi:10.17554/j.issn.2409-5680.2017.03.61
45. Jampol LM, Shankle J, Schroeder R, Tornambe P, Spaide RF, Hee MR. Diagnostic and therapeutic challenges. Retina. 2006;26:1072–1076. doi:10.1097/01.iae.0000248819.86737.a5
46. Obata R, Takahashi H, Ueta T, Yuda K, Kure K, Yanagi Y. Tomographic and angiographic characteristics of eyes with macular focal choroidal excavation. Retina. 2013;33:1201–1210. doi:10.1097/IAE.0b013e31827b6452
47. Wang Y, Chen ZQ, Wang W, Fang XY. Multimodal imaging evaluations of focal choroidal excavations in eyes with central serous chorioretinopathy. J Ophthalmol. 2016;2016:7073083. doi:10.1155/2016/7073083
48. Kou S, Rett D. Various SD-OCT features of focal choroidal excavations. Optom Vis Sci. 2015;92:S59–66. doi:10.1097/OPX.0000000000000530
49. Lim FP, Loh BK, Cheung CM, Lim LS, Chan CM, Wong DW. Evaluation of focal choroidal excavation in the macula using swept-source optical coherence tomography. Eye (Lond). 2014;28:1088–1094. doi:10.1038/eye.2014.78
50. Lee CS, Woo SJ, Kim YK, et al. Clinical and spectral-domain optical coherence tomography findings in patients with focal choroidal excavation. Ophthalmology. 2014;121:1029–1035. doi:10.1016/j.ophtha.2013.11.043
51. Ellabban AA, Tsujikawa A, Ooto S, et al. Focal choroidal excavation in eyes with central serous chorioretinopathy. Am J Ophthalmol. 2013;156:673–683. doi:10.1016/j.ajo.2013.05.010
52. Xu H, Zeng F, Shi D, Sun X, Chen X, Bai Y. Focal choroidal excavation complicated by choroidal neovascularization. Ophthalmology. 2014;121:246–250. doi:10.1016/j.ophtha.2013.08.014
53. Chung CY, Li SH, Li KKW. Focal choroidal excavation-morphological features and clinical correlation. Eye (Lond). 2017;31:1373–1379. doi:10.1038/eye.2017.71
54. Chen YC, Chou YB, Lin CK, et al. Characterization and functional correlation of multiple imaging modalities with focal choroidal excavation. J Chin Med Assoc. 2018;81:487–495. doi:10.1016/j.jcma.2017.07.017
55. Shinojima A, Kawamura A, Mori R, Yuzawa M. Morphologic features of focal choroidal excavation on spectral domain optical coherence tomography with simultaneous angiography. Retina. 2014;34:1407–1414. doi:10.1097/IAE.0000000000000108
56. Or C, Forooghian F. Vitelliform focal choroidal excavation. Ophthalmic Surg Lasers Imaging Retina. 2014;45:e26–28. doi:10.3928/23258160-20140522-01
57. Kumano Y, Nagai H, Enaida H, Ueno A, Matsui T. Symptomatic and morphological differences between choroidal excavations. Optom Vis Sci. 2013;90:e110–118. doi:10.1097/OPX.0b013e31828736f3
58. Cebeci Z, Bayraktar S, Oray M, Kir N. Focal choroidal excavation. Turk J Ophthalmol. 2016;46:296–298. doi:10.4274/tjo.24445
59. Cheung CMG, Lee WK, Koizumi H, Dansingani K, Lai TYY, Freund KB. Pachychoroid disease. Eye (Lond). 2018;33:14–33.
60. Luk FO, Fok AC, Lee A, Liu AT, Lai TY. Medscape. Focal choroidal excavation in patients with central serous chorioretinopathy. Eye (Lond). 2015;29:453–459. doi:10.1038/eye.2015.31
61. Suzuki M, Gomi F, Hara C, Sawa M, Nishida K. Characteristics of central serous chorioretinopathy complicated by focal choroidal excavation. Retina. 2014;34:1216–1222. doi:10.1097/IAE.0000000000000045
62. Lee JH, Lee WK. Choroidal neovascularization associated with focal choroidal excavation. Am J Ophthalmol. 2014;157:710–718 e711. doi:10.1016/j.ajo.2013.12.011
63. Lim FP, Wong CW, Loh BK, et al. Prevalence and clinical correlates of focal choroidal excavation in eyes with age-related macular degeneration, polypoidal choroidal vasculopathy and central serous chorioretinopathy. Br J Ophthalmol. 2016;100:918–923. doi:10.1136/bjophthalmol-2015-307055
64. Kobayashi W, Abe T, Tamai H, Nakazawa T. Choroidal excavation with polypoidal choroidal vasculopathy: a case report. Clin Ophthalmol. 2012;6:1373–1376. doi:10.2147/OPTH.S33879
65. Say EA, Jani PD, Appenzeller MF, Houghton OM. Focal choroidal excavation associated with polypoidal choroidal vasculopathy. Ophthalmic Surg Lasers Imaging Retina. 2013;44:409–411. doi:10.3928/23258160-20130715-12
66. Okubo A, Unoki K, Sameshima M, Sakamoto T. Focal choroidal excavation with changes in shape and alterations of inner retina during long follow-up in an eye with polypoidal choroidal vasculopathy. Clin Exp Optom. 2015;98:478–480. doi:10.1111/cxo.12265
67. Chawla R, Azad SV, Takkar B, Sharma A, Kashyap B. Nonconforming deep focal choroidal excavation in a patient with choroidal osteoma: a diagnostic dilemma. Ophthalmic Surg Lasers Imaging Retina. 2017;48:944–947. doi:10.3928/23258160-20171030-12
68. Hashimoto Y, Saito W, Noda K, Ishida S. Acquired focal choroidal excavation associated with multiple evanescent white dot syndrome: observations at onset and a pathogenic hypothesis. BMC Ophthalmol. 2014;14:135. doi:10.1186/1471-2415-14-135
69. Jabbarpoor Bonyadi MH, Hassanpour K, Soheilian M. Recurrent focal choroidal excavation following multiple evanescent white dot syndrome (MEWDS) associated with acute idiopathic blind spot enlargement. Int Ophthalmol. 2018;38:815–821. doi:10.1007/s10792-017-0511-9
70. Matsubara H, Uchiyama E, Suzuki K, Matsuda Y, Kondo M. A case of focal choroidal excavation development associated with multiple evanescent white dot syndrome. Case Rep Ophthalmol. 2018;9:388–394. doi:10.1159/000492747
71. Kim H, Woo SJ, Kim YK, Lee SC, Lee CS. Focal choroidal excavation in multifocal choroiditis and punctate inner choroidopathy. Ophthalmology. 2015;122:1534–1535. doi:10.1016/j.ophtha.2015.01.012
72. Nishikawa Y, Fujinami K, Watanabe K, Noda T, Tsunoda K, Akiyama K. Clinical course of focal choroidal excavation in Vogt-Koyanagi-Harada disease. Clin Ophthalmol. 2014;8:2461–2465. doi:10.2147/OPTH.S75558
73. Kuroda Y, Tsujikawa A, Ooto S, et al. Association of focal choroidal excavation with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:6046–6054. doi:10.1167/iovs.14-14723
74. Battaglia Parodi M, Casalino G, Iacono P, Introini U, Adamyan T, Bandello F. The expanding clinical spectrum of choroidal excavation in macular dystrophies. Retina. 2017;38:2030–2034.
75. Esfahani MR, Esfahani HR, Mahmoudi A, Johari MK, Hemati K. Focal choroidal excavation in best vitelliform macular dystrophy: case report. J Clin Diagn Res. 2015;9:ND01–02. doi:10.7860/JCDR/2015/12861.5993
76. Kumar V, Chatra K. Fibrotic pillar leads to focal choroidal excavation in best vitelliform dystrophy. Graefes Arch Clin Exp Ophthalmol. 2018;256(11):2083–2087. doi:10.1007/s00417-018-4120-8
77. Roy R, Saurabh K, Chandrasekharan DP, Sharma P, Vyas C. Bilateral focal choroidal excavation in cone dystrophy. Clin Exp Optom. 2016;99:198–199. doi:10.1111/cxo.12309
78. Hsu CKLC, Chen JT, Chang YH, Chang Y-H. Foveoschisis with focal choroidal excavation. Taiwan J Ophthalmol. 2014;4:189–190. doi:10.1016/j.tjo.2013.12.006
79. Sivalingam MD, Say EA, Shields CL. Evolution of focal choroidal excavation underlying combined hamartoma of the retina and retinal pigment epithelium in a child. J AAPOS. 2015;19:379–381. doi:10.1016/j.jaapos.2015.03.016
80. Wang Q, Liu JH, Jiao X, Wei WB, Lu H. Case report: focal choroidal excavation underlying combined hamartoma of the retina and retinal pigment epithelium. Optom Vis Sci. 2019;96:233–235. doi:10.1097/OPX.0000000000001348
81. Fayyad OF, Al-Hashimi MR, Fayyad FT. A case of full-thickness macular hole in eye with focal choroidal excavation. Retin Cases Brief Rep. 2018;12:291–293. doi:10.1097/ICB.0000000000000497
82. Fukumoto M, Morishita S, Okuda Y, et al. A case of a vitreomacular traction-associated macular microhole in an eye with focal choroidal excavation. Case Rep Ophthalmol. 2015;6:71–75. doi:10.1159/000377667
83. Guo J, Zhong L, Jiang C, et al. Clinical and optic coherence tomography findings of focal choroidal excavation in Chinese patients. BMC Ophthalmol. 2014;14:63. doi:10.1186/1471-2415-14-63
84. Savastano MC, Rispoli M, Di Antonio L, Mastropasqua L, Lumbroso B. Observed positive correlation between Epstein-Barr virus infection and focal choroidal excavation. Int Ophthalmol. 2014;34:927–932. doi:10.1007/s10792-013-9874-8
85. Liu GH, Lin B, Sun XQ, et al. Focal choroidal excavation: a preliminary interpretation based on clinic and review. Int J Ophthalmol. 2015;8:513–521. doi:10.3980/j.issn.2222-3959.2015.03.14
86. Park KA, Oh SY. The absence of focal choroidal excavation in children and adolescents without retinal or choroidal disorders or ocular trauma. Eye (Lond). 2015;29:841–842. doi:10.1038/eye.2015.6
87. Golchet PR, Jampol LM, Mathura JR, Daily MJ. Torpedo maculopathy. Br J Ophthalmol. 2010;94:302–306. doi:10.1136/bjo.2009.162669
88. Gass JD. Focal congenital anomalies of the retinal pigment epithelium. Eye (Lond). 1989;3(Pt 1):1–18. doi:10.1038/eye.1989.2
89. Roseman RL, Gass JD. Solitary hypopigmented nevus of the retinal pigment epithelium in the macula. Arch Ophthalmol. 1992;110:1358–1359. doi:10.1001/archopht.1992.01080220020005
90. Daily MJ Torpedo maculopathy or paramacular spot syndrome.
91. Grimaldi G, Scupola A, Sammarco MG, Marullo M, Blasi MA. Morpho-functional evaluation of torpedo maculopathy with optical coherence tomography angiography and microperimetry. Am J Ophthalmol Case Rep. 2018;10:165–168. doi:10.1016/j.ajoc.2018.02.019
92. Shirley K, O’Neill M, Gamble R, Ramsey A, McLoone E. Torpedo maculopathy: disease spectrum and associated choroidal neovascularisation in a paediatric population. Eye (Lond). 2018;32:1315–1320. doi:10.1038/s41433-018-0074-7
93. Sharma S, Naqvi A, Cruess AF. Bilateral macular colobomas. Can J Ophthalmol. 1996;31:27–28.
94. Richez F, Gueudry J, Brasseur G, Muraine M. [Bilateral torpedo maculopathy]. J Fr Ophtalmol. 2010;33:296. doi:10.1016/j.jfo.2009.12.013. French.
95. Rohl A, Vance S. Hyperpigmented torpedo maculopathy with pseudo-lacuna: a 5-year follow-up. Case Rep Ophthalmol. 2016;7:184–190. doi:10.1159/000445497
96. Sanabria MR, Coco RM, Sanchidrian M. Oct findings in torpedo maculopathy. Retin Cases Brief Rep. 2008;2:109–111. doi:10.1097/ICB.0b013e318033a130
97. Wong EN, Fraser-Bell S, Hunyor AP, Chen FK. Novel optical coherence tomography classification of torpedo maculopathy. Clin Exp Ophthalmol. 2015;43:342–348. doi:10.1111/ceo.12435
98. Jurjevic D, Boni C, Barthelmes D, et al. Torpedo maculopathy associated with choroidal neovascularization. Klin Monbl Augenheilkd. 2017;234:508–514. doi:10.1055/s-0043-100230
99. Pian D, Ferrucci S, Anderson SF, Wu C. Paramacular coloboma. Optom Vis Sci. 2003;80:556–563. doi:10.1097/00006324-200308000-00008
100. Rigotti M, Babighian S, Carcereri De Prati E, Marchini G. Three cases of a rare congenital abnormality of the retinal pigment epithelium: torpedo maculopathy. Ophthalmologica. 2002;216:226–227. doi:10.1159/000059639
101. Shields CL, Guzman JM, Shapiro MJ, Fogel LE, Shields JA. Torpedo maculopathy at the site of the fetal “bulge”. Arch Ophthalmol. 2010;128:499–501. doi:10.1001/archophthalmol.2010.29
102. Trevino R, Kiani S, Raveendranathan P. The expanding clinical spectrum of torpedo maculopathy. Optom Vis Sci. 2014;91:S71–78. doi:10.1097/OPX.0000000000000181
103. Bedar MS, Holz FG, Lischka T. [Hypopigmentation temporal to the macula. Case report: torpedo maculopathy]. Der Ophthalmologe. 2013;110:173–174. doi:10.1007/s00347-012-2705-x. German.
104. Pilotto E, Zannin ME, Convento E, Cortese M, Midena E. Torpedo maculopathy: a morphofunctional evaluation. Int Ophthalmol. 2013;33:71–74. doi:10.1007/s10792-012-9618-1
105. Venkatesh R, Jain K, Pereira A, Thirumalesh YNK. Torpedo retinopathy. J Ophthalmic Vis Res. 2020;15:187–194. doi:10.18502/jovr.v15i2.6736
106. Tripathy K, Sarma B, Mazumdar S. Commentary: inner retinal excavation in torpedo maculopathy and proposed type 3 lesions in optical coherence tomography. Indian J Ophthalmol. 2018;66:1213–1214. doi:10.4103/ijo.IJO_656_18
107. Venkatesh R, Bavaharan B, Yadav NK. Multicolor imaging findings in torpedo maculopathy. Indian J Ophthalmol. 2019;67:295–297. doi:10.4103/ijo.IJO_1317_18
108. Hamm C, Shechtman D, Reynolds S. A deeper look at torpedo maculopathy. Clin Exp Optom. 2017;100:563–568. doi:10.1111/cxo.12540
109. Thomas AS, Flaxel CJ, Pennesi ME. Spectral-domain optical coherence tomography and fundus autofluorescence evaluation of torpedo maculopathy. J Pediatr Ophthalmol Strabismus. 2015;52 Online:e8–10. doi:10.3928/01913913-20150303-01
110. Venkatesh R, Yadav NK, Sinha S, Mehta R, Akkali MC. Structural-functional correlation using adaptive optics, visual fields, optical coherence tomography and multifocal electroretinogram in a case of torpedo maculopathy. Indian J Ophthalmol. 2019;67:1502–1505. doi:10.4103/ijo.IJO_2044_18
111. Charbel Issa P, Gillies MC, Chew EY, et al. Macular telangiectasia type 2. Prog Retin Eye Res. 2013;34:49–77. doi:10.1016/j.preteyeres.2012.11.002
112. Leng T, Marmor MF, Kellner U, et al. Foveal cavitation as an optical coherence tomography finding in central cone dysfunction. Retina. 2012;32:1411–1419. doi:10.1097/IAE.0b013e318236e4ea
113. Su Y, Gurwood AS. Neurosensory retinal detachment secondary to torpedo maculopathy. Optometry. 2010;81:405–407. doi:10.1016/j.optm.2010.06.001
114. Giannakaki-Zimmermann H, Munk MR, Dysli C, Ebneter A, Wolf S, Zinkernagel MS. Optical coherence tomography angiography features of torpedo maculopathy. Retin Cases Brief Rep. 2017;Publish Ahead of Print. doi:10.1097/ICB.0000000000000589
115. Teitelbaum BA, Hachey DL, Messner LV. Torpedo maculopathy. J Am Optom Assoc. 1997;68:373–376.
116. Streeten BW. Development of the human retinal pigment epithelium and the posterior segment. Arch Ophthalmol. 1969;81:383–394. doi:10.1001/archopht.1969.00990010385017
117. Papastefanou VP, Vazquez-Alfageme C, Keane PA, Sagoo MS. Multimodality imaging of torpedo maculopathy with swept-source, en face optical coherence tomography and optical coherence tomography angiography. Retin Cases Brief Rep. 2018;12:153–157. doi:10.1097/ICB.0000000000000456
118. Chawla R, Pujari A, Rakheja V, Kumar A. Torpedo maculopathy: a primary choroidal capillary abnormality? Indian J Ophthalmol. 2018;66:328–329. doi:10.4103/ijo.IJO_784_17
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.